Abstract
The antioxidant principles isolated from the various parts of the plant are verminoside (leaf, stem bark and flowers; EC50 = 2.04 µg/ml), Specioside (flowers; EC50 = 17.44 µg/ml), Kampeferol diglucoside (leaf; EC50 = 8.87 µg/ml) and Caffeic acid (leaf and fruits). The non anti-oxidant components isolated in the study include ajugol (stem bark and fruits) and phytol (leaf).
Keywords: Spathodea campanulata, Bignoniaceae, Verminoside, Specioside, Ajugol, Kaempeferol diglucoside, Caffeic acid, antioxidant activity, DPPH
Introduction
Iridoids are monoterpene derivatives with cyclopentane pyran rings. They have been found as natural constituents in a large number of plant families (Tietze, 1983). The major iridoid yielding families include Oleaceae, Fontanesieae, Myxopyreae, Oleeae, Gentianales and Lamiales. Iridoids were not considered for a long time as having any interesting or important pharmacological activities (Ghisalberti, 1998). They have been shown to be present in a number of folk medicines used as bitter tonics, sedatives, febrifuges, cough medicines, remedies for wounds, skin disorders and hypotensives. Iridoids have also been revealed to exhibit a wide range of bioactivity and pharmacological activities including anti-inflammatory, antiamoebic (Bharti, et al., 2006), cardiovascular (Pennacchio, et al., 1996) and hepatoprotective activity (Gary et al., 1994).
The various plant parts of S. campanulata (leaves, stem bark, flowers, and roots) have been used in ethnomedicine for the treatment of diseases (ulcers, dysentery, oedemas, skin eruptions, scabies, wound healing and urethral discharge) and veterinary application have been attributed to the plant in different cultures (Burkill, 1985; Hutchinson and Dalziel, 1954). Previous phytochemical studies of the plant have revealed the isolation of triterpenes of the ursane and oleanane types from the bark (Niyonzima, et al., 1999; Amusan, et al., 1996; Ngouela et al., 1990). Spathodol, flavonoids and other phenolic acids have been reported from the leaves (Ngouela et al., 1991) and anthocyanins (Scogin, 1980) from the flowers. Verminoside was previously reported from the stem bark (Ngouela et al., 1988) and ajugol was only recently reported from the root bark (Adriana et al., 2007). Biological activities such as hypoglycaemic, anticomplement and anti-HIV were reported for the polar fractions of this plant. However, in spite of these interesting activities, the constituents responsible were unknown. The present study examines the stem bark, leaves, flowers and fruits of the plant for anti-oxidant principles.
Experimental and Results
Plant materials and general experimental details
Spathodea campanulata leaf, stem bark, fruits and flowers were collected from the campus of Obafemi Awolowo University (O.A.U.), Ile-Ife, Nigeria. The flowers were obtained during the flowering season between November and February. The plant was identified by Dr. Illoh and Mr. Adaramola, Department of Botany where a voucher specimen (13598) was deposited. TLC (normal and reverse phase) were performed on Silica gel 60F234 plates (0.2mm) Merck while silica gel 230-400 mesh was used for medium pressure liquid chromatography. Spots were generally detected under UV or visualized by spraying with vanillin-H2SO4 (v/s spray) followed by heating, while the detection, isolation and purification of antioxidant spots was by DPPH-guided bioautography method employing solvent systems A (Toluene: acetone: H2O, 10:25:1), B (Toluene: acetone: H2O, 10:30:1), C (50% MeOH) and D (Hexane: CHCl3, 7:3) as appropriate.
Extraction and isolation procedure
Isolation of Ajugol from the dried stem bark: Stem bark (600g) was oven-dried at 40°C for 3 days. The powered material was extracted with 50% EtOH for 72 hrs to yield the crude extract, SDB (40g, 6.7%). The extract was partitioned between CH2Cl2, n-BuOH and distilled H2O. The n-BuOH fraction (4.73g) was further fractionated by medium pressure liquid chromatography (MPLC) on silica employing toluene: acetone: MeOH solvent mixtures in gradient. Fraction SDB1 (236 mg), eluted from acetone was purified by column chromatography using sephadex LH-20 to obtain Ajugol (99mg), which eluted from 25% ethanol. It was identified as a pink reacting substance on silica (v/s spray) with Rf value of 0.1 (system A). It is non-UV visible compound and showed no antioxidant property.
Isolation of Verminoside from fresh stem bark: Fresh bark (2.5Kg) were chopped and extracted with MeOH at room temperature for 72 hours. The extract was evaporated to dryness in vacuo to yield a crude material, SFB (140 g, 5.6 %). SFB was partitioned on column chromatography using animal charcoal. 5 fractions were obtained; however, the aqueous fraction (SFB1) had the highest yield (52.5 g) and the strongest antioxidant reaction (Tlc). It was further separated by column chromatography using sephadex LH-20 employing aq. EtOH mixture in gradient. Two major sub-fractions SFB2 (1.4g) and SFB3 (66mg) were obtained. Repeated medium pressure liquid chromatography purification of SFB2 gave Verminoside (525 mg) which eluted with 70% CHCl3: MeOH. It was identified by NMR and by comparison with literature data. It is strongly anti-oxidant and strongly phenolic (FeCl3 reaction). It had an Rf values of 0.31, reverse phase (system C) and 0.33, silica (system B).
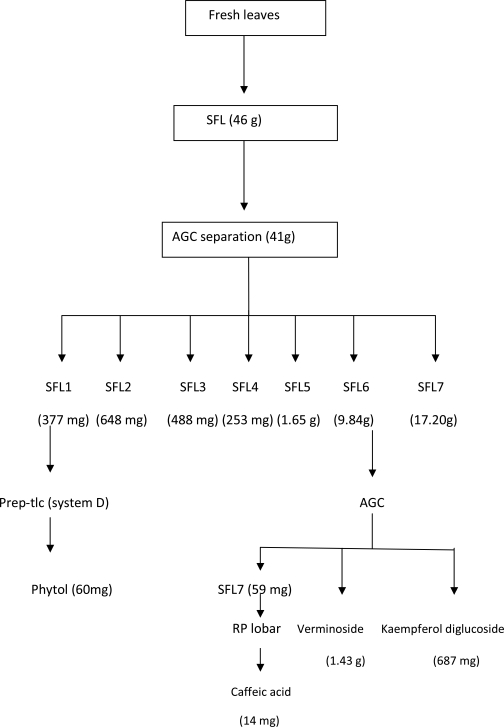
Compounds from the leaves: The preliminary examination of the leaves revealed 2 strongly anti-oxidant spots and other minor components. Fresh leaves were macerated in MeOH and extracted for 24 hours to generate a crude leaf extract (46 g). The crude extract, SFL was separated by direct accelerated gradient chromatography on silica (a form of MPLC) employing hexane: EtOAc: MeOH in gradient. A total of 129 fractions were collected and fractions with similar Tlc profile (v/s spray and DPPH reaction) were pooled into 7 main fractions (SFL1-7). Fraction SFL6 (9.8 g) eluted from EtOAc: MeOH (50%–20%) step gradient. It was purified by another AGC separation using toluene: EtOAc: acetone solvent mixtures in gradient. The two major anti-oxidant principles of the leaves earlier identified were isolated in this process. The least polar component (Rf = 0.36) eluted from EtOAc:acetone (3:7, 2:8 and 1:9) was later identified by spectroscopy as verminoside (1.4g). The second spot (Rf = 0.19), a flavonoid, eluted from acetone (100%) and was identified as Kaempferol diglucoside (687 mg). Fraction SFL7 was purified on Rp-18 (lobar) and the column was eluted isocratically with 50% MeOH to yield a third antioxidant component identified as caffeic acid (14 mg). The only non-antioxidant constituent isolated from the leaves was obtained from fraction SFL1 by preparative-tlc employing solvent system D. The major band was eluted with EtOAc: MeOH (8:2), yielded a purple reacting compound identified as phytol (60 mg).
Specioside and caffeic acid from the flowers: Fresh flowers were macerated using MeOH and allowed to extract for 72 hours. The crude flower extract, SFF, (14.5 g) was separated by direct AGC on silica using hexane: EtOAc: MeOH in gradient. Fractions obtained were combined based on their Tlc profile resulting in 5 main fractions. Fractions SFF1 (926 mg) eluted from (EtOAc: MeOH; 2:1) and SFF2 (1.5 g) eluted from (EtOAc: MeOH; 8:2 and 7.5:2.5) were combined and purified on lobar Rp-18 column to afford caffeic acid (20mg) and specioside (60 mg) as the third iridiod from the plant. Both compounds eluted from 20% MeOH.
Isolation of ajugol and caffeic acid from the fruits: The fresh fruits (4.9kg) of S. campanulata were chopped into smaller bits, macerated in 50% EtOH (7L) and extracted for 72hr. The extract was concentrated in-vacuo to yield a brown sticky crude material, SFU (254 g, 5.18 %). An aliquot of SFU (58 g) was applied on AGC (silica) for a direct fractionation of its constituents. The column was eluted in gradient using hexane: EtOAc: MeOH solvent mixtures. Collected fractions were combined based on their similar Tlc reactions (DPPH, FeCl3 and v/s spray) to afford six fractions (SFU1-6). Fraction SFU5 (4.1 g) with the highest yield was further fractionated on another AGC column employing similar solvent compositions. Five sub-fractions (SFU5a-e) were recovered, with sub-fraction SFU5e (671 mg) finally purified on a Rp-18 (lobar) column using gradient of aq. methanol to afford Ajugol (45 mg). Another batch of SFU (41 g) was adsorbed on silica and eluted in gradient of hexane: EtOAc: MeOH solvent mixtures. Four fractions SFU7-10 were obtained and SFU8 (255 mg) having strongest antioxidant activity was purified on Rp-18 lobar column employing aq. MeOH mixtures in gradient. Sub-fraction SFU8b afforded a dirty yellow powdery substance identified as caffeic acid (24 mg). It developed a faint pink colour reaction with v/s spray and a positive reaction with DPPH and FeCl3, Rf = 0.8, system C.
Antioxidant spectroscopic assay of the isolated compounds: The antioxidant properties of the isolated compounds were evaluated by measuring their activities against the stable free radical 2, 2-diphenyl-2-picryhydazyl (DPPH), using the method described by Mensor et al., 2001.
Sample preparation
The pure compounds (25mg) were dissolved in methanol (25ml) as the stock solution (1000µg/ml). Sample solutions were prepared from the stock and made up to final concentrations of 500, 250, 125, 50, 25, 10, 5, 2.5µg/ml in volumetric flasks (10ml) using MeOH.
Preparation of DPPH solution (0.3 mM)
DPPH (12mg) was dissolved in MeOH in a volumetric flask (100ml) and kept in the dark until use.
DPPH spectrophotometric assay
To DPPH/MeOH solution (1ml) was added the sample solution (2.5ml) of different concentrations. The absorbance values were measured at 520 nm in triplicate, and the average converted into the percentage antioxidant activity (AA) using the following formula:
AA% = 100 − {[(Abssample − Absblank) × 100] / Abscontrol}
MeOH (2.5ml) plus DPPH solution was used as negative control. The blank was the absorbance of MeOH (1ml) plus sample (2.5ml) solution.
Statistical analysis: The EC50 was calculated by linear regression of plots where the abscissa represents concentration of tested pure isolated compound and the ordinate represents average percent of antioxidant activity of three independent tests. Ascorbic acid served as the reference standard.
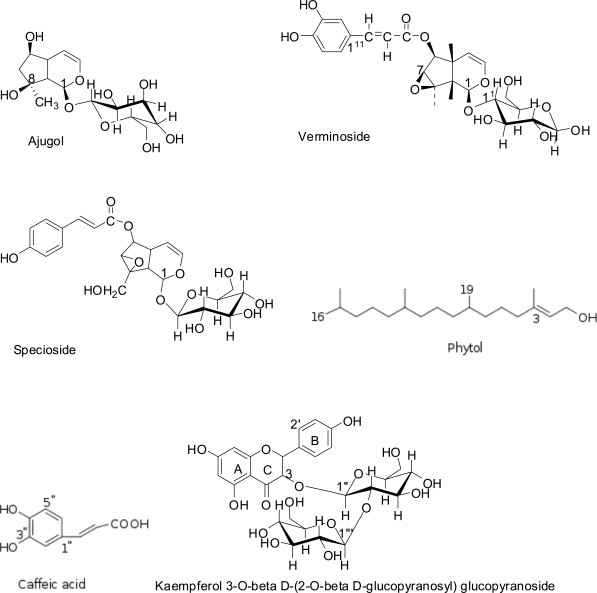
Ajugol: whitish powdery material soluble in methanol, Rf 0.1 (silica gel 60 F254, system B) and pink TLC reaction with v/s spray. 1H NMR (CD3OD, 200 MHz) δ 5.47 (1H, d, J = 1.8 Hz, H-1), 6.17 (1H, d, J = 2.4 Hz, H-3), 4.9 (1H, obscure, H-4), 2.04, 1.79 (2H, dd, J = 14.0, 4.0 Hz, H-7), 1.32 (3H, s), 4.65 (1H, d, J = 8.0 Hz); 13C NMR (CD3OD, 200 MHz) δ 99.26 (CH anomeric), 74.56, 77.85, 71.54 and 78.03 (other O-CH signals), 62.73 (CH2, C-6), 140.35 (CH, C-3), 105.78 (CH, C-4), 25.24 (CH3, C-10). The NMR data are consistent with literature values (Akbay et al., 2003; Guiso et al., 1974).
Verminoside: yellow powdery substance, hygroscopic on exposure, soluble in methanol and ethanol; Rf 0.31(Rp-18 F254, system E), 0.33 (silica gel 60 F254, system C); It developed a golden yellow colour reaction after v/s spray and a positive reaction with FeCl3 spray; IR (KBr), Vmax 1703, 1283, 3411, 2931, 1077 cm−1; 1H NMR (CD3OD, 200 MHz) δ 5.20 (1H, d, J = 6.5Hz, H-1), 6.35 (1H, d, 5.0 Hz, H-3), 2.6 (2H, m, H-5 and H-9), 3.69 (1H, s, H-7), 3.83, 4.17 (ABq, 2H, J = 13.0 Hz, H-10), 4.80 (1H, d, J = 6.5 Hz, H-11), 3.65, 3.9 (2H, dd, J = 12.2 Hz, H-61), 3.25–3.40 (m, H-21-H-51), 7.2, (1H, brs, H-211), 6.80 (1H, d, J = 8.0 Hz, H-511), 6.95 (1H, brd, J = 8.0 Hz, H-611), 6.32, (1H, d, J = 16.0 Hz), 7.60 (1H, d, J = 16.0 Hz); 13C NMR (CD3OD, 200 MHz) δ 95.02 (CH, C-1), 142.32 (CH, C-3), 102.91 (CH, C-4), 60.24 (CH, C-7), 66.78 (C, C-8), anomeric 99.61 ( CH, C-11), 74.76 (CH, C-21), 77.56 (CH, C-31), 71.66 (CH, C-41), 78.50 (CH, C-51), 62.84 (CH2, C-61), 114.44 (C, C-311), 149.68 (C, C-411), 147.59, 123.19 (CH, α, β olefinic); 168.93 (C=O). The NMR data are consistent with literature values (Sticher and Afifi-Yazar, 1979; Niyonzima et al., 1991).
Specioside: light pink solid, soluble in methanol, Rf 0.71 (Rp-18 F254, system E), Rf 0.14 (silica gel 60 F254, system C), positive reaction with FeCl3 spray; 1H NMR (CD3OD, 200 MHz) δ 5.08 (1H, d, J = 10 Hz, H-1), 6.79 (1H, d, J =8 Hz, H-2), 5.17 (1H, d, J =8 Hz, H-6), 4.18 (1H, d, J =8 Hz, H-7), 3.43 (1H, d, J =6 Hz, H-9), 3.83, 3.75 (2H, d s J = 12 Hz, H-10), 6.35 (1H, d, J = 8Hz, H-3), 5.10 (1H, dd, obscure, 7Hz, H-4), 6.85 (1H, d, J=16 Hz, α ), 8.08 (1H, d, J=16 Hz, β); 13C NMR (CD3OD, 200 MHz) δ 93.9 (CH, C-1), 141.2 (CH, C-3), 101.8 (CH, C-4), 60.1 (CH2, C-10), 98.9 (CH, C-11), 73.7 (CH, C-21), 77.4 (CH, C-31), 70.6 (CH, C-41), 76.5 (CH, C-51), 61.7 (CH2, C-61), 115.7 (CH, C-311), 160.3 (C, C-411), 113.4 (CH), 146.1 (CH), 167.8 (C=O). The NMR data are consistent with literature values (El-Naggar and Doskotch, 1980).
Kampeferol diglucoside: light brown solid material, soluble in methanol, Rf = 0.51 (Rp-18 F254, system E), developed a characteristic golden yellow colour (v/s spray) which turned reddish/orange after heating and a gave positive phenolic reaction with FeCl3 spray; IR (KBr), Vmax 1660, 3396, 2926, 1075 cm-1. 1H NMR (CD3OD, 200 MHz); δ 6.24 (1H, d, J = 1.5Hz, H-6), 6.43 (1H, s, H-8), 6.95 (2H, J = 9.0Hz, H-21, H-61) and δ 8.04 (2H, J = 9.0Hz, H-31, H-51) ABX system, 13C NMR; δ 133.84 (C-3), 103.02, 99.40 (CH, anomeric carbons), 178.45 (C-4, C=O). The NMR data are consistent with literature values (Norbæk and Kondo, 1999).
Phytol: soluble in EtOAc and CHCl3, Rf 0.32 (silica gel, system D) and a purple colour reaction with v/s spray. 1H NMR (CD3OD, 200 MHz); δ 4.15 (2H, d, J=10Hz, H-1), 5.42 (1H, t, H-2), 2.0 (2H, t, H-4), 0.8 (6H, d, H-16, 17), 1.65-0.8 (m), 1.65 (3H, s, H-20), 13C NMR; δ 16.41–22.97 (CH3, C-16 - C-20), 123.29 ( CH, C-2), 59.59 (CH2, C-1).
Caffeic acid: light yellow powder, soluble in CH3OH and CHCl3, Rf 0.6 (Rp-18 F254, system E), 0.3 (silica gel, system C). It has a natural yellow colour on Tlc which disappeared after v/s spray and later developed pink colour after heating. 1H-NMR: aromatic signals δ (6.22–7.53), splitting pattern and coupling constants of which indicated the presence of a 1,3,4-trisubtituted benzene ring, 7.04 (1 H, brs, J=1.6 Hz, H-2) indicating a meta coupling, 6.78 (1 H, d, J = 8.0 Hz, H-5), 6.92 (1 H, d, J = 8.0 Hz, H-6), 7.53, 6.22 (H-α and H-β, d, J=16 Hz). 13C NMR (apt spectrum); δ 115.2 – 149.4 (5 methine carbon signals), 168 (C=O). The NMR data are consistent with literature values for caffeic acid (Ramaiah, 1984).
Discussion
Ajugol was isolated from the stem bark and fruits of S. campanulata and was identified by NMR 1H/13C data and by comparison with literature values (Boros and Stermitz, 1990). Recently, Adriana et al. (2007), reported the isolation of Ajugol from the roots of S. campanulata. Ajugol is being reported here for the first time from its stem bark and fruits. Verminoside was isolated from the various fresh plant parts and identified by comparison of its spectroscopic data with literature. It is a yellow luster powder which is hygroscopic on exposure and detectable under UV light, strongly phenolic (FeCl3) and strongly antioxidant (DPPH bioautography). It is being reported for the first time from the leaves and the flowers but had been previously reported from the stem bark.
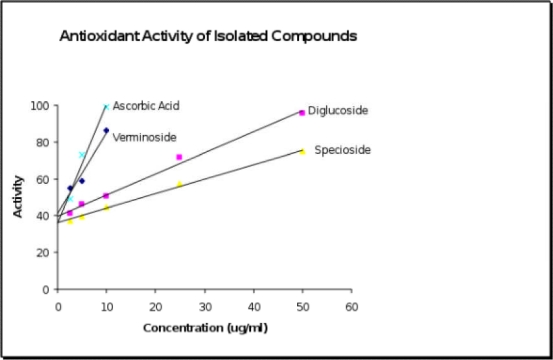
Verminoside earlier isolated from the stem bark of Kigelia pinnata (another bignoniaceae plant) has been reported to be a potent anti-amoebic agent against Entamoeba histolytica, showing better activity than the standard drug (Metronidazole), (Bharti et al., 2006). The presence and distribution of verminoside in the plant parts studied could further validate some of the ethno-medicinal uses ascribed to the plant, especially in the treatment of dysentery amongst certain cultures in West Africa (Burkill, 1985). From this study, it was found to be the major antioxidant principle of the plant. Its antioxidant property (EC50 = 2.038 µg/ml), Figure 1 compares with that of the standard ascorbic acid (EC50 = 2.178 µg/ml) in the test system used. Specioside was isolated from the fresh flowers of the plant and identified by NMR. Its spectroscopic data (Table 1) compares with the previously published data (El-Naggar & Doskotch, 1980). It has a moderate antioxidant activity (EC50 = 17.435 µg/ml). Previous report has also shown specioside to be moderately anti-amoebic (Bharti et al., 2006).
Figure 1.
Structures of compounds isolated from Spathodea campanulata
The presence of verminoside and specioside in various parts of the plant was also significant in rationalizing the traditional use of the plant in amoebic dysentery. Kaempferol diglucoside (EC50 = 8.86 µg/ml) and caffeic acid were also isolated from this study as the non-iridiod antioxidant principles of S. campanulata while phytol was obtained as a minor non-antioxidant component.
Figure 2.
Scheme for the isolation of the antioxidant principles (verminoside, kaempferol diglucoside and caffeic acid) and phytol as the non-active component of the fresh leaves of S. campanulata
Figure 3.
Linear regression of % antioxidant activity of pure isolated compounds
Acknowledgement
Thanks to Dr. Illoh and Mr. Adaramola, Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria for plant identification. Support provided by the International Programme in Chemical Sciences (IPICS), Uppsala, Sweden through the Nig. 01 project is duly acknowledged.
References
- 1.Adriana P, Pinto JP, Ferreira DT, Ishikawa NK, Braz-Filho R. Iridiod glucoside and antifungal phenolic compounds from Spathodea campanulata roots. Semina: Ciencias Agrarias, Londrina. 2007;28(2):251–256. [Google Scholar]
- 2.Akbay P, Ihsan C, Heilmann J, Sticher O, Ionone Iridiod and Phenylethaniod Glycosides from Ajuga salicifolia. Verlag der Zeitschrift fur Naturforschung. 2003;58c:177–180. doi: 10.1515/znc-2003-3-406. (Ger). [DOI] [PubMed] [Google Scholar]
- 3.Amusan OOG, Adesogan EK, Makinde JM. Antimalarial active principles of Spathodea campanulata stem bark. Phytother Res. 1996;10(8):692–693. [Google Scholar]
- 4.Bharti N, Singh S, Naqvi F, Azam A. Isolation and in vitro anti-amoebic activity of iridiods isolated from Kigelia pinnata. ARKIVOC. 2006;x:69–76. [Google Scholar]
- 5.Boros CA, Stermitz FR. Iridiods. An update review. Part 1. Journal of Natural Products. 1990;53(5):1055–1147. [Google Scholar]
- 6.Burkill HM. The useful plants of West Tropical Africa. Vol. 1. Royal Botanic Gardens, Kew, London; 1985. pp. 252–269. [Google Scholar]
- 7.El Naggar SF, Doskotch RW. Specioside: a new iridoid glycoside from Catalpa speciosa. Journal of Natural Product, Llyodia. 1980;43(4):524–526. [Google Scholar]
- 8.Garg HS, Blandani SPS, Tripathi SC, Patnaik GK, Puri A, Saxema R, Saxema R P. Antihepatotoxic and immunostimulant properties of Scrophularia koelzii. Phytotherapy Research. 1994;8:224–228. [Google Scholar]
- 9.Ghisalberti EL. Biological and pharmacological activity of naturally occurring Iridiods and secoiridiods. Phytomedicine. 1998;5(2):147–163. doi: 10.1016/S0944-7113(98)80012-3. [DOI] [PubMed] [Google Scholar]
- 10.Guiso M, Marini-Bettolo R, Agostini A. Ajugoside and ajugol: structure and configuration. Gazz Chim Ital. 1974;104:25–33. [Google Scholar]
- 11.Hutchinson J, Dalziel JM. Flora of West Tropical Africa. Vol. 1. London: The Whitefrars Press Limited; 1954. pp. 237–242. [Google Scholar]
- 12.Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG. Screening of Brazillian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy research. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 13.Ngouela S, Tsamo E, Sondengam BL. Extractives from Bignoniaceae: constituents of the stem bark of Spathodea campanulata. Planta medica. 1988;54:476. doi: 10.1055/s-2006-962516. [DOI] [PubMed] [Google Scholar]
- 14.Ngouela S, Tsamo E, Sondengam BL, Connolly JD. Spathodol, a new polyhydroxysterol from the leaves of Spathodea campanulata. Journal of Natural Products. 1991;54(3):873–876. [Google Scholar]
- 15.Ngouela S, Nyasse B, Tsamo E, Sondengam BL, Connolly JD. Spathodic acid: A triterpene acid from the stem bark of Spathodea campanulata. Phytochemistry. 1990;29(12):3959–3961. [Google Scholar]
- 16.Niyonzima G, Pieters L, Balde AM, Claeys M, Laekeman GM, Vlietinck AJ. Isolation of 6-O-Caffeoyl catalpol and some other compounds from Spathodea campanulata. Planta Medica. 1991;57(Supplement Issue 2):A85–A86. [Google Scholar]
- 17.Niyonzima G, Laekeman G, Witorouw M, van Poel B, Pieters L, Paper D, de Clercq E, Franz G, Vlietinck AJ. Hypoglycaemic, anti-compliment and anti-HIV activities of Spathodea campanulata stem bark. Phytomedicine. 1999;6(1):45–49. doi: 10.1016/S0944-7113(99)80034-8. [DOI] [PubMed] [Google Scholar]
- 18.Norbæk R, Kondo T. Flavonol glycosides from flowers of Crocus speciosus and Crocus antalyensis. Phytochemistry. 1999;51:1113–1119. doi: 10.1016/s0031-9422(99)00109-0. [DOI] [PubMed] [Google Scholar]
- 19.Pennacchio M, Syah YM, Ghisalberti EL, Alexander E. Cardioactive compounds from Eremophlia species. Journal of Ethnopharmacology. 1996;53:21–27. doi: 10.1016/0378-8741(96)01422-5. [DOI] [PubMed] [Google Scholar]
- 20.Ramaiah PA, Devi PU, Frolow F. Lavie D. 3-Oxo-friedelan-20a-oic acid from Gymnosporia emarginata. Phytochemistry. 1984;23:2251–2255. [Google Scholar]
- 21.Scogin R. Anthocyanins of the Bignoniaceae. Biochemical Systematics and Ecology. 1980;8:273–276. [Google Scholar]
- 22.Sticher O, Afifi-Yazar FU. Minecoside and verminoside, two new iridoid glucosides from Veronica officinalis L. (Scrophulariaceae) Helvetica Chimica Acta. 1979;62(56):535–539. [Google Scholar]
- 23.Tietze LF. Secologanin, a biogenetic key compound - synthesis and biogenesis of the Iridiods and Secoirid oidal glycosides. Angewandte Chemie (Int Ed) 1983;22:828–841. [Google Scholar]