Abstract
Serotonin (5-hydroxytryptamine; SER) is one of the well-studied indoleamine neurotransmitters in vertebrates. Recently SER has also been reported in wide range of plant species. The precise function of SER at the physiological level, particularly growth regulation, flowering, xylem sap exudation, ion permeability and plant morphogenesis in plant system has not been clear. Though SER is found in different parts of plant species including leaves, stems, roots, fruits and seeds, the quantity of SER within plant tissues varies widely. SER has been recently shown as a plant hormone in view of its auxin-like activity. This brief review provides an overview of SER biosynthesis, localization, its role in plant morphogenesis and possible physiological functions in plants. This would certainly help to elucidate further the multiple roles of SER in plant morphogenesis. In the future it may form the basis for studies on involvement of SER in cellular signaling mechanisms in plants. Apart from these gaps in understanding the role of SER in ontogeny of plant physiology and ecological, adaptations have been emphasized. Thus, overall perspectives in this area of research and its possible implications have been presented.
Key words: biosynthesis, growth regulation, occurrence, physiological functions, phytoserotonin, plant morphogenesis, serotonin
Serotonin—Its Occurrence
Serotonin is a physiologically active amine which is well-known neurotransmitter that regulates mood, sleep and anxiety in mammals.1 Its action as a hallucinogenic drug is well known from biochemical, electro-physiological and behavioral studies. It was initially identified as a vasoconstrictor substance in blood serum and later chemically identified as 5-hydroxytryptamine (5-HT) by Rapport et al. (1958).2 In plants it was first identified in the legume Mucuna pruriens3 and later it has been reported in ∼42 plant species from 20 families.4 The highest values of 25–400 mg kg of SER have been found in walnuts (Juglans regia) and hickory (Carya sps.). However, in plantain, pineapple, plums, banana, kiwifruit and tomatoes SER levels were moderate i.e. 3–30 mg/kg.5 In spite of so many diverse roles of SER in animal systems, their role in plant systems are not known precisely. This review focuses on the phytoserotonin and its occurrence, localization, quantities, biosynthesis, functions as well as its beneficial effects as dietary SER to humans. It is an attempt for a compilation of available literature to bring to focus the perspectives on this topic.
SER has been implicated in diverse physiological functions in plants viz. growth regulation,6 flowering, xylem sap exudation,7 ion permeability8 and plant morphogenesis.9,10 It is well established that the levels of SER vary in different plant part and also varied in respective tissues. In Table 1 the list of plants identified to contain SER is provided. In leaves, SER was reported to be 0.007 µg/g fresh weight, but seeds harbored 2,000 µg/g in West Africa leguminous plant Griffonia simplicifolia.11 Fruits, vegetables and seeds are the major tissues in which SER occurs abundantly.12 SER occurs in fruits (e.g., pineapple, banana and tomato) avocado, eggplant and seeds (walnuts) and also in the sting of the nettle Urtica dioica13 and in the pods of cowhage3 in which SER is suggested to play a protective role against predators.
Table 1.
Occurrence of serotonin in plants
| Family | Common name | Scientific name | Serotonin content | Reference |
| Alliaceae | Green onion | Allium fistulosum | 8 ± 0.8 µg/g fw | 71 |
| Amaranthaceae | Spinach | Spinacia oleracea | 34.4 ± 2.4 µg/g fw | 71 |
| Asteraceae | Lettuce | Lactuca serriola | 3.3 ± 0.6 µg/g fw | 71 |
| Chicory | Cichorium intybus | 8.5 ± 3.2 µg/g fw | 71 | |
| Brassicaceae | Chinese cabbage | Brassica rapa | 110.9 ± 22.5 µg/g fw | 71 |
| Bromeliaceae | Pineapple | Ananas comosus | Fruit 1.5 µg/g fw | 72 |
| Caricaceae | Papaya | Carica papaya | Fruit 1.1–2.1 µg/g fw | 16 |
| Crassulaceae | Sedum | Sedum pachyphyllum | Leaves 0.02–0.03 µg/g fw | 73 |
| Fabaceae | Griffonia | Griffonia Simplicifolia | Leaves (0.0017–0.007 µg/g) Seeds (20,000 µg/g) |
11 |
| Scarlet runner bean | Phaseolus multiflorus | Leaves 0.6 µg/g fw |
74 | |
| Rain tree | Samanea saman | Stem 0.1–4 µg/g fw |
74 | |
| Garden pea | Pisum sativum | Leaves,stem 0.9–1 µg/g fw |
74 | |
| Lygophyllaceae | Harmal | Peganum harmala | Leaf 18,200 µg/g fw |
75 |
| Juglandaceae | Heartseed walnut Japanese walnut Black walnut |
Juglans ailanthifolia Juglans mandshurica Juglans nigra |
Embryo of fruit 95 µg/g fw Embryo of fruit 251 µg/g fw Embryo of fruit 180 µg/g fw |
28 |
| Mimosaceae | Mimosa |
Mimosa tenuiflora Mimosa pudica |
Leaves 9% In vitro leavesn 8.3 µg/g fw Ex vitro leaves 17.3 µg/g fw Seeds 80.4 µg/g fw |
18 10 |
| Musaceae | French plantain banana. | Musa sapientum | Fruit (peel) 40–150 µg/g fw Fruit (pulp) 19–28 µg/g fw Ripened 18.5 ng/g |
23 21 19 |
| Passifloraceae | Wild maracuja | Passiflora foetida | Fruit 1.4–3.5 µg/g fw Tendrils 1.0 µg/g fw |
74 |
| Rosaceae | Garden plum | Prunus domestica | Red fruit 10 µg/g fw Blue-red Fruit 8 µg/g fw |
21 |
| Strawberry | Fragaria x ananassa | 3.77 ± 0.66 µg/g fw | 71 | |
| Sweet cherry |
Prunus avium Cultivar Burlat Navalinda Van Pico Limon Negro Sweetheart Pico Negro Ambrunes Pico Colorado |
12.6 ng/100 g fw 30.7 ng/100 g fw 10.6 ng/100 g fw 27.1 ng/100 g fw 19.2 ng/100 g fw 2.8 ng/100 g fw 37.6 ng/100 g fw 36.6 ng/100 g fw |
25 | |
| Rubiaceae | Coffee | Coffea arabica (Green) | 2.5 mg/kg dry wt | 62 |
| Coffea arabica (Roasted) | 2.3 mg/kg dry wt | 62 | ||
| Coffea canephora (Green) | 2.1 mg/kg dry wt | 62 | ||
| Coffe canephora (Roasted) | 3.3 mg/kg dry wt | 62 | ||
| Polished coffee (Astra) | 20–40 mg/100g | 62 | ||
| Coffee wax | 4.1 mg g-1 | 29 | ||
| Instant coffee beverage | 0.04–1.80 mg/100g | 76 | ||
| Solanaceae | Tomato Cherry tomato Hot pepper Paprika Egg plant |
Lycopersicon esculentum Lycopersicon esculentum Capsicum annuum Capsicum annuum Solanum melongena |
221.9 µg/g fw 156.1 µg/g fw 17.9 µg/g fw 1.8 µg/g fw 1.5–12 µg/g fw |
71 71 71 14 |
| Urticaceae | Himalayan nettle Stinging bush Wood nettle Bull nettle Tree nettle |
Girardinia heterophylla Laportea moroides Utrica cubensis Utrica dioica Utricaferox Forst |
Stinging trichomes 0.15 µg/g fw Stinging trichomes 1.0 µg/g fw Shoots with leaves 0.42 µg/g fw Stinging trichomes 3–5 µg/g fw Shoots with leaves 0.33 µg/g fw |
77 77 13 77 |
In fruits of pineapple SER distribution in tissues is not uniform, with great amount in the outer skin of fruit, considerably less in inner skin and pulp.14 SER levels are known to increase as the fruits ripen in many species, including tomato, although the inverse is true of the fruit of pineapple (Ananas comosus).15 SER was analyzed in fruits of Juglans regia, a temperate species where its amount was found to be lowest in spring and sharply increases during autumn which levels is more than that found in animal tissues,16 coinciding with seed dispersal period.17 Even in embryos of Juglans regia (walnut) SER was profiled (0.4–0.6 µg/g fresh mass).17 In Griffonia simplicifolia leaves, high quantities of SER accumulated in the reproductive period (March) up to 1.2–1.3 µmole g/fresh weight tissue, whereas in vegetative period (November and December) they accumulate only up to 0.3 µmole g/fresh weight of tissue.11 SER in Sedum morganianum and S. Pachyphyllum accumulates more in light period than in the dark. Variations in the accumulation levels of SER and N,N-dimethyltryptamine (DMT) in micropropagated trees and in in vitro cultures of Mimosa tenuiflora was reported.18 In rice plant roots, SER accumulated with age. Although SER levels varied among the tissue types, SER was abundantly synthesized in senescent rice tissues, and the induced synthesis of SER was closely associated with symptoms of senescence.7 SER occurs in M. pudica and M. verrucosa, with concentrations varying from 0.06 to 0.86% dry weight (DW).18 In our recent report in M. pudica, high levels of SER (80.4 µg/g fresh weight, FW) was found in seeds compared to in vitro leaves (8.3 µg/g FW) and ex vitro leaves (17.3 µg/g FW).10
Pulp of underripe and ripe yellow banana contains SER at concentrations of 31.4 and 18.5 ng/g, respectively.19,20 However, Vettorazi (1974),20 reported that SER levels decreased during ripening of Musa cavendish. Udenfriend et al. (1959),21 reported the higher concentrations of SER in the peel (150,000 ng g−1) of the banana compared to the pulp 24,000 ng g−1. During ripening, concentrations of SER increased significantly in both the inner and outer banana peel (Table 2). However, in French plantain (Musa paradisica var. sapientum) though very high SER concentrations were found during ripening (from 49,900 to 56,700 ng g−1), but during ripening it decrease (12,000 ng g−1).22 It was reported that SER levels decreased during ripening in M. Cavendish.20 In Prata bananas (M. acuminate × M. balbisiana) during ripening, SER levels were between 15,000 and 20,000 ng g−1 until the fourteenth day of storage, after which SER levels decreased to about 7,500 ng g−1 after 35 days storage.23 The content of SER in maturing fruits of Ananas comosus L. and Lycopersicon esculentum L. were shown in Table 3. Recently, Murch et al. (2009),24 reported the occurrence of both melatonin and SER in D. metel. The flowers and smallest flower buds of D. metel had the highest concentrations of both melatonin and SER. The exposure of the flower buds to a cold stress significantly increased the concentrations of both SER and melatonin in the youngest buds at the most sensitive stage of reproductive development.24 Both melatonin and SER have been detected and quantified for the first time in eight different sweet cherry cultivars using high performance liquid chromatography (HPLC) with mass spectrometry detection. The highest SER contents were found in the cultivar “Ambrunes” (37.6 ± 1.4 ng/100 g of fresh fruit). Sweet cherries, which contain substantial amounts of melatonin and SER, may have a great number of health benefits and would be important to incorporate in a healthy diet.25 Moreover, changes in the levels of both SER and melatonin during ripening of wine grapes was reported.26
Table 2.
Serotonin content in different tissues of Banana (Musa sapientum L.) during unripened and ripened stage
| Plant part | Serotonin content (ng g−1) | Reference |
| Musa sapientum L. | ||
| Banana peel | 150,000 | 21 |
| Inner peel (hard green) | 74,000 | |
| Ripe | 96,000 | |
| over ripe | 161,000 | |
| Outer peel (hard green) | 13,000 | |
| ripe | 38,000 | |
| over ripe | 170,000 | |
| Banana pulp (hard green) | 24,000 | |
| ripe | 36,000 | |
| over ripe | 35,000 | |
| French plantain Musa paradisiaca var. sapientum | ||
| Ripening | 49,900 to 56,700 | 22 |
| Over ripening | 12,000 | |
| Prata banana (M. acuminatea × M. balbisiana) | ||
| Ripened (Until the 14th day of storage) | 15,000 and 20,000 | 21 |
| Ripened (After 35 days storage) | 7,500 |
Table 3.
Content of serotonin in maturing fruits
| Family | Common name | Scientific name | Immatured Matured (µg/g fw) | Matured (µg/g fw) | Super matured (µg/g fw) | References |
| Bromeliaceae | Pineapple | Ananas comosus L. (pulp) | 50–60 | 19 | Nil | 15 |
| Solanaceae | Tomato | Lycopersicon esculentum L. (pulp) | 0.18 | 3.75 | 2.9 | 72 |
Adapted from Roshchina (2001).4
Localization of Serotonin in Plants
Immunolocalization analysis has revealed that SER was abundant in the vascular parenchyma cells, including companion and xylem cells of rice, suggesting its involvement in maintaining the cellular integrity for facilitating efficient nutrient recycling from senescing leaves to sink tissues during senescence.7 Moreover, the distribution of SER in various tissues of Musa paradisica L., Allium cepa L. and other plants viz. lemon was reported.27 The SER fluorescence was also detected in the vascular bundles of the fruit wall of banana.27 It is reported to accumulate in protein bodies of cotyledons of developing embryos of Juglans regia in which there are still no vacuole formation.28
Quantification of Serotonin in Plant Tissues
HPLC is widely used for SER determination in different samples, usually on reversed phase (RP) or cation exchange analytical columns, with electrochemical, fluorimetric or UV.29–33 Accurate analysis of melatonin, SER and auxin in plant samples using liquid chromatography-tandem mass spectrometry has been proposed. SER was detected and quantified in the ESI, APCI and APPI positive mode.33 SER was also assayed fluorimetrically (excitation 394 nm, emission 505 nm) after incubation (75°C, 30 min) with ninhydrin.34 The studies on influence of SER on physiological processes is governed by our ability to detect minute endogenous levels. Hence detection method play crucial role. The amount of SER in these natural products has not been perfectly established due to great variability of this amine in fruit types and also its degree of ripeness during the harvest period.35
Biosynthesis and Metabolism of Serotonin
In animals, SER is synthesized from tryptophan in two successive steps involving tryptophan hydroxylase (TPH; EC 1.14.16.4) and aromatic L-amino acid decarboxylase (AADC; EC 4.1.1.28) in which tryptophan hydroxylase acts as the rate-limiting enzyme.1 In plants, SER is synthesized in a differentially whereby tryptophan is first catalyzed into tryptamine by tryptophan decarboxylase (TDC; EC 4.1.1.28), followed by the catalysis of tryptamine by tryptamine 5-hydroxylase (T5H) to form SER.36 However, in St. John's wort (Hypericum perforatum), SER synthesis was reported to occur via 5-hydroxytryptophan as in mammals.37 However another pathway of transformation of tryptophan to SER in plants can not be ruled out which is similar to that found typically in animals. Hydroxylation of tryptophan leads to formation of 5-oxytryptophan in the presence of Tryptophan-5-hydroxylase. Subsequently 5-oxytryptophan is decorboxylated by decorboxylase to yield SER11 (Fig. 1). The biosynthetic pathway of SER in plants and animals is shown in Figure 2.
Figure 1.
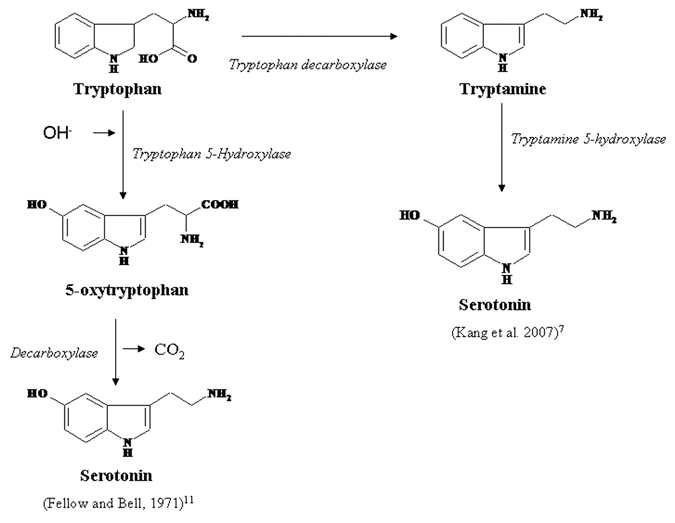
Figure 2.
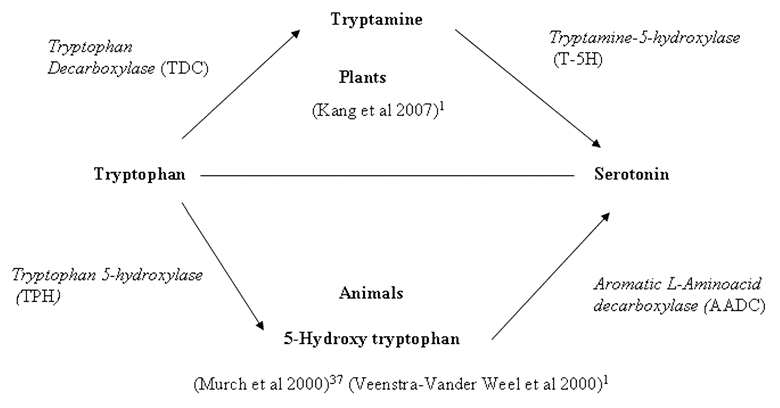
Biosynthetic pathway of serotonin in animals and plants.1,37
Recently, Tryptamine 5-hydroxylase (T5H) and SER synthesis in rice plants has been reported by Kang et al. (2007).7 The abundant production of SER in roots (∼6.5 mg g−1 fresh weight) and (1 mg g−1 fresh weight) in stem of rice is reported. Synthesis of SER also occurs in rice seedlings and requires tryptamine as a substrate for the T5H enzyme activity. Thus the tryptamine content is closely related to SER synthesis.7
Catabolism of Serotonin
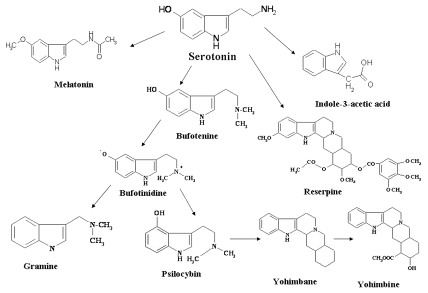
Catabolism of SER in plants is beginning to be understood. SER to N-acetyl SER, catabolized by hydroxytryptamine acetyl transferase and then its methylation to form 5-methoxy N-acetyl tryptamine (melatonin) implicate several physiological functions in plants viz. photoperiodic responses, growth promoter, reproductive physiology, defense of plant cells against apoptosis, phytoremediative capacity, and free radical scavenging agent.38–41 There is a possibility that the simple dehydroxylation of 5-hydroxyindolylacetic acid leads to the formation of indoleacetic acid. Methylated derivatives of SER and the alkaloids bufotenine, bufotenidine, psilocin and psilocybin were found in amphibians and plants.42 SER is precursor of bufotenine which occurs in seeds Piptadenia peregrina, P. colubrina, P. macrocorpa (up to 9.4 µg/g fresh weight).11 Bufotenine is also found in the South American and Carribean tree P. peregrina, which is rich in indoleamines. In this species, 80% 14C-SER is incorporated into bufotenine.11 Psilocin and psilocybin are formed from SER in fungi Psilocybe aztecorum. The Figure 3 shows catabolism of SER in plants. Moreover, metabolism condensation of the isopentyl group with the indole nucleus of SER may give rise to synthesis of alkaloids viz. reserpine, yohimbane and yohimbine, vinblastine. These alkaloids may induce human hallucinations and formation of these endogenous alkaloids in humans may cause psychic disorders.43
Figure 3.
Catabolism of serotonin in plants.4
Possible Physiological Functions of Serotonin in Plants
Although several different roles have been proposed, the function of SER is not yet clear. Furthermore, SER is implicated in flowering, ion permeability and in a protective role as an antioxidant.4 Kang et al. (2009),44 reported that tryptophan levels were significantly induced upon senescence and the increased tryptophan was readily converted into SER by the induction of Tryptophan decorboxylase (TDC). SER is a preferable mediator of senescence. The physiological functions of SER has been depicted in Table 4 and Figure 4.
Table 4.
Possible physiological functions of serotonin in plants
| S.No. | Function | Name of the plant | References |
| 1 | Detoxification Secondary plant product |
Walnut seeds (Juglans regia) | 28, 36 |
| 2 | Ion permeability Antioxidant Maintain the cellular integrity of xylem parenchyma and companion cells |
Rice (Oryza sativa) |
38 7 44 |
| 3 | Protective agent against predation | Cowhage (Mucuna pruriens) Tree nettle (Urtica dioica) | 3 |
| 4 | Auxin-like effect, Regulation of organogenesis, root growth and development | St. John's wort (Hypericum perforatum) | 6, 46, 49 |
| 5 | Stimulation of radish seeds germination | Radish seeds (Hippeastrum hybridum) | 4, 47 |
| 6 | Root growth and development | Oat (Avena sativa) | 46 |
| 7 | Plant seed development | Barley (Hordeum vulgare) | 9 |
| 8 | Adaptation to environmental changes | Albizzia julibrissin Garden pea (P. sativum) Mimosa (Mimosa pudica) Wild maracuja (Passiflora quadrangularis) |
8 |
| 9 | Photo morphogenetic responses | Corn (Zea mays) | 50, 51 |
Figure 4.
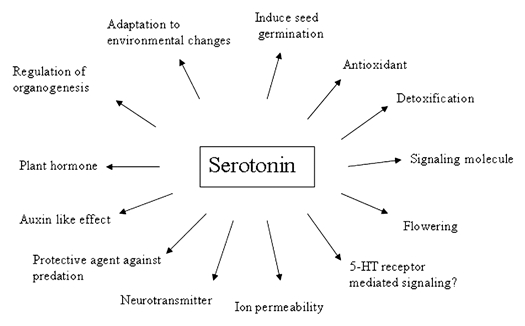
Possible physiological functions of serotonin in plants.
Role of Serotonin in Plant Morphogenesis
SER may act as a growth regulator and stimulates the growth of roots9 and the hook of oat coleoptiles.45 It also stimulate the germination of both radish seeds4 and the pollen of Hippeastrum hybridum.46 In in vitro studies of root organogenesis, the relative balance of melatonin to SER was found to mediate the morphogenetic responses such that decreased melatonin reduced root organogenesis and increased SER increased shoot organogenesis in the presence of exogenous auxin.6 The presence of indoleamines and their potential role in morphogenesis in vitro was investigated in axenic cultures of St. John's wort (Hypericum perforatum L.). De novo shoot regeneration was induced on etiolated hypocotyl explants after 9 days of exposure to thidiazuron. The balance of the endogenous concentrations of SER and melatonin may play a role in in vitro plant morphogenesis.6 Endogenous synthesis of melatonin and SER had been established and a series of inhibitors of indoleamine action and transport were found to effectively mediate regenerative responses in St. John's wort tissues.6 Selective SER reuptake inhibitors previously shown to effect morphogenesis in St. John's wort were investigated in the presence of TDZ.37 Recently, the highest SER levels were found in the rice roots7 and its possible involvement in root growth and development are mentioned in previous reports.6,10,47 Our recent report suggest that SER, MEL and calcium channels are having influence on morphogenesis in in vitro cultures of M. pudica L. The synergistic influence of SER and calcium treatment was noticed in eliciting morphogenesis. Higher levels of SER were noticed in seeds compared to in vitro leaves and ex vitro leaves. This may be of significance in seed germination and its viability.10 Recently we have studied indoleamine mediated regulation of secondary metabolites in plants. Caffeine alkaloids and polyamine levels were augmented by exogenous indoleamines (SER and melatonin) in Coffea canephora P. Ex. Fr. in vitro callus cultures.48 Moreover we have studied the influence of SER and melatonin on somatic embryogenesis in C. canephora.49
Photomorphogenetic Responses of Serotonin
SER also stimulates phosophoinositide (PI) turnover, which is found to mimic the red light effect in enhancing the nitrate reductase (NR) transcript levels and inhibiting phyI transcript accumulation and releasing second messengers in maize.50,51 The rate of recovery of the SER in St. John's wort is higher under low light than under supplemental light condition.37 The differential synthesis of indoleamines in light and dark observed in St. John's wort may suggests analogy with mammalian system, wherein the relative ratio of SER to melotonin may play a role in light mediated responses in plants.37 The indoleamine biosynthetic pathway and the relative roles of SER and melotonin have been well characterized in yeast, bacteria and mammals.52 A recent study demonstrates how endogenous pools of indoleamines varies under changes in photoperiod in green alga D. bardawil.53 SER concentrations are found to be higher during day times. They exhibit a striking diurnal rhythm remaining at a maximum level during the daylight hours and falling by more than 80% soon after the onset of darkness.54 Moreover, addition of SER and the effect of darkness were studied on biomass and metabolite production in Tetrahymena thermophila.55
Protective Role of Serotonin in Plants
Protective role for SER has also been suggested. SER has been reported to be one of the physiologically active compounds accumulated in the sting nettle of U. dioica and in trichomes in the pods of Mucuna pruriens.28 The high concentration of noradrenalin and SER found in organs of movements, the pulvini and tendrils of Albizzia julibrissin, P. sativum, Mimosa pudica13 compared with other vegetative parts. Increased synthesis of SER was also observed in rice leaves challenged with pathogenic infection. Specifically, the SER accumulated upon pathogenic infection is incorporated into the cell walls leading to strengthening of the wall.44 Phenylpropanoid amides of SER derivative i.e., p-coumaroyl SER and N-ferulyl SER accumulate in bamboo with witches broom disease.56 Rice plant accumulated SER, tryptamine and their amides coupled with phenolic acids in response to fungal pathogen infection. These compounds possible to play an important role in the formation of physical barrier to the invading pathogens.57
Serotonin Detoxify Excess Ammonia and Induce Seed Germination
One interesting study on SER synthesis and its possible biological function was reported for walnut ( Juglans regia) seeds, in which SER is mainly accumulated during the process of fruit abscission.17 This abscission period is accompanied by proteolysis and deamination of amino acids giving rise to ammonium accumulation in walnut seeds. To circumvent the toxic accumulation of ammonia, glutamine synthase assimilates ammonia together with glutamic acid via the synthesis of glutamine, which directly serves as a substrate for tryptophan synthesis. SER synthesis is closely associated either with ripening or maturation of plant organs or with the accumulation of ammonia, which occurs predominantly during the process of plant senescence.58
Serotonin Delaying Senescence
SER, with its high antioxidant activity, may play an important role in maintaining the cellular integrity of xylem parenchyma and companion cells by protecting them from the oxidative damage caused by the process of senescence and thus facilitate efficient nutrient recycling from senescing leaves into sink tissues.7 Predominant production of SER has been reported in reproductive organs and enormous induction of SER synthesis in senescing rice leaves, which is characterized by chlorophyll loss, membrane lipid peroxidation, increased reactive oxygen species (ROS) and induced senescence-related genes. SER synthesized upon senescence was found in the soluble fraction of senescent tissues, especially in the vascular bundle cells, such accumulation of SER is believed to play a protective role against ROS, leading to a delay in the process of senescence as demonstrated by analyses of transgenic rice plants, such as tryptophan decarboxylase (TDC) overexpression and TDC RNA interference (RNAi) in rice.44
Serotonin as a Potent Antioxidant
SER plays a role as an antioxidant by scavenging reactive oxygen species (ROS) and shows strong in vitro antioxidant activity. The antioxidant activity of SER far exceeds that of tryptophan, tryptamine and SER derivatives. SER relieves the accumulation of the toxic metabolite tryptamine and maintains the reducing potential of cells through its powerful antioxidant activity in the senesced leaves.44 So that SER plays a practical role in delaying senescence by scavenging ROS efficiently. SER derivatives viz. N-(p-Coumaroyl) serotonin (CS) and N-feruroylserotonin (FS) with antioxidative activity are present in safflower oil.59 SER derivatives viz. Cinnamoyl serotonin, 4-Coumaroyl serotonin, Caffeoyl serotonin, Feruloylserotonin and Sinapoyl serotonin were shown in Figure 5. In Datura metel SER acts as an antioxidant in protecting the young reproductive tissues from environmental stress. The exposure of Datura flower to a cold stress significantly increased the concentrations of SER.24
Figure 5.
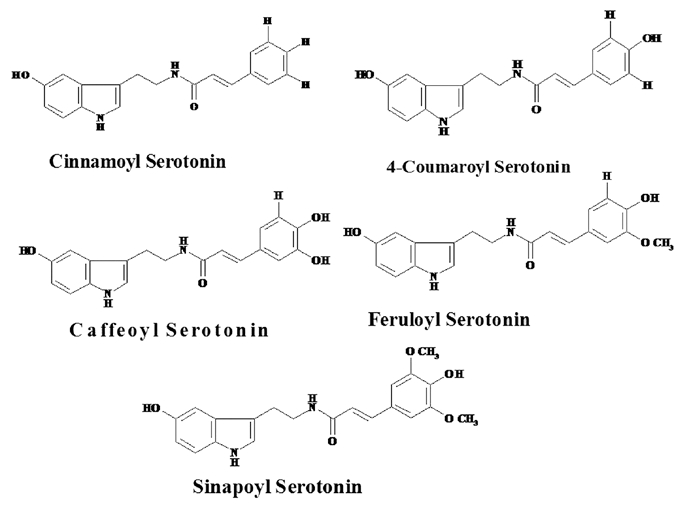
Serotonin derivatives in plants.
Health Benefits of Serotonin
SER is involved in the regulation of a number of important functions in humans, including sleeping, hunger, thirst, mood and sexual activity.60 Some of the foods (banana, pineapple, plum, nuts and milk) rich in SER and tryptophan may elevate mood by raising brain SER levels.61 SER, was reported in coffee, its presence in seeds and leaf wax29 and also in polished coffee called Astra, contain 20–40 mg SER/100 g and marketed as “low irritant” coffee in Germany and Switzerland.62 Due to the presence of SER in roasted coffee and in brew, it has physiological action in humans.63 SER was the amine most resistant to the effects of roasting and it was observed for some samples in higher amount after roast than in the green beans. The predominant amines that are reported in green coffee are SER and putrescine, followed by spermidine and spermine.63 Sweet cherries contain substantial amount of SER, and may have a great number of health benefits if incorporated in a healthy diet.25 Moreover, SER was also found in grape and wine which supports the health benefits due to their bioactive chemical diversity.64 Many plant species that are neurologically active in humans have been found to contain SER as well as other neurotransmitters. Such data indicates that SER in our diets and plant based medicines can affect the human health and may have an impact on several chronic diseases.60,68 FS and CS have been isolated from safflower seeds and possess antioxidant, anti-inflamatory activities,59 anti-tumor,65 anti-bacterial66 and anti-stress potential and also involved in reducing depression and anxiety.67
SER was administered to boost its levels in human brain in treating SER deficiency syndrome.68 Some of the extensive uses of SER include treating patients suffering from the parkinson-like symptoms68 and controlling obesity.69 SER action of hallucinogenic drugs is known from the early 1950s to the present day. There is now converging evidence from biochemical, electro- physiological and behavioral studies reported that the two major classes of psychedelic hallucinogens, (e.g., LSD) and the phenethylamines (e.g., mescaline) have a common site of action as partial agonists at 5-HT2A and other 5-HT2 receptors in the central nervous system.70
Future Perspectives
Although, SER has been identified in various plant species, many unanswered questions remain. In which part of the plant SER synthesis does occur? How important is the consumption of SER containing plant products for the health of humans? Do SER levels in plants change on a seasonal basis when they are grown in their natural habitat? Seasonal variations in day length would it influence the levels of SER in plant? Variations in the SER levels of plants grown at different latitudes should be studied. The discovery of mammalian neurohormones in plants used in the treatment of human ailments provides new avenues for investigation of medicinally active compounds. It is important to determine some of the positive health effects of plant SER. And also bioavailability studies of SER should be performed. Although interesting results have been obtained in the past five years, data on SER in plants are incomplete and more detailed studies are needed to elucidate the role of this compound in plants. All considerations with respect to the metabolism of SER in plants are still open. Isolation, characterization of the enzymes of biosynthesis and their modulators, the biosynthetic origin at tissue and cellular level has to be elucidated. The possible implication of SER catabolites in plant responses should be clearly elucidated. The pathway of SER transport, its possible conjugated compounds and its catabolic pathway(s) and also its interaction with IAA metabolism would be interesting. The existing data on the possible role of SER are very interesting but these investigations should be extended to other plant species with the aim of confirming the physiological action. Perhaps, the relation between SER and phytohormones will open up new perspectives in the possible role of SER in plant morphogenesis, flowering, dormancy and some stress-situations. The influence of stress factors viz. biotic and abiotic elicitors on SER synthesis should also be investigated. More studies on SER as growth modulator are necessary. The involvement of SER in organogenesis, both in vivo and in vitro, seems probable. The protective role of SER, data on apical dominance, SER's role in tropisms (photo-, geo- and others) and its relation with other plant growth regulators needs to be investigated. The possible presence of specific SER receptors in plant cells has to be researched. In animals, various subtypes of receptors (5HT1) have been characterized, and their genes have been sequenced. Another perspective is the possible interaction of SER with the postulated auxin receptor ABP-1. Thus, the information presented here make it conceivable that SER might play physiological role in plants and the indepth understanding is still to come through extensive research in the lines suggested in this review.
Acknowledgments
The authors (G.A.R. & P.G.) are thankful to the Department of Science & Technology, New Delhi, India for financial assistance. R.A. acknowledges CSIR, New Delhi for awarding Senior Research Fellowship.
References
- 1.Veenstra-VanderWeele J, Anderson GM, Cook EH. Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharm. 2000;410:165–181. doi: 10.1016/s0014-2999(00)00814-1. [DOI] [PubMed] [Google Scholar]
- 2.Rapport MM, Green AA, Pages IH. Crystalline serotonin. Science. 1948;108:329–330. doi: 10.1126/science.108.2804.329. [DOI] [PubMed] [Google Scholar]
- 3.Bowden K, Brown BG, Batty JE. 5-hydroxytryptamine: Its occurrence in cowhage (Mucuna pruriens) Nature. 1954;174:925–926. doi: 10.1038/174925a0. [DOI] [PubMed] [Google Scholar]
- 4.Roshchina VV. Neurotransmitters in plant life. Science Publishers, Enfield; 2001. pp. 4–81. [Google Scholar]
- 5.Feldman JM, Lee EM. Serotonin content of foods: Effect on urinary excretion of 5-hydroxy indoleacetic acid. Am J Clin Nutr. 1985;42:639–643. doi: 10.1093/ajcn/42.4.639. [DOI] [PubMed] [Google Scholar]
- 6.Murch SJ, Campbell SSB, Saxena PK. The role of serotonin and melatonin in plant morphogenesis: Regulation of auxin induced root organogenesis in in vitro-cultured explants of St. John's wort (Hypericum perforatum L.) In Vitro Cell Dev Biol Plant. 2001;37:786–793. [Google Scholar]
- 7.Kang S, Kang K, Lee K, Back K. Characterization of tryptamine-5-hydroxylase and serotonin synthesis in rice plants. Plant Cell Rep. 2007;26:2009–2015. doi: 10.1007/s00299-007-0405-9. [DOI] [PubMed] [Google Scholar]
- 8.Odjakova M, Hadjiivanova C. Animal neurotransmitter substances in plants. Bulg J Plant Physiol. 1997;23:94–102. [Google Scholar]
- 9.Csaba G, Pal K. Effect of insulin triiodothyronine and serotonin on plant seed development. Protoplasma. 1982;110:20–22. [Google Scholar]
- 10.Ramakrishna A, Giridhar P, Ravishankar GA. Indoleamines and calcium channels influence morphogenesis in in vitro cultures of Mimosa pudica L. Plant Signal Behav. 2009;12:1–6. doi: 10.4161/psb.4.12.10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fellows LE, Bell EA. Indole metabolism in Piptadenia peregrina. Phytochem. 1971;10:2083–2091. [Google Scholar]
- 12.Kema IP, de Vries EG, Muskiet FAJ. Clinical chemistry of serotonin and metabolites. J Chromatogra B. 2000;747:33–48. doi: 10.1016/s0378-4347(00)00341-8. [DOI] [PubMed] [Google Scholar]
- 13.Collier HOJ, Chesher GB. Identification of 5-hydroxytryptamine in the sting of the nettle (Utrica dioica) Brit J Pharmacol Chemother. 1956;11:186–189. doi: 10.1111/j.1476-5381.1956.tb01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udenfriend S, Weissbach H, Clark CT. The estimation of 5-hydroxytryptamine (serotonin) in biological tissues. J Biol Chem. 1955;215:337–344. [PubMed] [Google Scholar]
- 15.Foy JM, Parratt JR. 5-hydroxytryptamine in pineapples. J Pharm and Pharm. 1961;13:382–383. doi: 10.1111/j.2042-7158.1961.tb11840.x. [DOI] [PubMed] [Google Scholar]
- 16.Erspamer V. Recent research in the field of 5-Hydroxy tryptamine and related indolealkylamines. In: Jucker E, editor. Progress in Drug Research. Basel, Stuttgart: Birkhaser Verlag; 1961. pp. 151–367. [DOI] [PubMed] [Google Scholar]
- 17.Bergmann L, Grosse W, Ruppel HG. Serotonin in Juglans regia L. Planta. 1970;94:47–59. doi: 10.1007/BF00386608. [DOI] [PubMed] [Google Scholar]
- 18.Nicasio MDP, Villrreal ML, Gillet F, Bensaddek L, Fliniaux MA. Variation in the accumulation levels of N,N-Dimethyltryptamine in micropropagated trees and in in vitro cultures of Mimosa Tenuiflore. Nat Prod Res. 2005;19:61–67. doi: 10.1080/14786410410001658860. [DOI] [PubMed] [Google Scholar]
- 19.Badria FA. Melatonin, serotonin and tryptamine in some Egyptian food and medicinal plants. J Med Food. 2002;5:153–157. doi: 10.1089/10966200260398189. [DOI] [PubMed] [Google Scholar]
- 20.Vetorazzi G. 5-hydroxytryptamine content of bananas and banana products. Food Cosm Toxicol. 1974;12:107–113. doi: 10.1016/0015-6264(74)90326-5. [DOI] [PubMed] [Google Scholar]
- 21.Udenfriend S, Lovenberg W, Sjoerdsma A. Physiologically active amines in common fruits and vegetables. Archives in Biochem and Biophy. 1959;85:487–490. doi: 10.1016/0003-9861(59)90516-8. [DOI] [PubMed] [Google Scholar]
- 22.Foy JM, Parratt JR. Noradrenaline and 5-hydroxytryptamine in plantain (Musa sapientum, var. paradisiaca) J Pharm Pharmacol. 1960;12:360–364. doi: 10.1111/j.2042-7158.1960.tb12675.x. [DOI] [PubMed] [Google Scholar]
- 23.Waalkes TP, Sjoerdsma A, Creveling CR, Weissbach H, Udenfriend S. Serotonin, norepinephrine and related compounds in Banana. Science. 1958;127:648–650. doi: 10.1126/science.127.3299.648. [DOI] [PubMed] [Google Scholar]
- 24.Murch SJ, Alan AR, Cao J, Saxena PK. Melatonin and serotonin in flowers and fruits of Datura metel L. J Pineal Res. 2009;47:277–83. doi: 10.1111/j.1600-079X.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Gomez D, Lozano M, Fernandez-Leon MF, Ayuso MC, Bernalte MJ, Rodriguez AB. Detection and quantification of melatonin and serotonin in eight sweet Cherry cultivars (Prunus avium L.) Eur Food Res Tech. 2009;229:223–229. [Google Scholar]
- 26.Murch SJ, Hall BA, Le CH, Saxena PK. Changes in the levels of indoleamine phytocemicals during veraison and ripening of wine grapes. J Pineal Res. 2010;49:95–100. doi: 10.1111/j.1600-079X.2010.00774.x. [DOI] [PubMed] [Google Scholar]
- 27.Kimura M. Fluorescence histochemical study on serotonin and catecholamine in some plants. Jap J Pharma. 1968;18:162–168. doi: 10.1254/jjp.18.162. [DOI] [PubMed] [Google Scholar]
- 28.Grobe W. Function of serotonin in seeds of walnuts. Phytochem. 1982;21:819–822. [Google Scholar]
- 29.Kele M, Ochmacht R. Determination of serotonin released from coffee wax by Liquid Chromatography. J Chromatogra A. 1996;730:59–62. doi: 10.1016/0021-9673(95)01186-2. [DOI] [PubMed] [Google Scholar]
- 30.Traiffort E, Hubert P, Tayeb N, Aymard N. Rapid determination of 5-hydroxytryptamine in whole blood by liquid chromatography with fluorimetric detection. J Chromatogra. 1991;571:231–234. doi: 10.1016/0378-4347(91)80449-m. [DOI] [PubMed] [Google Scholar]
- 31.Battini ML, Careri M, Casoli A, Mangia A, Lugari MT. Determination of N-alkanoyl-5- hydroxytryptamides (C5HT) in coffee beans by means of HPLC and TLC. Ann Chim. 1989;79:369–377. [Google Scholar]
- 32.Lagana A, Curini L, de Angelis Curtis S, Marino A. Rapid liquid chromatographic analysis of carboxylic acid 5-hydroxytryptamides in coffee. Chromatographia. 1989;28:593–596. [Google Scholar]
- 33.Cao J, Murch SJ, O'Brien R, Saxena PK. Rapid method for accurate analysis of melatonin, serotonin and auxin in plant samples using liquid chromatographytandem mass spectrometry. J Chromatogra A. 2006;1134:333–337. doi: 10.1016/j.chroma.2006.09.079. [DOI] [PubMed] [Google Scholar]
- 34.Vanable JW. A ninhydrin reaction giving a sensitive quantitative fluorescence assay for 5-hydroxytryptamine. Anal Biochem. 1963;6:393–403. doi: 10.1016/0003-2697(63)90092-7. [DOI] [PubMed] [Google Scholar]
- 35.Garcia C, Marine A. Serotonin contents in fresh and processed foods. Rev Agroquim Tecnol Aliment. 1983;23:60–70. [Google Scholar]
- 36.Schroder P, Abele C, Gohr P, Stuhlfauth-Roisch U, Grosse W. Latest on the enzymology of serotonin biosynthesis in walnut seeds. Adv Exp Med Biol. 1999;467:637–644. doi: 10.1007/978-1-4615-4709-9_81. [DOI] [PubMed] [Google Scholar]
- 37.Murch SJ, KrishnaRaj S, Saxena PK. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John's wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000;19:698–704. doi: 10.1007/s002990000206. [DOI] [PubMed] [Google Scholar]
- 38.Arnao MB, Hernandez Ruiz J. The physiological function of melatonin in plants. Plant Signal Behav. 2006;1:89–95. doi: 10.4161/psb.1.3.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnao MB, Hernandez-Ruiz J. Melatonin in Plants. More Studies are Necessary. Plant Signal Behav. 2007;5:381–382. doi: 10.4161/psb.2.5.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei XY, Zhu RY, Zhang GY, Dai YR. Attenuation of cold induced apoptosis by exogenous melatonin in carrot suspension cells: The possible involvement of polyamines. J Pineal Res. 2004;36:126–131. doi: 10.1046/j.1600-079x.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 41.Tan DX, Manchester LC, Helton P, Reiter RJ. Phytoremediative capacity of plants enriched with melatonin. Plant Signal Behav. 2007;2:514–516. doi: 10.4161/psb.2.6.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atta-ur-Rahman, Bash A. Indole Alkaloids. Berks: Harwood Academic Publishers; 1999. p. 324. [Google Scholar]
- 43.Metzler DE. The chemical reactions of living cells. Vol. 3. New York, San Francisco: Academic Press; 1997. Biochemistry; p. 100. [Google Scholar]
- 44.Kang K, Kim YS, Park S, Back K. Senescence-induced serotonin biosynthesis and its role in delaying senescence in rice leaves. Plant Phy. 2009;150:1380–1393. doi: 10.1104/pp.109.138552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niaussat P, Laborit H, Dubois C, Hiaussat M. Action de la serotonine sur la croissance des jeunes plantules d'Avoine. Compt Rend Soc Biol. 1958;152:945–947. [PubMed] [Google Scholar]
- 46.Roshchina VV, Melnikova EV. Spectral analysis of intact secretory cells and excretion of plants. Allelop J. 1995;2:179–188. [Google Scholar]
- 47.Hernandez-Ruiz J, Cano A, Arnao MB. Melatonin: A growth stimulating compound present in lupin tissues. Planta. 2004;220:140–144. doi: 10.1007/s00425-004-1317-3. [DOI] [PubMed] [Google Scholar]
- 48.Giridhar P, Ramakrishna A, Sridevi V, Ravishankar GA. Augmentation of caffeine alkaloids by exogenous indoleamines in Coffea canephora P. Ex. Fr. in vitro cultures: The possible involvement of polyamines; Bali: In 23rd International conference on Coffee Science, (Association for Science and Information on Coffee, ASIC-2010). [Google Scholar]
- 49.Ramakrishna A, Giridhar P, Ravishankar GA. Serotonin and melatonin influence somatic embryogenesis in Coffea canephora; Florence: 5th International Symposium on Plant Neurobiology (SPNB-2009); p. 69. [Google Scholar]
- 50.Raghuram N, Sopory SK. Evidence for some common signal transduction events for opposite regulation of nitrate reductase and phytochrome-I gene expression by light. Plant Mol Biol. 1995;29:25–35. doi: 10.1007/BF00019116. [DOI] [PubMed] [Google Scholar]
- 51.Chandok MR, Sopory SK. 5-Hydroxytryptamine affects turnover of polyphosphoinositides in maize and stimulates nitrate reductase in the absence of light. FEBS Lett. 1994;36:39–42. doi: 10.1016/0014-5793(94)01213-x. [DOI] [PubMed] [Google Scholar]
- 52.Yu HS, Reiter RJ. Melatonin: Biosynthesis, physiological effects and clinical applications. Boca Raton: CRC Press; 1993. pp. 1–550. [Google Scholar]
- 53.Ramakrishna A, Dayananda C, Giridhar P, Rajasekaran T, Ravishankar GA. Photoperiod influences endogenous indoleamines in cultured green alga Dunaliella bardawil. Indian J Exp Biol. 2011;49:234–240. [PubMed] [Google Scholar]
- 54.Josefsson LG, Rask L. Cloning of a putative G-protein-coupled receptor from Arabidopsis thaliana. Eur J Biochem. 1997;249:415–420. doi: 10.1111/j.1432-1033.1997.t01-1-00415.x. [DOI] [PubMed] [Google Scholar]
- 55.Leclercq B, Exbrayat JM, Duyme F, De Coninck J. New approach to model effects of darkness, melatonin and serotonin on Tetrahymena thermophila growth and production of hydrolytic enzymes. Biotech Lett. 2002;24:769–774. [Google Scholar]
- 56.Tanaka E, Tanaka C, Mori N, Kuwahara Y, Tsuda M. Phenylpropanoid amides of serotonin accumulate in witchers' broom diseased bamboo. Phytochem. 2003;64:965–969. doi: 10.1016/s0031-9422(03)00429-1. [DOI] [PubMed] [Google Scholar]
- 57.Ishihara A, Hashimoto Y, Miyagawa H, Wakasa K. Induction of serotonin accumulation by feeding of rice striped stem borer in rice leaves. Plant Signal Behav. 2008;3:714–716. doi: 10.4161/psb.3.9.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peeters KMU, Van Laere AJ. Ammonium and amino acid metabolism in excised leaves of wheat (Triticum aestivum) senescing in the dark. Physiol Plant. 1992;84:243–249. [Google Scholar]
- 59.Hotta Y, Nagatsu A, Liu W, Muto T, Narumiya C, Lu X, et al. Protective effects of antioxidative serotonin derivatives isolated from safflower against postischemic myocardial dysfunction. Mol Cell Biochem. 2002;238:151–162. doi: 10.1023/a:1019992124986. [DOI] [PubMed] [Google Scholar]
- 60.Coutts RT, Baker GB, Pasutto FM. Food stuffs as sources of psychoactive amines and their precursors: Content, significance and identification. Adv Drug Res. 1986;15:169–232. [Google Scholar]
- 61.Spring B, Chiodo J, Bowen DJ. Carbohydrates, tryptophan and behavior: A methodological review. Psych Bull. 1987;102:234–256. [PubMed] [Google Scholar]
- 62.Stranc A. Astra—a natural coffee with a reduced irritant content. Przemysl spozywezy. 1993;47:76–77. [Google Scholar]
- 63.Casal S, Mendes E, Aalves RM, Alves RC, Ferreira MA. Free and conjugated biogenic amines in green and roasted coffee beans. J Agric Food Chem. 2004;52:6188–6192. doi: 10.1021/jf049509u. [DOI] [PubMed] [Google Scholar]
- 64.Iriti M, Faoro F. Grape phytochemicals: A bouquet of old and new nutraceuticals for human health. Med Hyp. 2006;67:833–838. doi: 10.1016/j.mehy.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 65.Nagatsu A, Zhang HL, Mizukami H. Tyrosinase inhibitory and antitumor promoting activities of compounds isolated from safflower (Carthamus tinctorius L.) and cotton (Gossypium hirsutum L.) oil cakes. Nat Prod Res. 2000;14:153–158. [Google Scholar]
- 66.Kumarasamya Y, Middletona M, Reida RG, Naharb L, Sarkera SD. Biological activity of serotonin conjugates from the seeds of Centaurea nigra. Fitoterapia. 2003;74:609–612. doi: 10.1016/s0367-326x(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 67.Yamamotova A, Pometlova M, Harmatha J, Raskova H, Rokyta R. The selective effect of N-feruloylserotonins isolated from Leuzea carthamoides on nociception and anxiety in rats. J Ethno pharm. 2007;112:368–374. doi: 10.1016/j.jep.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 68.Bell EA, Janzen DH. Medical and ecological considerations of L-DOPA and 5-HTP in seeds. Nature. 1966;210:529. doi: 10.1038/229136a0. [DOI] [PubMed] [Google Scholar]
- 69.Cangiano C, Ceci F, Cascino A, Del Ben M, Laviano A, Muscaritoli M, et al. Eating behavior and adherence to dietary prescriptions in obese adult subjects treated with 5-hydroxytryptophan. Ame J Clin Nut. 1992;56:863–867. doi: 10.1093/ajcn/56.5.863. [DOI] [PubMed] [Google Scholar]
- 70.Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- 71.Ly D, Kang K, Choi JY, Ishihara A, Back K, Lee SG. HPLC analysis of serotonin, tryptamine, tyramine and the hydroxycinnamic acid amides of serotonin and tyramine in food vegetables. J Med Food. 2008;11:385–289. doi: 10.1089/jmf.2007.514. [DOI] [PubMed] [Google Scholar]
- 72.West GB. Carcinoid tumors and pineapples. J Pharm Pharmacol. 1960;12:768–769. doi: 10.1111/j.2042-7158.1960.tb12747.x. [DOI] [PubMed] [Google Scholar]
- 73.Kimbrough TD, Reynolds JD, Humphereys KJ, Weekly LB. Diuranal changes in tissue leaf levels of tryptophan, tyrosine and amine metabolites in sedum morganium and sedum pachyphyllum. Biochemie Physiolgie der Pflanzen. 1987;182:67–72. [Google Scholar]
- 74.Applewhite PB. Serotonin and norepinephrine in plant tissues. Phytochem. 1973;12:191–192. [Google Scholar]
- 75.Nettleship L, Slaytor M. Limitation of feeding experiments in studying alkaloid biosynthesis in Peganum harmala callus cultures. Phytochem. 1974;13:735–742. [Google Scholar]
- 76.da Silveira TML, Tavares E, Gloria MBA. Profiles and levels of bioactive amines in instant coffee. J Food Comp Anal. 2007;20:451–457. [Google Scholar]
- 77.Regula I, Devide Z. The presence of serotonin in some species of genus Urtica. Acta Bot Croa. 1980;39:47–50. [Google Scholar]
- 78.Rayne S. Concentrations and profiles of melatonin and serotonin in fruits and vegetables during ripening: A mini-review 2010. Nature Precedings. 2010 doi: 10.1038/npre.2010.4722.1. [DOI] [Google Scholar]
- 79.Adao RC, Gloria BA. Bioactive amines and carbohydrate changes during ripening of 'Prata' banana (Musa acuminates x M. balbisiana) Food Chem. 2000;90:705–711. [Google Scholar]