Abstract
Plants harbor a variety of signaling molecules which are members of a vast array of signaling networks in maintaining their physiological balance. The well known members up till now are salicylic acid (SA), jasmonic acid (JA), ethylene (ET), abscissic acid (ABA) and reactive oxygen species (ROS) which are employed by plants for their adaptation to various environmental stresses in order to survive. GSH is gradually gaining importance and becoming a molecule of interest to a number of researchers especially in relation to plant defense to pathogens. Although the role of GSH in plant defense has long been known, a dearth of information still exists regarding the mechanism underlying this defense response. This review highlights on the progress made in the cross-communication of GSH with other established signaling molecules through which GSH acts in abating biotic stress.
Key words: glutathione, salicylic acid, biotrophic pathogen, NPR1, crosstalk, signaling
Glutathione, popularly known as the “master anioxidant” or “super defender,” is a nearly ubiquitous non-protein tripeptide thiol compound found in both prokaryotes and eukaryotes1–3 except for some organisms which use different other thiol cofactors. This molecule helps to prevent or even reduce the effect of certain human diseases which are of major concern in today's world including cancer, inflammation, kwashiorkor, Alzheimer's disease, Parkinson's disease, sickle cell anaemia, liver disease, cystic fibrosis, HIV, AIDS, infection, heart attack, stroke and diabetes. GSH, in animals, participates in the detoxification of ROS and xenobiotics, plays a major role in cell proliferation and death, DNA synthesis and repair, regulation of protein synthesis, prostaglandin synthesis, amino acid transport and enzyme activation, maintains essential thiol status, regulates immune functions, plays a role in spermatogenesis and sperm maturation and so on. In prokaryotes, GSH is one of the most abundant thiols as well and is present in cyanobacteria and proteobacteria. In bacteria, in addition to its key role in maintaining the proper oxidation state of protein thiols, GSH also serves a key function in protecting the cell from the action of low pH, chlorine compounds and oxidative as well as osmotic stresses.
The well known functions performed by this molecule in plants are as a major player in redox chemistry, heavy metals and electrophilic xenobiotics elimination, serving as electron donor for biochemical reactions, long-distance transport of reduced sulfur, stress defense gene expression, posttranslational modifications through glutathionylation, role in biotic and abiotic stresses and so on.4–13 Over the past three decades, GSH has been known to be involved in defense reactions against a variety of pathogens in addition to the induction of various defense genes. GSH when supplied at various concentrations to the cell suspension culture of bean induced several genes encoding enzymes that participate in the biosynthesis of lignin and phytoalexins.14 Early reports also found that GSH supplementation partly mimicked induction of chalcone synthase, the expression of which occurs as a result of fungal elicitor stimulation in soybean.15 Bean and soybean cells treated with fungal elicitor or GSH causes the rapid insolubilization of hydroxy-proline-rich structural proteins in the cell wall.16 Previous report also revealed that significant increase in GSH levels occurred as a result of enhanced resistance of melon and tomato roots against Fusarium oxysporum brought about by herbicides.17 In compatible barley-barley powdery mildew interactions the ascorbate-GSH cycle and other antioxidative enzymes (e.g., glutathione S-transferase) are activated and these processes might diminish the damaging effects of oxidative stress. However, in incompatible interactions these antioxidative reactions are not or are only slightly activated.18 A considerable accumulation of GSH and, in particular, oxidized glutathione (GSSG) has been observed in tomato cells carrying Cf-9 or Cf-2 resistance genes after treatment with race-specific elicitors of the fungus Cladosporium fulvum.19 Ball et al. reported that 32 stress-responsive genes were altered due to changed GSH metabolism in Arabidopsis rax1-1 and cad2-1, mutants of γ-ECS. Previous studies also reported that Arabidopsis pad2-1 mutant with only 22% of wild-type amounts of GSH were susceptible to Pseudomonas syringae as well as Phytophthora brassicae.20–22 Additionally, according to a recent report, enhanced resistance of transgenic tobacco with enhanced level of GSH was observed against P. syringae.23
Recent reports have found the role of GSH in cell signaling and in signaling pathways induced by different phytohormones. This review was an effort to throw some light on understanding the role of GSH as a signaling molecule by discussing the interrelationship of this multi-faceted molecule with other established signaling molecules which have well documented signaling roles in plants against biotic and abiotic stresses.
Crosstalk with SA
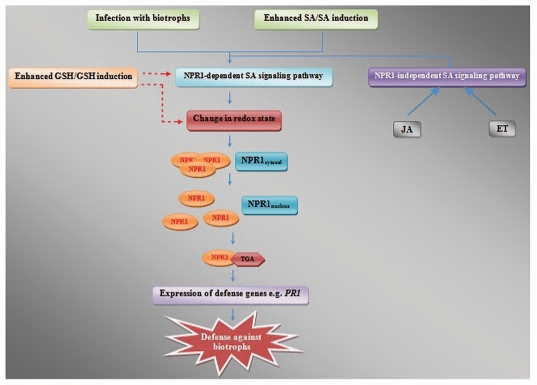
When plants face pathogen ingress, a blend of various defense reactions come into play. One of the major players that come into the scenario in this situation is SA. A substantial body of evidence indicates that SA is a critical signaling molecule in the pathway(s) leading to local and systemic disease resistance.24 SA is synthesized by plants in response to a variety of phytopathogens thus leading to the establishment to both local and systemic acquired resistance (SAR) for its own survival. SAR is characterized by the coordinate activation of a set of PR genes which encode for proteins, many of which have antimicrobial activity.25 SA signaling is mediated by at least two mechanisms, one requiring NPR1 and a second that is independent of NPR1.26,27 NPR1 is an essential regulator of plant SAR which contributes to the immunity of plants. It is a central positive regulator of systemic defense and functions in multiple nodes of SA signaling network. In uninduced state, NPR1 exists as an oligomer, but after SAR induction, NPR1 is reduced to its monomeric i.e., the active form. Increase in SA triggers the reduction of disulphide bonds located on both the regulatory protein NPR1 and on certain TGA transcription factors with which NPR1 interacts.28,29 Additionally, it has also been demonstrated that the SA-induced NPR1 oligomer-to-monomer reaction is regulated by thioredoxin through reduction or oxidation of its intermolecular disulphide bonds.30 This reduction stimulates both the translocation of NPR1 from the cytosol to the nucleus and the physical interaction of NPR1-TGA1 that is necessary for the activation of PR gene transcription.28,29 Interestingly, it has been observed that the resistance of plants to biotrophic pathogens is classically thought to be mediated through SA signaling, while that of necrotrophs is dealt with by JA or ET.31,32 Crosstalk among the signaling pathways provides the plant facing pathogen ingress with the power to tailor its defense responses according to its needs and antagonism between SA and JA is a widely accepted fact.32,33
Studies over the past decades have evidenced the relationship between GSH and SA in various stress responses. Measurement of glutathione levels in response to SA treatment revealed an increase in the GSH content and a decrease in the GSSG, along with an increased GSH:GSSG ratio in pea seedlings.34 According to a previous report, the induction of SAR by treatment with isonicotinic acid (INA), the analog of SA as well as infection with P. syringae, the biotrophic pathogen led to the increase in total glutathione content as well as GSH:GSSG ratio.28 Constitutive overexpression of SA was shown to induce GSH-mediated nickel tolerance in Thlaspi hyperaccumulators.35 The GSH biosynthesis inhibitor l-buthionine sulfoximine (BSO) strongly reduced the suppression of the JA-responsive gene PDF1.2 by SA, which suggests that SA-mediated modulation of the cellular redox state is an important trigger for the attenuation of JA signaling.36 According to yet another study, protection of ozone-induced leaf injury in Arabidopsis by SA occurred by increasing de novo biosynthesis of GSH.37 A recent report has shown that GSH status regulates SA and other pathways involved in biotic stress responses at several levels in Arabidopsis, including SA-accumulation and JA-linked gene expression.8 A recent report has shown that transgenic tobacco with enhanced level of GSH were resistant to P. syringae and constitutively expressed genes of the NPR1-dependent SA-mediated pathway.23 Interestingly, studies with a number of pathogens as discussed earlier, to decipher the role of GSH in biotic stress tolerance, are biotrophic in nature. Considering these studies, it is clear that crosstalk between GSH and SA exists in plants (Fig. 1).
Figure 1.
Position and involvement of GSH in the signaling web in plants. GSH has been known to participate in biotic stress tolerance by NPR1-dependent SA-mediated pathway. Only those signal transduction components relevant to this review has been shown. Continuous lines: established signaling pathways; broken lines: proposed signaling pathway.
Crosstalk with JA
The role of JA in defense has been reported in previous studies in reference 38–40. JA-dependent signaling also comes into play in pathogen attack, especially necrotrophs, through increased JA synthesis and marked by consequent increases in expression of defense effector genes such as PDF1.2. JA signaling also results from wounding and insect feeding. The JA signal pathway involves several signal transduction events: the perception of the primary wound or stress stimulus and transduction of the signal locally and systemically, the perception of this signal and induction of JA biosynthesis, the perception of JA and induction of responses and finally, integration of JA signaling with outputs from the SA, ET and other signaling pathways.40 JA is better known to play antagonistic actions with SA in defense signaling.
JA treatment increased mRNA levels and the capacity for GSH synthesis but did not alter the GSH content in unstressed plants.41 In tobacco, GSH-dependent formaldehyde dehydrogenase (FALDH) levels and enzymatic activity decreased after JA treatment and increased in response to SA.42 Recent study has shown that JA led to the increase in GSH metabolism under water stress in Agropyron cristatum, thus suggesting that water stress-induced JA is a signal that leads to the regulation of GSH metabolism and has important role for acquisition of water stress tolerance in A. cristatum.43 The involvement of intracellular GSH has also been studied in methyl jasmonate (MeJA) signaling.44
Crosstalk with ET
ET is a gaseous phytohormone that is well known for its role in developmental processes in plants. In addition, it has evolved to play signaling roles in plants and participate in crosstalk with other signaling molecules. In most of the cases, the relationship between ET and JA is synergistic one like in case of defense against necrotrophic pathogens.32,33 In contrast, synergistic interaction of ET with SA, the antagonist of JA has also been reported. ET has been shown to be essential for the onset of SA-dependent SAR that is triggered upon infection by tobacco mosaic virus.45 Moreover, ET was shown to enhance the response of Arabidopsis to SA, resulting in a potentiated expression of the SA-responsive marker gene PR1. This synergistic effect of ET on SA-induced PR1 expression was blocked in the ET-insensitive mutant ein2.46,47
ET has been reported to control GSH biosynthesis positively in ozone exposed Arabidopsis leaves.35 In ET signaling mutants, ein2-1, GSH-dependent Pb(II) resistance, which was related to constitutive reduction of expression of gamma-glutamyl synthetase (γ-ECS) gene involved in GSH synthesis consequently led to reduced GSH content.48 In transgenic tobacco with genetically enhanced level of GSH, the expression levels of PR4, the marker of ethylene signaling and ACC oxidase, that oxidizes ACC to ET were found to be enhanced thus demonstrating the synergism between GSH and ET.23 However, further study is required in this field before making a statement on the interrelationship.
Crosstalk with ROS
Aerobic life forms are known for continuous formation of ROS, the damaging end-products of aerobic energy which were first and still now widely described as deleterious since they can provoke cellular damage.49 One of the earliest observable aspects of plant's defense strategy is oxidative burst, which is a rapid and transient production of huge amounts of ROS.50 Although ROS have been mainly associated with pathogen attack, they are also detected in other biotic interactions including beneficial symbiotic interactions with bacteria or mycorrhiza, suggesting that ROS production is a common feature of different biotic interactions.51 They are powerful signaling components linking growth, metabolism and defense responses in cells.52–55 ROS is now largely admitted to play a signaling role in stomatal closure,56 various cellular mechanisms,57 regulation of gene expression,57,58 cellular growth59 and plant's defense system against pathogens.60 The role of GSH in the regulation of abiotic stress conditions have been reviewed in reference 61.
GSH being the most abundant non-protein thiol, qualifies as the “master antioxidant” among researchers, serves as a redox buffer and occurs to be at the centre of a complex antioxidant network in plants thus maintaining the intracellular environment reduced.62,63 Redox state of cells is largely maintained by the concentration and ratio of GSH and GSSG. Activation of GSH synthesis and accumulation of GSH is a general feature of enhanced oxidation of the cytosol. Exposure to GSH is involved in both the direct and indirect control of ROS concentrations.5,64,65 For instance, GSH takes part in the removal of H2O2 in a reaction in which the thiol group of cysteine in GSH is oxidized to form GSSG.64 The reverse reaction is catalyzed by glutathione reductase (GR) using NADPH. A highly reduced GSH pool is required for active protein function. Because both the levels and oxidation state of GSH are directly influenced by ROS, GSH is a key redox-signaling component.6,65–68 In addition to its participation in direct detoxification of ROS, GSH may also protect cells against unfavorable stress effects through the activation of various defense mechanisms due to its involvement in redox signaling.4,60,65,69–71 In this signaling pathway, GSH interacts with ROS, redox molecules (thioredoxins [Trxs], glutaredoxins [Grxs]) and plant hormones (SA, ABA). Additionally, regulatory functions for ROS in defense particularly the response to pathogen infection occur in conjunction with other plant signaling molecules, especially with SA and nitric oxide (NO).69,72 Increasing evidence supports the idea that SA also plays a role in controlling the cellular redox balance at the onset of the SAR28,73,74 thus demonstrating the crosstalk between these two signals. It is well known that production of ROS is a key event in hypersensitive response (HR) and antioxidant redox systems like that of GSH are involved in the activation of both compatible and incompatible plant responses by alterations in its level, redox state as well as in the activity of redox enzymes.75 NO is also a major player in defense to pathogens.76 It has been reported earlier that changes in the ascorbate and GSH metabolisms caused by the interplay between NO, ROS and plant cells can be part of the transduction signals that triggers programmed cell death.77
Crosstalk with ABA
Apart from SA, JA, ET and ROS which are most well documented in defense signaling, ABA also has a prominent role in biotic stress. Earlier it was known that although it acts as a negative regulator of disease resistance, ABA can also promote plant defense and is involved in a complicated network of synergistic and antagonistic interactions depending upon the nature of pathogen.78 ABA is connected to the SA-JA-ET network, as it was shown to attenuate JA/ET-dependent gene expression79 and to affect JA biosynthesis and resistance against JA-inducing necrotrophic pathogens.80,81 Moreover, ABA was demonstrated to antagonize the onset of SA-dependent defenses and SAR.82,83
Relationship between GSH and ABA has been studied earlier. In two maize genotypes differing in their stress tolerance, ABA differentially affected the GSH content, GSH:GSSG ratio, GR activity and γ-ECS transcript level.84 However, according to a recent report, the GSH content did not vary in potato tubers treated with ABA.85
Conclusions and Perspectives
In plant systems, though extensive research has been carried out to elucidate the roles of the multi-faceted molecule, GSH, the mechanism through which biotic stress tolerance against various phytopathogens occurs, is still a potential area of research. Considering the studies undergone to decipher the cross-communication of GSH with other established signaling molecules, it is clear that GSH has a distinct standpoint in the rather complicated signaling pathway crosstalk that exists in plant system. Substantial body of evidence has been gathered regarding the synergism of GSH with the much acclaimed signaling molecule SA. Accumulated evidence have indicated that GSH plays an important role in mitigating biotic stress likely through NPR1-dependent SA-mediated pathway. However, further studies and experimental evidences are essential to elucidate the relationship of GSH with JA and ET as well as the involvement of other molecular players of signaling, to gather a complete scenario about the signaling web in plants and the defense mechanisms in response to pathogen invasion.
Acknowledgements
This work was supported by grants from Department of Science and Technology (DST) and Council of Scientific and Industrial Research (CSIR), New Delhi, India. S.G. acknowledges CSIR for her fellowship.
References
- 1.Meister A. Metabolism and functions of glutathione. Trends Biochem Sci. 1981;6:231–234. [Google Scholar]
- 2.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 3.Sharma KG, Sharma V, Bourbouloux A, Delrot S, Bachhawat AK. Glutathione depletion leads to delayed growth stasis in Saccharomyces cerevisiae: evidence of a partially overlapping role for thioredoxin. Curr Genet. 2000;38:71–77. doi: 10.1007/s002940000137. [DOI] [PubMed] [Google Scholar]
- 4.Foyer CH, Lopez-Delgado H, Dat JF, Schott IM. Hydrogen peroxide- and glutathione-associated mechanisms of acclamatory stress tolerance and signaling. Physiol Plant. 1997;100:241–254. [Google Scholar]
- 5.May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D. Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot. 1998;49:649–667. [Google Scholar]
- 6.Ball L, Accotto GP, Bechtold U, Creissen G, Funck D, Jimenez A, et al. Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell. 2004;16:2448–2462. doi: 10.1105/tpc.104.022608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa K, Yoshimura K, Harada K, Fukusaki E, Ogawa T, Tamoi M, et al. AtNUDX6, an ADP-ribose/NADH pyrophosphohydrolase in Arabidopsis, positively regulates NPR1-dependent salicylic acid signaling. Plant Physiol. 2010;152:2000–2012. doi: 10.1104/pp.110.153569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, et al. Arabidopsis GLUTATHIONE REDUCTASE 1 plays a crucial role in leaf responses to intracellular H2O2 and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol. 2010;153:1144–1160. doi: 10.1104/pp.110.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar B, Singla-Pareek SL, Sopory SK. Glutathione homeostasis and abiotic stresses in plants: Physiological, biochemical and molecular approaches. In: Kumar A, Sopory SK, editors. Recent Advances in Plant Biotechnology and its Applications. New Delhi: IK International Publishing House; 2008. pp. 373–390. [Google Scholar]
- 10.Kocsy G, Szalai G, Vagujfalvi A, Stehli L, Orosz G, Galiba G. Genetic study of glutathione accumulation during cold hardening in wheat. Planta. 2000;210:295–301. doi: 10.1007/PL00008137. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz JM, Blumwald E. Salinity-induced glutathione synthesis in Brassica napus. Planta. 2002;214:965–969. doi: 10.1007/s00425-002-0748-y. [DOI] [PubMed] [Google Scholar]
- 12.Gomez LD, Noctor G, Knight MR, Foyer CH. Regulation of calcium signaling and gene expression by glutathione. J Exp Bot. 2004;55:1851–1859. doi: 10.1093/jxb/erh202. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Chakraborty A, Ghanta S, Chattopadhyay S. Agrobacterium-mediated genetic transformation of mint with E. coli glutathione synthetase gene. Plant Cell Tissue Organ Cult. 2009;96:117–126. [Google Scholar]
- 14.Wingate VMP, Lawton MA, Lamb CJ. Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol. 1988;87:206–210. doi: 10.1104/pp.87.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dron M, Clouse SD, Dixon RA, Lawton MA, Lamb CJ. Glutathione and fungal elicitor regulation of a plant defense gene promoter in electroporated protoplasts. Proc Natl Acad Sci USA. 1988;85:6738–6742. doi: 10.1073/pnas.85.18.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: A novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- 17.Bolter C, Brammall RA, Cohen R, Lazarovits G. Glutathione alterations in melon and tomato roots following treatment with chemicals which induce disease resistance to Fusarium wilt. Physiol Mol Plant Pathol. 1993;42:321–336. [Google Scholar]
- 18.El-Zahaby HM, Gullner G, Kiraly Z. Effects of powdery mildew infection of barley on the ascorbate-glutathione cycle and other antioxidants in different host-pathogen interactions. Phytopathology. 1995;85:1225–1230. [Google Scholar]
- 19.May MJ, Hammond-Kosack KE, Jones JDG. Involvement of reactive oxygen species, glutathione metabolism and lipid peroxidation in the Cf-gene-dependent defense response of tomato cotyledons induced by race-specific elicitors of Cladosporium fulvum. Plant Physiol. 1996;110:1367–1379. doi: 10.1104/pp.110.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glazebrook J, Ausubel FM. Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci USA. 1994;91:8955–8959. doi: 10.1073/pnas.91.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glazebrook J, Zook M, Merrit F, Kagan I, Rogers EE, Crute IR, et al. Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics. 1997;146:381–392. doi: 10.1093/genetics/146.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parisy V, Poinssot B, Owsianowski L, Buchala A, Glazebrook J, Mauch F. Identification of PAD2 as a γ-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J. 2007;49:159–172. doi: 10.1111/j.1365-313X.2006.02938.x. [DOI] [PubMed] [Google Scholar]
- 23.Ghanta S, Bhattacharyya D, Sinha R, Banerjee A, Chattopadhyay S. Nicotiana tabacum overexpressing γ-ECS exhibits biotic stress tolerance likely through NPR1-dependent salicylic acid mediated pathway. Planta. 2011;233:895–910. doi: 10.1007/s00425-011-1349-4. [DOI] [PubMed] [Google Scholar]
- 24.Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, et al. Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci USA. 2000;97:8849–8855. doi: 10.1073/pnas.97.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Loon LC, Rep M, Pieterse CMJ. Significance of inducible defense-related proteins in infected plants. Ann Rev Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 26.Shah J. The salicylic acid loop in plant defense. Curr Opin Plant Biol. 2003;6:365–371. doi: 10.1016/s1369-5266(03)00058-x. [DOI] [PubMed] [Google Scholar]
- 27.Blanco F, Salinas P, Cecchini NM, Jordana X, Van Hummelen P, Alvarez ME, et al. Early genomic responses to salicylic acid in Arabidopsis. Plant Mol Biol. 2009;70:79–102. doi: 10.1007/s11103-009-9458-1. [DOI] [PubMed] [Google Scholar]
- 28.Mou Z, Fan W, Dong X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell. 2003;27:935–944. doi: 10.1016/s0092-8674(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 29.Després C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, et al. The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell. 2003;15:2181–2191. doi: 10.1105/tpc.012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tada T, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, et al. Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science. 2008;321:952–956. doi: 10.1126/science.1156970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 32.Loake G, Grant M. Salicylic acid in plant defence—the players and protagonists. Curr Opin Plant Biol. 2007;10:466–472. doi: 10.1016/j.pbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5:308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava MK, Dwivedi UN. Salicylic acid modulates glutathione metabolism in pea seedlings. J Plant Physiol. 1998;153:409–414. [Google Scholar]
- 35.Freeman JL, Garcia D, Kim D, Hopf A, Salt DE. Constitutively elevated salicylic acid signals glutathione-mediated nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Physiol. 2005;137:1082–1091. doi: 10.1104/pp.104.055293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koornneef A, Pieterse CMJ. Cross talk in defense signaling. Plant Physiol. 2008;146:839–844. doi: 10.1104/pp.107.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida S, Tamaoki M, Ioki M, Ogawa D, Sato Y, Aono M, et al. Ethylene and salicylic acid control glutathione biosynthesis in ozone-exposed Arabidopsis thaliana. Physiol Plant. 2009;136:284–298. doi: 10.1111/j.1399-3054.2009.01220.x. [DOI] [PubMed] [Google Scholar]
- 38.Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gundlach H, Müller M, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. Plant Cell. 2002;14:153–164. doi: 10.1105/tpc.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiang C, Oliver DJ. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell. 1998;10:1539–1550. doi: 10.1105/tpc.10.9.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diaz M, Achkor H, Titarenko E, Martinez MC. The gene encoding glutathione-dependent formaldehyde dehydrogenase/GSNO reductase is responsive to wounding, jasmonic acid and salicylic acid. FEBS Lett. 2003;22:136–139. doi: 10.1016/s0014-5793(03)00426-5. [DOI] [PubMed] [Google Scholar]
- 43.Shan C, Liang Z. Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci. 2010;178:130–139. [Google Scholar]
- 44.Akter N, Sobahan MA, Hossain MA, Uraji M, Nakamura Y, Mori IC, et al. The involvement of intracellular glutathione in methyl jasmonate signaling in Arabidopsis guard cells. Biosci Biotechnol Biochem. 2010;74:2504–2506. doi: 10.1271/bbb.100513. [DOI] [PubMed] [Google Scholar]
- 45.Verberne MC, Hoekstra J, Bol JF, Linthorst HJM. Signaling of systemic acquired resistance in tobacco depends on ethylene perception. Plant J. 2003;35:27–32. doi: 10.1046/j.1365-313x.2003.01778.x. [DOI] [PubMed] [Google Scholar]
- 46.Lawton KA, Potter SL, Uknes S, Ryals J. Acquired resistance signal transduction in Arabidopsis is ethylene independent. Plant Cell. 1994;6:581–588. doi: 10.1105/tpc.6.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Vos M, Van Zaanen W, Koornneef A, Korzelius JP, Dicke M, Van Loon LC, et al. Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiol. 2006;142:352–363. doi: 10.1104/pp.106.083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao S, Chen Z, Liu G, Jiang L, Yuan H, Ren G, et al. The Arabidopsis Ethylene-Insensitive 2 gene is required for lead resistance. Plant Physiol Biochem. 2009;47:308–312. doi: 10.1016/j.plaphy.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Halliwell B, Gutteridge JM. Oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts. Arch Biochem Biophys. 1986;246:501–514. doi: 10.1016/j.abb.2022.109246. [DOI] [PubMed] [Google Scholar]
- 50.Wojtaszek P. Oxidative burst: An early plant response to pathogen infection. Biochem J. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres MA. ROS in biotic interactions. Physiol Plant. 2010;138:414–429. doi: 10.1111/j.1399-3054.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 52.May MJ, Vernoux T, Sánchez-Fernández R, Van Montagu M, Inzé D. Evidence for posttranscriptional activation of gamma-glutamylcysteine synthetase during plant stress responses. Proc Natl Acad Sci USA. 1998;95:12049–12054. doi: 10.1073/pnas.95.20.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner D, Przybyla D, den Camp RO, Kim C, Landgraf F, Lee KP, et al. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science. 2004;306:1183–1185. doi: 10.1126/science.1103178. [DOI] [PubMed] [Google Scholar]
- 54.Torres MA, Jones JD, Dangl JL. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet. 2005;37:1130–1134. doi: 10.1038/ng1639. [DOI] [PubMed] [Google Scholar]
- 55.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 56.Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 57.Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT. Hydrogen peroxide and nitric oxide as signaling molecules in plants. J Exp Bot. 2002;53:1237–1247. [PubMed] [Google Scholar]
- 58.Vranova E, Inzé D, Van Breusegem F. Signal transduction during oxidative stress. J Exp Bot. 2002;53:1227–1236. [PubMed] [Google Scholar]
- 59.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 60.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 61.Szalai G, Kellös T, Galiba G, Kocsy G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul. 2009;28:66–80. [Google Scholar]
- 62.Rouhier N, Lemaire SD, Jacquot JP. The role of glutathione in photosynthetic organisms: Emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol. 2008;59:143–166. doi: 10.1146/annurev.arplant.59.032607.092811. [DOI] [PubMed] [Google Scholar]
- 63.Maughan SC, Pasternak M, Cairns N, Kiddle G, Brach T, Jarvis R, et al. Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc Natl Acad Sci USA. 2010;107:2331–2336. doi: 10.1073/pnas.0913689107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noctor G, Foyer CH. Ascorbate and glutathione: Keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 65.Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buchanan BB, Balmer Y. Redox regulation: A broadening horizon. Annu Rev Plant Biol. 2005;56:187–220. doi: 10.1146/annurev.arplant.56.032604.144246. [DOI] [PubMed] [Google Scholar]
- 67.Michelet L, Zaffagnini M, Marchand C, Collin V, Decottignies P, Tsan P, et al. Glutathionylation of chloroplast thioredoxin f is a redox signaling mechanism in plants. Proc Natl Acad Sci USA. 2005;102:16478–16483. doi: 10.1073/pnas.0507498102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, Jacquot JP, et al. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J. 2007;52:973–986. doi: 10.1111/j.1365-313X.2007.03280.x. [DOI] [PubMed] [Google Scholar]
- 69.Mittler R, Vanderauwera S, Gollery M, van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 70.Mullineaux PM, Rausch T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth Res. 2005;86:459–474. doi: 10.1007/s11120-005-8811-8. [DOI] [PubMed] [Google Scholar]
- 71.Pitzschke A, Forzani C, Hirt H. Reactive oxygen species signaling in plants. Antioxid Redox Signal. 2006;8:1757–1764. doi: 10.1089/ars.2006.8.1757. [DOI] [PubMed] [Google Scholar]
- 72.Bolwell GP, Daudi A. Reactive oxygen species in plant pathogen interactions. In: del Río LA, Puppo A, editors. Reactive Oxygen Species in Plant Signaling, Signaling and Communication in Plants. Berlin, Heidelberg: Springer-Verlag; 2009. [Google Scholar]
- 73.Mateo A, Funck D, Mühlenbock P, Kular B, Mullineaux PM, Karpinski S. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J Exp Bot. 2006;57:1795–1807. doi: 10.1093/jxb/erj196. [DOI] [PubMed] [Google Scholar]
- 74.Holuigue L, Salinas P, Blanco F, GarretÓn V. Salicylic acid and reactive oxygen species in the activation of stress defense genes. In: Hayat S, Ahmed A, editors. Salicylic Acid: A Plant Hormone. Netherlands: Springer; 2007. pp. 197–246. [Google Scholar]
- 75.De Gara L, de Pinto MC, Tommasi F. The antioxidant systems vis-à-vis reactive oxygen species during plant-pathogen interaction. Plant Physiol Biochem. 2003;41:863–870. [Google Scholar]
- 76.Durner J, Klessig DF. Nitric oxide as a signal in plants. Curr Opin Plant Biol. 1999;2:369–374. doi: 10.1016/s1369-5266(99)00007-2. [DOI] [PubMed] [Google Scholar]
- 77.de Pinto MC, Tommasi F, De Gara L. Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco Bright-Yellow 2 cells. Plant Physiol. 2002;130:698–708. doi: 10.1104/pp.005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ton J, Flors V, Mauch-Mani B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009;14:310–317. doi: 10.1016/j.tplants.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 79.Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, et al. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004;16:3460–3479. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adie BAT, Pérez-Pérez J, Pérez-Pérez MM, Godoy M, Sánchez-Serrano JJ, Schmelz EA, et al. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell. 2007;19:1665–1681. doi: 10.1105/tpc.106.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flors V, van Doorn R, Jakab G, García-Agustín P, Mauch-Mani B. Interplay between JA, SA and ABA signaling during basal and induced resistance against Pseudomonas syringae and Alternaria brassicicola. Plant J. 2008;54:81–92. doi: 10.1111/j.1365-313X.2007.03397.x. [DOI] [PubMed] [Google Scholar]
- 82.Yasuda M, Ishikawa A, Jikumaru Y, Seki M, Umezawa T, Asami T, et al. Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell. 2008;20:1678–1692. doi: 10.1105/tpc.107.054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohr PG, Cahill DM. Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes, in Arabidopsis infected with Pseudomonas syringae pv. tomato. Funct Integr Genomics. 2007;7:181–191. doi: 10.1007/s10142-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 84.Kellos T, Timar I, Szilagyi V, Szalai G, Galiba G, Kocsy G. Stress hormones and abiotic stresses have different effects on antioxidants in maize lines with different sensitivity. Plant Biol. 2008;10:563–572. doi: 10.1111/j.1438-8677.2008.00071.x. [DOI] [PubMed] [Google Scholar]
- 85.Stroinski A, Chadzinikolau T, Gizewska K, Zielezinska M. ABA or cadmium induced phytochelatin synthesis in potato tubers. Biol Plant. 2010;54:117–120. [Google Scholar]