Abstract
The human neurotropic polyomavirus JC (JCV) induces a broad range of neural-origin tumors in experimental animals and has been repeatedly detected in several human cancers, most notably neural crest–origin tumors including medulloblastomas and glioblastomas. The oncogenic activity of JCV is attributed to the viral early gene products, large T and small t antigens, as evident by results from in vitro cell culture and in vivo animal studies. Recently, we have shown that alternative splicing factor, SF2/ASF, has the capacity to exert a negative effect on transcription and splicing of JCV genes in glial cells through direct association with a specific DNA motif within the viral promoter region. Here, we demonstrate that SF2/ASF suppresses large T antigen expression in JCV-transformed tumor cell lines, and the expression of SF2/ASF in such tumor cells thereby inhibits the transforming capacity of the viral tumor antigens. Moreover, down-regulation of SF2/ASF in viral-transformed tumor cell lines induces growth and proliferation of the tumor cells. Mapping analysis of the minimal peptide domain of SF2/ASF responsible for JCV promoter silencing and tumor suppressor activity suggests that amino acid residues 76 to 100 of SF2/ASF are functionally sufficient to suppress the growth of the tumor cells. These observations demonstrate a role for SF2/ASF in JCV-mediated cellular transformation and provide a new avenue of research to pathogenic mechanisms of JCV-induced tumors.
Keywords: SF2/ASF, JC virus, transcription, tumor antigen
Introduction
JCV is a human polyomavirus, which actively infects individuals mostly under immunosuppressive conditions, leading to the development of progressive multifocal leukoencephalopathy (PML). In addition to its role in the development of PML, JCV has also been shown to be associated with various tumors in laboratory animals and humans. JCV can transform primary human fetal glial cells in a manner similar to SV40.1,2 JCV-transformed primary human cells express viral-early genes and exhibit a transformed phenotype.3 Inoculation of JCV into owl and squirrel monkeys induces glioblastomas, neuroblastomas, and astrocytomas.4,5 Transgenic animals expressing the JCV-early genome under the control of the JCV promoter develop neural-origin tumors including adrenal neuroblastoma, medulloblastoma, malignant peripheral nerve sheath tumors, and pituitary adenomas.6-9 The oncogenic potential of JCV is strongly related to the expression of viral large and small tumor antigens. Ample evidence suggests that the mechanism of JCV-mediated transformation relies on the sequestration and suppression of the tumor suppressor proteins, p53 and the pRb family, by the viral large T antigen. Binding of these tumor suppressor proteins with large T antigen appears to interfere with the cell cycle regulatory properties of these proteins.
SF2/ASF (splicing factor 2/alternative splicing factor) is a member of the arginine/serine-rich splicing factor family and is one of the key regulators of alternative splicing of many genes.10 Aside from its role in the regulation of gene expression through the modulation of pre-mRNA alternative splicing, SF2/ASF has also been shown to be an inducer of translation initiation by suppressing the activity of 4E-BP1, an inhibitor of cap-dependent translation.11 We have recently demonstrated that SF2/ASF strongly regulates JCV transcription by directly targeting a double-stranded DNA motif within the viral promoter region.12 In this study, the expression of SF2/ASF in glial cells suppressed the transcription of the JCV-early proteins (large T antigen and small t antigen), as well as the viral-late proteins (agnoprotein, VP1, VP2, and VP3), resulting in abrogation of JCV propagation.
Here, we investigated the impact of SF2/ASF on JCV-induced transformation of glial cells and its effect on the maintenance of a transformed phenotype mediated by the JCV tumor antigens. Our results show that expression of SF2/ASF in tumor cell lines, transformed by JCV, strongly suppresses the expression of large T antigen, causes growth arrest, and induces apoptosis. In contrast, down-regulation of SF2/ASF in such tumor cell lines increases the growth and expansion rates of the cells under anchorage-independent conditions. Collectively, these observations may suggest a significant role of SF2/ASF in JCV-mediated cellular transformation and provide a novel approach to target JCV-induced tumors.
Results
JCV tumor antigen expression is suppressed by SF2/ASF in viral-transformed cell lines
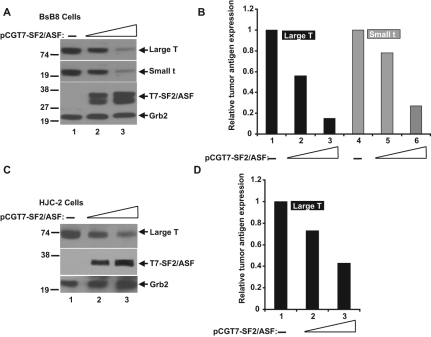
The impact of SF2/ASF on JCV tumor antigen expression was tested in BsB8 cells, a cell line that originated from a medulloblastoma developed in a transgenic mouse expressing the JCV-early region,8 and in HJC-2 cells, a cell line obtained from a glioblastoma induced by intracranial injection of JCV in newborn hamsters.13,14 Both cell lines express the JCV-early gene products, large T and small t antigens, under the control of the JCV-early promoter. To investigate the possible role of SF2/ASF in regulating the expression of large T antigen, T7-tagged SF2/ASF was introduced into BsB8 and HJC-2 cells by transient transfection (Fig. 1). Western blot analyses of whole cell extracts from BsB8 and HJC-2 cells revealed that SF2/ASF suppressed the protein expression levels of large T antigen as well as small t antigen in a dose-dependent manner. These results show that SF2/ASF down-regulates JCV tumor antigen expression in the viral-transformed tumors.
Figure 1.
SF2/ASF inhibits tumor antigen expression in JCV-transformed cell lines. (A) Western blot analysis of JCV tumor antigen expression in BsB8 cells. BsB8 cells were transfected with increasing concentrations of an SF2/ASF expression plasmid, which produces SF2/ASF in fusion with T7 as an expression tag. Expressions of large tumor antigen, small tumor antigen, and SF2/ASF were detected by specific antibodies in whole cell lysates at 48 hours posttransfection. GRB2 was probed as loading control. (B) Band intensities of large T antigen and small t antigen proteins from A were quantified by densitometric analyses and shown as a bar graph. (C) Western blot analysis of JCV large T antigen expression in HJC-2 cells overexpressing T7-tagged SF2/ASF. (D) Band intensity of large T antigen expression from C is shown as a bar graph. Levels of tumor antigen expression in all panels are presented as percentages relative to levels detected in untransfected control cells.
SF2/ASF inhibits proliferation of JCV-transformed cells
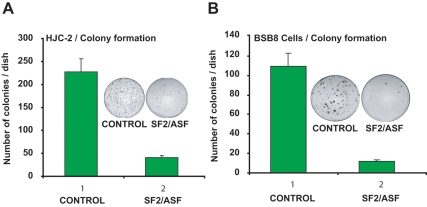
Previous studies have suggested that the transformed phenotype of BsB8 cells is due to the expression of viral tumor antigens.15 To determine the effect of SF2/ASF-mediated suppression of large T antigen expression on the transformed phenotype, a colony formation assay was conducted. BsB8 and HJC-2 cells were transiently cotransfected with T7-SF2/ASF expression vector in combination with a plasmid carrying the G418 resistance gene, and cells were grown for 2 to 3 weeks in G418 containing medium. As seen in Figure 2, control groups (transfected with pCGT7-empty plasmid) of BsB8 and HJC-2 cells formed colonies as expected. However, expression of SF2/ASF in these cell lines greatly suppressed the growth of the cells when compared to the control groups (Fig. 2A and 2B, compare lane 1 with lane 2).
Figure 2.
Growth inhibition of JCV-transformed tumor cells by SF2/ASF. Colony formation of HJC-2 and BsB8 cells overexpressing SF2/ASF. HJC-2 (A) and BsB8 (B) cells were seeded in 100-mm plates and transfected with 10 μg of either empty plasmid or SF2/ASF expression plasmid along with a plasmid encoding resistance to G418. At 24 hours posttransfection, cultures were harvested, and equal numbers of cells (1 × 105 cells/dish) were plated in 100-mm plates containing DMEM plus fetal calf serum (10%) and 1 mM G418 for selection of the transfected cells. After incubation for 2 weeks, cells were washed with 1x PBS and fixed with solution containing 1% methylene blue for 10 minutes. All experiments were carried out in triplicate. Images depict representative data.
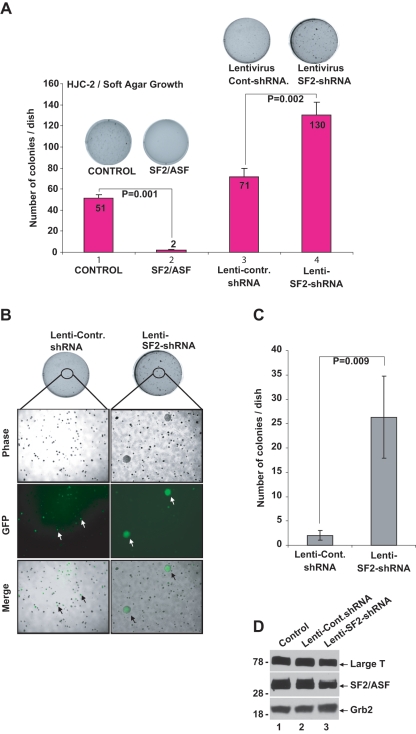
Next, we tested the effect of SF2/ASF on the growth of HJC-2 cells under anchorage-independent conditions. HJC-2 cells were either transfected with T7-SF2/ASF expression plasmid or infected with a lentivirus encoding shRNAs against SF2/ASF. After the optimal incubation time, cells were fixed and stained to visualize the colonies. As expected, HJC-2 cells formed numerous colonies in soft agar under anchorage-independent conditions (Fig. 3A, lane 1). Results from three independent experiments revealed that expression of SF2/ASF significantly inhibited colony formation on soft agar (Fig. 3A, lane 2). Moreover, HJC-2 cells infected with lentiviruses, expressing shRNA sequences against SF2/ASF, formed considerably more colonies than the control cells and those infected with control for lentivirus (Fig. 3A, compare lane 4 with lanes 1 and 3, respectively). Interestingly, down-regulation of SF2/ASF in HJC-2 cells not only increased the number of the colonies formed but also led to the formation of colonies that were much larger in size (Fig. 3B and 3C), suggesting more aggressive growth of these cells under anchorage-independent conditions.
Figure 3.
SF2/ASF inhibits anchorage-independent growth of HJC-2 cells in soft agar. (A) Approximately 5 × 105 HJC-2 cells were transfected with pCDNA 3.1 plus pCGT7 (control) or pCGT7-SF2/ASF (SF2/ASF) constructs. Twenty-four hours after transfection, cells were trypsinized and counted, and 10,000 cells were seeded into soft agar media containing 1 mM G418, as described in Materials and Methods. In a separate set of soft agar experiments, HJC-2 cells were plated (5 × 105) in 60-mm dishes and infected with either control lentivirus (lenti-control) or with lentivirus containing shRNA sequence against SF2/ASF (lenti-SF2-shRNA). Forty-eight hours after infection, cells were trypsinized and counted, and 10,000 cells were seeded into soft agar media. Plates were incubated at 37°C for 3 weeks, and colonies were counted. Each experiment was performed in triplicate. (B) Down-regulation of SF2/ASF increased the growth rate of HJC-2 cells. HJC-2 cells were infected with lenti-SF2-shRNA as described in A. Phase, GFP, and merge images of colonies formed in soft agar were captured by fluorescence microscope. (C) Quantification of the size and number of the colonies from B. Numbers of colonies >10 mm2 were counted and shown as a bar graph. (D) Western blot analyses of SF2/ASF and large T antigen expression in HJC-2 cells infected with lenti-control and lenti-SF2/ASF-shRNA at 48 hours postinfections. Grb2 was probed in the same blots as loading control. The Student t test was performed to calculate P values in A and C.
Extinction of JCV tumor antigens by SF2/ASF results in loss of viability and induction of apoptosis in BsB8 and HJC-2 cells
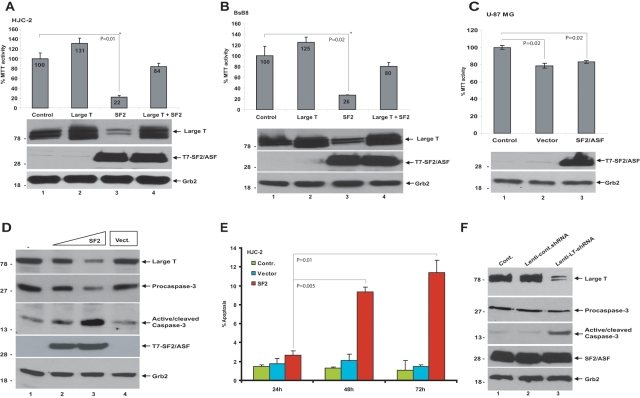
In the next series of experiments, we analyzed the effects of SF2/ASF on cellular viability of HJC-2, BsB8, and U-87 MG cells by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Cells were plated in 6-well plates and transfected either with pCG-T7 (empty vector) or pCG-T7-SF2/ASF expression plasmids. As shown in Figure 4, ectopic expression of SF2/ASF greatly suppressed the MTT activities of HJC-2 and BsB8 cells (Fig. 4A and 4B, respectively). The MTT activity of the human glioblastoma cell line, U-87 MG, ectopically expressing SF2/ASF was also analyzed. Unlike the JCV-transformed cells, the MTT activity of U-87 MG cells was not affected by SF2/ASF (Fig. 4C), suggesting that SF2/ASF-mediated suppression of tumor growth was specific to JCV-transformed cells. The selective growth inhibition by SF2/ASF in JCV-transformed cells but not in the U-87 MG cells suggests that the tumor suppressor action of SF2/ASF in these cells is mainly related to the large T antigen down-regulation.
Figure 4.
SF2/ASF-mediated loss of cell viability and induction of apoptosis in HJC-2 and BsB8 cells. HJC-2 (A) and BsB8 (B) cells were transiently transfected with expression plasmids encoding SF2/ASF and large T antigen either individually or in combination. Seventy-two hours after transfection, MTT activities were detected. MTT activity of untransfected cells is presented as 100%, and MTT activity of the treated groups is presented proportionally. In parallel to MTT assays, Western blot analyses of large T antigen and SF2/ASF expression were detected by specific antibodies. GRB2 was probed as loading control. (C) U-87 MG cells were transfected with empty vector or an SF2/ASF expression plasmid. MTT activities were detected as described in A and B. (D) Induction of apoptosis by SF2/ASF in HJC-2 cells was analyzed by transient transfection studies. Western blot analyses of large tumor antigen, procaspase-3 (inactive), and cleaved caspase-3 (active) and SF2/ASF expression were detected by specific antibodies. GRB2 was probed as loading control. (E) SF2/ASF-induced apoptosis in HJC-2 cells was analyzed by flow cytometry as described in Materials and Methods. Percentage of cells undergoing apoptosis is shown as a bar graph at 24, 48, and 72 hours after transfections. Standard deviations reflect the results from two independent experiments. (F) Induction of apoptosis by direct down-regulation of large T antigen in HJC-2 cells. HJC-2 cells were either infected with lenti-control and lenti-SF2/ASF-shRNA or left uninfected. At 48 hours postinfection, expression of large tumor antigen, procaspase-3 (inactive), and cleaved caspase-3 (active) and SF2/ASF was detected by specific antibodies. Grb2 was probed as loading control. The Student t test was performed to calculate P values in A, B, C, and E.
To further confirm this, we performed complementation studies by transiently expressing large T antigen under a CMV promoter. Coexpression of large T antigen under CMV promoter significantly complemented the SF2/ASF-mediated inhibition of cellular viability in HJC-2 and BsB8 cells (Fig. 4A [lane 4] and 4B [lane 4], respectively), suggesting that large T antigen expression was required to maintain cell viability.
To further demonstrate the effect of SF2/ASF on the survival of JCV-transformed cells, SF2/ASF was introduced into HJC-2 cells in increasing concentrations by transient transfection. Whole cell extracts were prepared at 72 hours posttransfection, and expression of cleaved caspase-3, a well-documented indicator of apoptosis, was analyzed by Western blotting. As shown in Figure 4D, overexpression of SF2/ASF led to a shift of caspase 3 from its inactive (procaspase) to active (cleaved) form. This induction of apoptosis in HJC-2 cells overexpressing SF2/ASF was further confirmed through flow cytometry analysis (Fig. 4E). In order to determine the direct involvement of large T antigen in the maintenance of tumor survival, we have developed a lentiviral shRNA construct for the down-regulation of large T antigen and investigated the impact of large T antigen down-regulation on the survival of JCV-transformed cells. As shown in Figure 4F, down-regulation of large T antigen also induced apoptosis in HJC-2 cells, as evidenced by the induction of active caspase-3. These studies suggest that expression of the viral tumor antigens is required for the proliferation and survival of these tumor cells and confirm the role of SF2/ASF as a tumor suppressor in JCV-induced tumor cells.
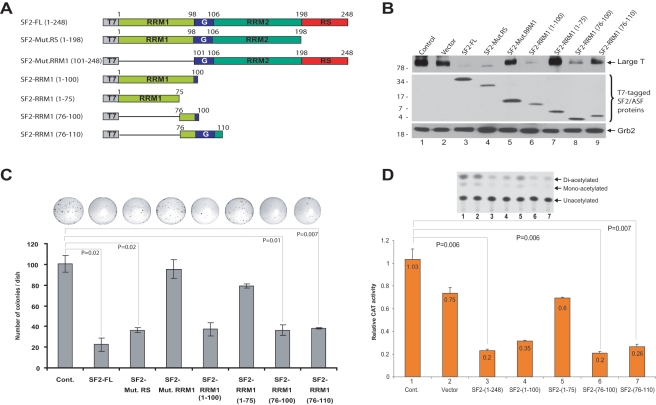
The 76-to-100–amino acid region of SF2/ASF is sufficient to suppress growth of JCV-transformed cells
SF2/ASF has a modular structure (Fig. 5A), consisting of two copies of N-terminal RNA recognition motifs (RRM1, RRM1), followed by a C-terminal arginine-serine–rich domain (RS). We have previously reported that the RRM1 domain has the capacity to interact with the JCV promoter and that this interaction mediates suppression of JCV gene transcription.12 To identify the region within SF2/ASF that is involved in the suppression of large T antigen expression, HJC-2 cells were transfected with plasmids expressing various deletion mutants of SF2/ASF, and large T antigen expression was analyzed by Western blotting (Fig. 5A and 5B). As shown in Figure 5B, wild-type SF2/ASF (SF2-FL, lane 3) and the RRM1 domain (lane 6) sufficiently suppressed the expression of large T antigen. On the other hand, removal of the RRM1 domain (lane 5) had very low, if any, inhibitory effect on large T antigen expression. Further truncation mutations within RRM1 domain revealed that the amino acid region from 76 to 100 was sufficient to suppress large T antigen expression (Fig. 5B, compare lane 8 with lanes 6, 7, and 9). To assess the importance of the SF2/ASF-(76-100) region in cell growth inhibitory function, we performed colony formation assay in which HJC-2 cells were transfected with plasmids expressing various mutations of SF2/ASF. In accordance with Western blot analyses (Fig. 5B), the plasmid encoding amino acid region 76 to 100 led to a greater level of growth suppression compared to the amino acid region 1 to 75 (Fig. 5C). SF2/ASF also contains a “glycine-rich domain” residing between amino acid residues 98 and 106. To test whether the glycine-rich domain had any impact on SF2/ASF-mediated inhibition of tumor growth, we compared the activity of a plasmid encoding SF2/ASF-(76-110) construct with the one encoding SF2/ASF-(76-100). As seen in Figure 5C, SF2/ASF-(76-110) also showed very similar growth inhibition to that seen with SF2/ASF-(76-100), suggesting that the glycine-rich domain does not alter the tumor suppressor activity of amino acid residues 76 to 100 (compare column 8 with column 7). Therefore, these results show that the 76-to-100–amino acid region of SF2/ASF is the minimal effective peptide for SF2/ASF-mediated growth inhibition of JCV-transformed cells.
Figure 5.
Characterization of the domain of SF2/ASF important for the suppression of large T antigen expression and cell growth inhibition. (A) Schematic representation of 248 amino acid full-length SF2/ASF and the deletion mutant constructs utilized. SF2/ASF deletion mutants were cloned into pCGT7 expression vector and tagged with T7. The “G” depicts the glycine-rich domain. (B) Western blot analysis of large T antigen in HJC-2 cells transfected with the SF2/ASF mutant constructs. Expression of SF2/ASF full-length and truncated mutants was analyzed by Western blot analysis with anti-T7 antibody. Grb2 was probed as a loading control. (C) The ability of SF2/ASF full-length and various mutants to inhibit HJC-2 cell growth was analyzed by colony formation as described above in Figure 2. (D) pBLCAT3 JCV-early reporter plasmid was transiently transfected into U-87 MG cells either alone or in combination with expression plasmids encoding various deletion mutants of SF2/ASF. CAT enzymatic activities were determined, and relative CAT activities are presented as a bar graph. The autoradiograph (above the graph) represents results from one of the three independent experiments presented in the graph. The Student t test was performed to calculate P values in C and D.
In the next series of experiments, we measured the effect of SF2/ASF deletion mutants on the transcription of the JCV-early promoter. U-87 MG cells were transiently transfected with expression plasmids encoding various deletion mutants of SF2/ASF and with a reporter plasmid encoding the CAT gene under the JCV-early regulatory region. In accordance with previous studies,12 the full-length SF2/ASF construct (amino acids 1-248) and the RRM1 domain (amino acids 1-100) suppressed transcription mediated by the JCV promoter (Fig. 5D, columns 3 and 4, respectively). To evaluate the importance of the regions within the RRM1 domain in the control of JCV gene transcription, we then examined the impact of the RRM1 deletion mutants on JCV promoter activity. The results indicate that amino acid region 1 to 75 of the RRM1 domain showed no significant effect on JCV transcription (Fig. 5D, column 5). On the other hand, amino acid region 76 to 100 of SF2/ASF effectively suppressed activity of the JCV promoter (Fig. 5D, column 6). Consistent with the tumor growth assays (Fig. 5C), expression of the glycine-rich domain in addition to amino acid region 76 to 100 of SF2/ASF did not cause any significant alteration in the rate of promoter suppression (Fig. 5D, compare column 7 with column 6). These results suggest that the amino acid region 76 to 100 of SF2/ASF is sufficient to suppress JCV transcription.
Discussion
We have previously reported that SF2/ASF, splicing factor 2/alternative splicing factor, inhibits JCV propagation in glial cells by targeting a specific DNA motif within the JCV bidirectional promoter region, leading to inhibition of viral-early and -late gene transcription.12 SF2/ASF-mediated inhibition of viral genes at the transcriptional level was specific to JCV and required the viral promoter. Previous studies have suggested that SF2/ASF is up-regulated in various human tumors, including lung, kidney, colon, pancreas, small intestine, and thyroid. Up-regulation of SF2/ASF in tumor cells indicates a possible role for this factor in malignant transformation. Furthermore, overexpression of SF2/ASF is sufficient to transform NIH-3T3 and Rat1 fibroblasts through aberrant splicing of the tumor suppressor, BIN1, and the kinases, MNK2 and S6K1.16
In addition to the role of JCV in the development of the demyelinating disease, PML, JCV has also been associated with a broad range of tumors in humans and laboratory animals. In order to investigate the role of SF2/ASF in JCV-induced tumors, we utilized HJC-2 and BsB8 cells, which contained an integrated portion of the JC viral genome, which encodes large T and small t antigens under the control of the JCV-early promoter. Western blot analyses of HJC-2 and BsB8 cells have revealed that overexpression of SF2/ASF caused inhibition of tumor antigen expression in both cell lines, suggesting that SF2/ASF could also inhibit expression of JCV genes while integrated within the host chromosomes. Analyses of the growth characteristics of these cell lines by colony formation and soft agar assays revealed that SF2/ASF-mediated extinction of tumor antigen expression resulted in loss of the transformed characteristics of these cell lines. Moreover, SF2/ASF expression decreased cell viability and induced apoptosis in the JCV-induced tumor cells, but not in the human glioblastoma cell line, U-87 MG, suggesting that the tumor suppressor effect of SF2/ASF was specific to tumors transformed by JCV. In other words, SF2/ASF-mediated suppression of tumor growth and induction of apoptosis were not related to any other cellular signal events that were specifically or directly affected by SF2/ASF. Indeed, it was highly related to the viral tumor antigen expression, which was suppressed by SF2/ASF at the level of transcription, suggesting that large T antigen expression was required for maintaining the transformed phenotype of these tumors. This was further supported by the experiments involving direct down- regulation of large T antigen by an shRNA construct, leading to induction of apoptosis in JCV-transformed cell lines. This observation is consistent with previous studies in JCV transgenic mice, which were created by JCV-early genes. Interestingly, immunohistochemical studies of various tumors induced in these transgenic animals revealed that a subset of tumor cells exhibited viral large T antigen expression,8,9 suggesting that expression of JCV tumor antigen is negatively regulated by host factors in a specific portion of these tumors. In addition, subcutaneous inoculation of the large T antigen positive cells, BsB8, in the flanks of nude mice resulted in rapid tumor development, while large T antigen negative cells did not proliferate significantly in nude mice, suggesting that large T antigen expression contributes to the growth of these tumors.8,9,15
JCV gene expression is a highly regulated process, which is under tight control of the viral promoter by inducible and ubiquitously expressed cellular transcription factors in permissive cells. The restricted tropism of JCV to the central nervous system (CNS) is attributed to the tissue-specific expression of the viral-early promoter and large T antigen, which is the main regulatory protein of viral replication and transcription. Identification of SF2/ASF, which is ubiquitously expressed in tissues throughout the body, as a negative regulator of JCV tumor antigen expression may suggest a very important checkpoint for the regulation of JCV gene expression in permissive as well as nonpermissive cells.
SF2/ASF has a modular structure consisting of two copies of N-terminal RNA recognition motifs (RRM1, RRM2), followed by a C-terminal domain rich in Arg and Ser residues known as the arginine-serine–rich (RS) domain. The RS domain interacts with the components of the core-splicing apparatus to form splice site pairing, whereas the RRM1 and the RRM2 domains determine the RNA-binding specificity.17,18 We previously demonstrated that the RRM1 domain of SF2/ASF is mainly responsible for the suppression of JCV gene transcription.12 Here, we further characterized the minimal region within RRM1 domain that is involved in the suppression of JCV tumor antigen expression in viral-transformed cells. Results from biochemical and functional studies have revealed that amino acids 76 to 100 of SF2/ASF are sufficient to inhibit expression of the tumor antigen and the viral-early promoter activity. Further investigation of the interaction between this small peptide with the JCV promoter region and employment of computational chemistry to decipher the structure of the crystallized peptide may lead to the development of small molecules to suppress the expression of JCV genes. These observations provide further evidence that SF2/ASF is a critical cellular protein that limits/controls expression of JCV genes and may suggest a novel target for the treatment of JCV-caused diseases.
Materials and Methods
Plasmid constructs
pcDNA3.1 large T antigen, pCGT7-SF2/ASF-FL, pCGT7-SF2/ASF-Mut.RS, pCGT7-SF2/ASF-Mut.RRM1, and pCGT7-SF2/ASF-RRM1 expression vectors were previously described.12 SF2/ASF-RRM1 mutants were cloned into pCGT7 vector at the Xba1 and BamH1 sites and were created using the following primer pairs: SF2/ASF-RRM1(1-75) forward: 5′-ACCTTCCATCTAGATCGGGAGGTGGTGTGATTCGT-3′, SF2/ASF-RRM1(1-75) reverse: 5′-TTCCAGGATCCTTAGTCGCGACCATACACCGCGTCTT-3′, SF2/ASF-RRM1 (76-100) forward: 5′-ACCTTCCATCTAGAGGCTATGATTACGATGGGTA-3′, SF2/ASF-RRM1 (76-100) reverse: 5′-ACCTTCCAGGATCCTTAGCCGCCGCCTCGGC CTGTT-3′, SF2/ASF-RRM1 (76-110) reverse: 5′-ACC TTCCAGGATCCTTAACCTCGG GGAGCTCCGC CA-3′.
Cell lines and cell culture
BsB8 cells were derived from primitive neuroectodermal tumors that developed in transgenic mice expressing the early genome of the JC virus.8 HJC-2 is a clonal subline of the HC-15 glioma cell line, which was established from a hamster brain tumor induced by intracerebral inoculation of neonatal hamsters with JCV.13,14,19 U-87 MG, a human malignant glioma cell line, was obtained from the American Type Culture Collection (ATCC). All cell lines and cultures were maintained at 37°C in a humidified atmosphere with 7% CO2.
Western blot analysis
Whole cell extracts were prepared in TNN buffer (150 mM NaC, 40 mM tris-HCL, pH 7.4, 1% NP-40, 1 mM DTT, 1 mM EDTA, and protease inhibitors) from BsB8 and HJC-2 cells transfected with pCG-T7-SF2/ASF and were separated by SDS-PAGE and transferred onto a 0.2-μm nitrocellulose membrane (BioExpress, Kaysville, UT). Blots were blocked in 10% nonfat dry milk in 1x PBST and then incubated with one of the following primary antibodies: anti-SV40 T-Ag Ab2 (1:5,000 in 2% milk/1x PBST, mouse; Calbiochem, San Diego, CA), anti-Grb2 (1:3,000 in 10% milk/1x PBST, mouse; BD Biosciences, Franklin Lakes, NJ), or anti-T7 (1:3,000 in 2% milk/1x PBST, mouse; Novagen, Gibbstown, NJ). Goat anti-mouse HRP secondary antibodies (1:5,000 in 2% milk/1x PBST) were applied, and an Amersham ECL Development kit (GE Healthcare, Fairfield, CT) was used as chemoluminescence.
Colony formation assay
BsB8 and HJC-2 cells (5 × 105/100-mm dishes) were cotransfected either with pCGT7 empty vector or pCGT7-SF2/ASF and pcDNA 3.1 zeo (+) plasmids (1 µg each). After 24 hours, transfected and untransfected control cells were collected by trypsinization and replated in 100-mm dishes (1 × 105/100-mm dishes) in DMEM medium containing 10% FBS and G418 for selection (1 mM). Cells were maintained for 3 weeks, and the number of colonies was determined by fixing and staining the cells with 1% methylene blue. All experiments were performed in triplicate.
Soft agar growth assay
BsB8 and HCJ-2 cells (3 × 105/60-mm dishes) were either cotransfected with pCGT7-SF2/ASF and pcDNA 3.1 zeo (+) plasmids or infected with lenti-SF2/ASF-shRNA. After 24 hours, the cells were harvested and reseeded in 60-mm dishes (5,000 cells/dish) containing 2 mL of a 0.3% agarose suspension in DMEM plus 10% fetal bovine serum and G418 (1 mM). Plates were incubated at 37°C with 7% CO2 for 3 weeks. All experiments were done in triplicate.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay for cell proliferation
HJC-2, BsB8, and U-87 MG cells were plated in 6-well plates (2 × 105 cells/plate) and transfected either with pCGT7 vector alone or pCGT7-SF2/ASF expression plasmid. Seventy-two hours after transfection, cells were washed with 1x PBS and incubated with 1 mL of MTT working solution (DMEM with 0.5 mg/mL MTT; Invitrogen, Carlsbad, CA) for 1 hour at 37°C. At the end of the incubation, the converted dye was vigorously resuspended with 1 mL acidic isopropanol (0.004 M HCL in isopropanol). The dye solution was then transferred into 1.5 mL eppendorf tubes and was centrifuged at 15,000 rpm for 5 minutes. The supernatant was transferred into new tubes, and absorbance of the converted dye was measured at a wavelength of 570 nm with background subtraction at 650 nm.
Flow cytometry analyses
HJC-2 cells were seeded at a density of 250,000 cells in 60-mm dishes and transfected with 3 µg of pCGT7 alone or with 3 µg of pCGT7-SF2/ASF expression plasmid. Untransfected cells were used as a control. Cells were harvested at 24, 48, and 72 hours posttransfection, then fixed in 70% ethanol, and stained for 45 minutes with Guava Cell Cycle Reagent (Guava Technologies, Hayward, CA), based on standard propidium iodide (PI) chemistry for quantification for DNA in whole cells. Cell acquisition and analyses were performed by the Guava Easy cycle Mini machine and software. All experiments were performed in triplicate.
Lentiviral infection and RNA interference
Lentivirus-based U6-promoted SF2/ASF shRNA constructs were generated by cloning PCR products carrying sense and antisense oligonucleotides of SF2/ASF into the pLL3.7 vector, which also express green fluorescent protein (GFP), as described previously.12 To knock down the expression of large T antigen, shRNA construct was generated to target the nucleotide sequences 139 to 162 of the JCV large T antigen cDNA. PCR products carrying sense and antisense oligonucleotides of large T antigen were cloned into the pLL3.7 vector. The viruses were packaged in 293T (human embryonic kidney) cells according to the procedure described previously.20,21 HJC-2 cells were seeded in 6-well plates at 50% confluency and were incubated with 1 mL of the viral supernatants. The infected cells were then kept in regular complete medium (1× DMEM, 10% FBS) for 48 hours. Inspection by fluorescence microscopy confirmed the presence of more than 80% of GFP-positive cells after viral infection.
Reporter gene assays
JCV-early promoter reporter gene construct containing the viral regulatory region has been described previously.22 U-87 MG cells were transfected with the reporter construct in the presence or absence of expression plasmids for SF2/ASF-FL and its various deletion mutants. At 48 hours posttransfection, cells were extracted with a series of freeze/thaw cycles, and the CAT activity of the samples was determined.
Acknowledgments
The authors thank the past and present members of the Department of Neuroscience/Center for Neurovirology for sharing their ideas and reagents, particularly Drs. Kamel Khalili and Mahmut Safak. They also thank Drs. Martyn White and George Tuszynski for critical reading of this article.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
This work was supported by the National Institutes of Health (NIH) [grant number R01 MH086358].
References
- 1. Mandl C, Walker DL, Frisque RJ. Derivation and characterization of POJ cells transformed human fetal glial cells that retain their permissivity for JC virus. J Virol. 1987;61(3):755-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Major EO, Miller AE, Mourrain P, Traub RG, de Widt E, Sever J. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci U S A. 1985;82(4):1257-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gallia GL, Gordon J, Khalili K. Tumor pathogenesis of human neurotropic JC virus in the CNS. J Neurovirol. 1998;4(2):175-81 [DOI] [PubMed] [Google Scholar]
- 4. London WT, Houff SA, Madden DL, et al. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus). Science. 1978;201:1246-9 [DOI] [PubMed] [Google Scholar]
- 5. London WT, Houff SA, McKeever PE, et al. Viral-induced astrocytomas in squirrel monkeys. Prog Clin Biol Res. 1983;105:227-37 [PubMed] [Google Scholar]
- 6. Franks RR, Rencic A, Gordon J, et al. Formation of undifferentiated mesenteric tumors in transgenic mice expressing human neurotropic polymavirus early protein. Oncogene. 1996;12:2573-8 [PubMed] [Google Scholar]
- 7. Shollar D, Del Valle L, Khalili K, Otte J, Gordon J. JCV T-antigen interacts with the neurofibromatosis type 2 gene product in a transgenic mouse model of malignant peripheral nerve sheath tumors. Oncogene. 2004;23(32):5459-67 [DOI] [PubMed] [Google Scholar]
- 8. Krynska B, Otte J, Franks R, Khalili K, Croul S. Human ubiquitous JCV (CY) T-antigen gene induces brain tumors in experimental animals. Oncogene. 1999;18(1):39-46 [DOI] [PubMed] [Google Scholar]
- 9. Gordon J, Del Valle L, Otte J, Khalili K. Pituitary neoplasia induced by expression of human neurotropic polyomavirus, JCV, early genome in transgenic mice. Oncogene. 2000;19:4840-6 [DOI] [PubMed] [Google Scholar]
- 10. Manley JL, Tacke R. SR proteins and splicing control. Genes Dev. 1996;10:1569-79 [DOI] [PubMed] [Google Scholar]
- 11. Michlewski G, Sanford JR, Caceres JF. The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E-BP1. Mol Cell. 2008;30(2):179-89 [DOI] [PubMed] [Google Scholar]
- 12. Sariyer IK, Khalili K. Regulation of human neurotropic JC virus replication by alternative splicing factor SF2/ASF in glial cells. PLoS One. 2011;6(1):e14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wold WS, Green M, Mackey JK, Martin JD, Padgett BL, Walker DL. Integration pattern of human JC virus sequences in two clones of a cell line established from a JC virus-induced hamster brain tumor. J Virol. 1980;33(3):1225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raj G, Gordon J, Logan T, et al. Characterization of glioma-cells derived from human polyomavirus-induced brain-tumors in hamsters. Int J Oncol. 1995;7(4):801-8 [DOI] [PubMed] [Google Scholar]
- 15. Krynska B, Del Valle L, Gordon J, Otte J, Croul S, Khalili K. Identification of a novel p53 mutation in JCV-induced mouse medulloblastoma. Virology. 2000;274(1):65-74 [DOI] [PubMed] [Google Scholar]
- 16. Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14(3):185-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graveley BR. Sorting out the complexity of SR protein functions. RNA. 2000;6:1197-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanford SR, Ellis JD, Cazalla D, Caceres JF. Reversible phosphorylation differentially affects nuclear and cytoplasmic functions of splicing factor 2/alternative splicing factor. PNAS. 2005;102(42):15042-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walker DL, Padgett BL, ZuRhein GM, Albert AE, Marsh RF. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973;181:674-6 [DOI] [PubMed] [Google Scholar]
- 20. Song Y, Wilkins P, Hu W, et al. Inhibition of calcium-independent phospholipase A2 suppresses proliferation and tumorigenicity of ovarian carcinoma cells. Biochem J. 2007;406(3):427-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubinson DA, Dillon CP, Kwiatkowski AV, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33(3):401-6 [DOI] [PubMed] [Google Scholar]
- 22. Akan I, Sariyer IK, Biffi R, et al. Human polyomavirus JCV late leader peptide region contains important regulatory elements. Virology. 2006;349(1):66-78 [DOI] [PubMed] [Google Scholar]