Abstract
Two wheat varieties HD-29 (resistant, R) and WH-542 (susceptible, S) were pretreated with jasmonic acid (JA) or jasmonate and then artificially inoculated with sporidial suspension of Tilletia indica to study its influence in reducing Karnal bunt (KB) infection by regulating cystatin gene expression. JA was found to improve the plant defense against KB as its exogenous application resulted in decrease in coefficient of infection (CI) in both susceptible and resistant varieties following pathogen inoculation. Transcript profiling of wheat cystatin genes at different days after inoculation (DAI) showed that JA pretreatment positively induced cystatin gene expression in both varieties with greater induction of expression in resistant variety than the susceptible one (p < 0.05). Different temporal expression of three wheat cystatin genes, WC2, WC3 and WCMD was observed with their increased expression at 1DAI in the boot emergence stage which is most susceptible to KB and then slowly declined gradually at 3, 7 and 15 DAI in both the varieties. Except WC2, higher expression of other two cystatins viz. WC3 and WCMD at 1DAI showed higher response (p < 0.05) to KB pathogenesis at the disease-prone boot emergence stage as also evident by decrease of CI in both varieties. The results of determination of specific activity of cystatins by inhibitor assay were found to be consistent with those of transcript profiling. These findings suggest that jasmonic acid (JA) may act as a potential activator of induced resistance against Karnal bunt of wheat by upregulating cystatin gene expression.
Key words: Karnal bunt, jasmonic acid, cysteine protease, cysteine proteinase inhibitor, phytocystatins, wheat cystatin
Introduction
Karnal bunt (KB), also known as partial bunt, is a major disease of wheat which is caused by hemibiotrophic fungus, Tilletia indica, syn. Neovossia indica,1 a floret-infecting organism that proliferates in kernels to complete disease cycle. The grains are partially destroyed by the pathogen attack starting at the hilum and running along the suture, leaving the endosperm intact and covered by the whole or partly ruptured seed coat. Since the disease spreads through infected seeds and seriously impairs grain quality, it acts as a genuine non-tariffs trade barrier in commercial wheat seed trade in the international markets. Plant breeders have been unsuccessful to develop disease resistant varieties though degree of resistance varies in different cultivated varieties. It is being felt that elucidation of molecular mechanism of plant defense against KB could help in development of disease resistant varieties through Biotechnological methods. In recent years, extensive studies on plant pathogen interaction have shown the involvement of programmed cell death (PCD) in both pathogenesis and defense.
During plant pathogen interaction in plants, proteases especially cysteine proteases may generate inducer signal specific to PCD2 by processing/releasing bioactive molecule or by activating cell surface receptors. In our lab, the induction of cysteine proteases especially caspase 3 like protease has been reported during PCD in response to KB colonization in wheat calli.3,4 It is known that induction of PCD, in plants, is regulated by cysteine protease inhibitors or phytocystatins (Phycys). Phytocystatins (PhyCys) or plant cystatins are 12–16 kDa in size, contain no disulfide bonds and share sequence homology with animal cystatins but lack disulphide bonds. PhyCys constitute a multigene family, different members of which inhibit the activity of endo- and/or exogenous cysteine proteinases to regulate PCD.5 For example, they have been demonstrated to regulate the protein turnover by preventing the degradation of storage protein by endogenous proteinases during endosperm development.6–8 They are also known to impart plant defense by counteracting exogenous proteases secreted by insects, nematodes and fungi.9–12 In wheat (Triticum aestivum) multiple cystatins like WC1-5 and multidomain wheat cystatin (WCMD) are known to be differentially expressed at different developmental stage of wheat seed maturation and germination and inhibit exo/endo cysteine proteases.13–15 The ability of a plant to withstand the attack of a potential pathogen depends upon inhbition of exogenous fungal cysteine proteases by the induction of appropriate cystatin besides other biochemical events. It has been demonstrated that the activity poised in between cysteine proteases and cysteine protease inhibitors or cystatins decides the ultimate fate of cell in form of PCD or cell survival during plant pathogen interaction.2
Jasmonic acid (JA) and it's derivatives—large family of oxylipin products of linolenic acid which are produced through the induction of octadecanoid pathway16—are known to induce the expression of cysteine proteinase inhibitors/cystatins in tomato and soybean.17,18 Furthermore, jasmonate (JA) dependent pathways defend plants against necrotrophic fungi by suppressing PCD whereas biotrophic fungi are known to be restricted by activation of salicylic acid (SA) dependent pathway which induces PCD during plant pathogen interaction. Tilletia indica, being hemibiotrophic and showing more propensity towards necrotrophy, requires deceased plant cells, particularly during the later stages of its life cycle when it ingresses the ovary cells to form teliospores. As one would expect, disease progression can be restricted by suppression of programmed cell death (PCD) through activation of jasmonate dependent pathway leading to resistance against KB. Hence, it's possible involvement in regulating PCD during pathogenesis of Karnal bunt through affecting critical balance between wheat cystatins and cysteine proteases can not be ruled out.19
KB pathogen, Tilletia indica, is slow growing fungal pathogen and its development is concomittantly dependent on development of host from flowering to grain filling stages. Thus the influence of JA signalling involved in induction of cystatins in plant's response to pathogen attack during developing spikes of wheat is obviously of great importance. In view of above, the present study was carried out to elucidate the possible role of different cystatin genes in imparting defense to KB at different stages of developing spikes and to detect if induction of defense and arrest of fungal colonization depends on JA signaling by modulating the expression of different cystatin genes.
Results and Discussion
The most favorable weather for KB infection coincides with wheat heading. It was demonstrated that infection occurred most reliably after hypodermically injecting a suspension of sporidia into the wheat boot at the awns emerging stage when the ear head just peeps out at the tip or from center.20 Percentage disease severity of this stage is 22.2 as compared to 6.97 of boot stage and 6.9 of ear head half outside boot leaf.21 The KB is slow growing fungal pathogen and its development is concomitantly dependent on the host's flowering to grain filling stages. The boot emergence stage of developing spikes at pre-anthesis is the most susceptible stage and disease infectivity significantly drops when ear head is completely out from boot leaf and at the anthesis or post anthesis stage. Hence, it is quite worthwhile to study the influence of JA on the expression of cystatin genes and registering the resistance against KB during pre-anthesis, anthesis and post-anthesis stages of developing spikes. Therefore, in order to check whether JA can reduce disease severity, JA pre-treatment was done a day prior to artificial inoculation of the KB pathogen in wheat spikes of both varieties when the ear head just peeped out of the boot.
Pathogenicity testing of cultivars after JA treatment and boot inoculation of sporidial suspension.
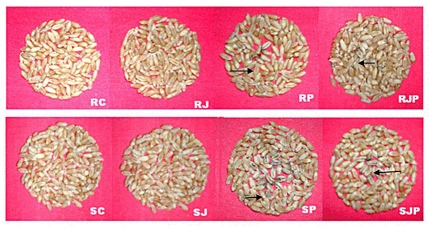
Results of infectivity tests carried out by boot injection are presented in Table 1. On comparing the pathogen inoculated plants of both varieties (RC and SC), the values of % infection, coefficient of infection and overall response of susceptible variety were found to be greater than the resistant variety. However, on JA application values were decreased in both susceptible and resistant varieties, showing inhibition of infection due to induction of defense during conditioning effect before pathogen inoculation. JA mediated defense was found to be more pronounced in resistant variety than the susceptible variety. It was observed that JA changed the overall response value towards pathogen from 43 to 6.4 in susceptible (SP vs. SJP) and from 12.4 to 1.7 in resistant variety (RP vs. RJP). The coefficient of infection (CI) was also high in the absence of JA in pathogen inoculated varieties. But, JA application decreases its value from 0.087 to 0.04 in case of susceptible variety and from 0.047 to 0.023 in case of resistant variety. The role of JA in reduction of disease incidence also became evidant from observation of seeds harvested from both varieties pretreated with JA but inoculated with pathogen (Fig. 1). It was found that JA considerably reduced amount of seed blackening in wheat seeds after pathogen inoculation in both varieties. This clearly suggests that JA can elicit the defense responses in favor of the host to enable it to resist KB pathogenesis.
Table 1.
Pathogenicity testing of two varieties under different treatments on the basis of disease scoring after the crop harvest
| Treatments | Total no. of seeds | No. of seeds with susceptible reaction | Percentage infection | Coefficient of infection in% | Overall response value*** |
| (1) | (2) | (3) | (4) | (5) | (6) |
| Resistant | |||||
| RC | 116 | 0 | 0 | 0 | 0 |
| RJ | 93 | 0 | 0 | 0 | 0 |
| RP | 85 | 15 (14* + 1**) | 17.65 | 0.047 | 12.44 |
| RJP | 88 | 8 (8*) | 9.09 | 0.023 | 1.67 |
| Susceptible | |||||
| SC | 109 | 0 | 0 | 0 | 0 |
| SJ | 100 | 0 | 0 | 0 | 0 |
| SP | 89 | 21 (11* + 10**) | 23.6 | 0.087 | 43 |
| SJP | 90 | 12 (10* + 2**) | 13.33 | 0.04 | 6.4 |
Incipient infection.
Blackening of seed upto ½.
Products of numerical values in column 3–5.
Figure 1.
Seeds collected after crop harvest of both varieties subjected to different treatments. SC, control susceptible variety; RC control resistant variety; SJ, jasmonic acid-treated susceptible variety; RJ, jasmonic acid-treated resistant variety; SP, pathogen inoculated susceptible variety; RP, pathogen inoculated resistant variety; and SJP, pathogen inoculated and JA conditioned susceptible variety; RJP, pathogen inoculated and JA conditioned resistant variety (Black arrows represent the susceptible reaction).
Molecular cloning of wheat cystatin gene families.
Since JA is known to trigger the expression of proteinase inhibitors, the preconditioning/priming effect could be the result of reinforcement/alteration in the downstream signaling pathways which ultimately leads to expression of cystatins which decrease/delay fungal growth and development. Therefore, in the present study, attempts were made to study the effect of jasmonate signaling on the expressions of three wheat cystatin WC2, WC3 and WCMD during resistance response against Karnal bunt. Different sets of primers used for amplification of three cystatin genes are given in Table 2. The primer sets used were efficient in reproducible amplification of expected sizes of amplicons. After two-step RT-PCR, products were checked on 1.8% agarose gel. The amplicon sizes of WC2, WC3 and WCMD were 250, 450 and 750 bp respectively. The amplicons were cloned and subsequently sequenced by Automated DNA Sequencing Service of GeNei (India). The obtained sequences were shorter than the size estimated by gel electrophoresis as the terminal sequences could not be read with confidence by using this technique. The sequences showed 80 to 100% homology to different wheat cystatins upon homology search using BLAST tool (Table 3). The multiple sequence alignment with other cystatins showed known conserved signature motifs for cystatin. The sequences of WC2, WC3 and WCMD were submitted as partial cDNA to NCBI database with accession numbers as DQ234766.1, FJ545271 and FJ545270 respectively. Further, phylogenetic analysis of wheat cystatins also reveals the distinctness of each members of cystatin gene family (Fig. 2).
Table 2.
Detail of primers used for the expression profiling of different wheat cystatin families
| S no. | Primer code | Sequence (5′→3′) | No. of bases | Tm (°C) | % GC |
| 1 | WC2F | CCG TCT CCG AGC ACA AGA | 18 | 55 | 61 |
| 2 | WC2R | ATC CTT GCA CAG CTG GGA | 18 | 55 | 55 |
| 3 | WC3F | CCA TCG TCG TGC CGT TTA CTC | 21 | 55 | 57 |
| 4 | WC3R | TAC ACG AGA ACA CGC TAA ACC A | 22 | 55 | 45 |
| 5 | WCMDF | CAT AAT ATC CAG GTA GAG ACA GGT | 24 | 55 | 45 |
| 6 | WCMDR | ATT CAC TGG CTG CTA GAT TCG TCA | 24 | 55 | 46 |
| 7 | Wac F | AGT GTC TGG ATC GGT GGC TCT ATT | 24 | 55 | 50 |
| 8 | Wac R | TCC CCT TCA CCG ACT CTT CAA AA | 23 | 55 | 48 |
Table 3.
Results of homology search of wheat cystatins by nucleotide blast
| Accession no. | Nucleotide name | Homology (%) |
| MDWC | ||
| AB223039.1 | Triticum aestivum multidomain cystatin | 94% |
| BT009401.1 | Triticum aestivum clone wlm96.pk045.d23:fis | 92% |
| AJ748344.1 | Hordeum vulgare ssp. vulgare cystatin Hv-CPI4 (icy4 gene | 91% |
| EU835902.1 | Triticum aestivum cultivar HD29 cystatin | 93% |
| WC2 | ||
| DQ127246.1 | Triticum aestivum cystatin WC2 | 100% |
| AB038395.1 | Triticum aestivum WC2 cysteine proteinase inhibitor | 99% |
| WC3 | ||
| BT009300.1 | Triticum aestivum clone wlm1.pk0011.f3:fis | 95% |
| AK333096.1 | Triticum aestivum cDNA, clone: WT005_K06 | 95% |
| AB038394.1 | Triticum aestivum WC3 cysteine proteinase inhibitor | 95% |
| AB038392.1 | Triticum aestivum WC1 cysteine proteinase inhibitor | 92% |
| DQ279929.1 | Triticum aestivum genotype WH-542 cystatin WC-1 | 97% |
| DQ279928.1 | Triticum aestivum genotype HD-29 cystatin WC-1 | 97% |
| AB038393.1 | Triticum aestivum WC4 cysteine proteinase inhibitor | 80% |
| DQ279931.1 | Triticum aestivum genotype WH-542 cystatin WC-4 | 80% |
| DQ279930.1 | Triticum aestivum genotype HD-29 cystatin WC-4 | 80% |
| AB038395.1 | Triticum aestivum WC2 cysteine proteinase inhibitor | 83% |
Figure 2.
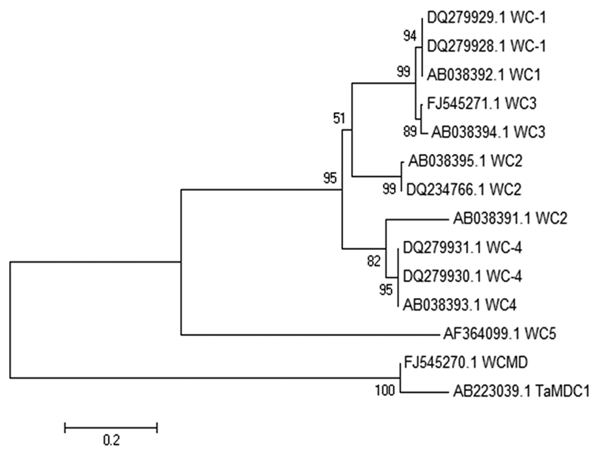
Phylogenetic tree construction using UPGMA method for establishing the similarity index between cystatin gene sequences isolated in the present work (DQ234766.1, FJ545271 and FJ545270) and different cystatins available in NCBI databases.
Expression profiling of cystatin at the transcriptional level.
In order to assess the regulatory mechanism of cystatin genes at the transcriptional level in the wheat spikes, the expression profiling of WCs was monitored at different developmental stages of wheat spikes of resistant and susceptible varieties under the influence of JA/pathogen or both using the technique of semi-quantitative RT-PCR taking actin gene as the internal standard.
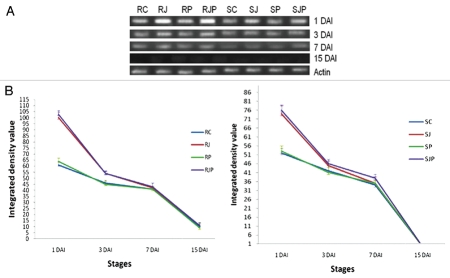
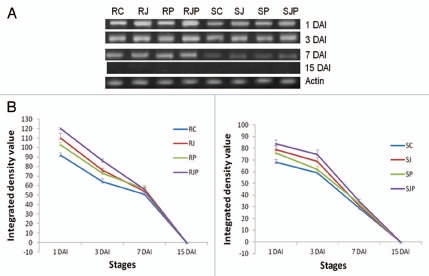
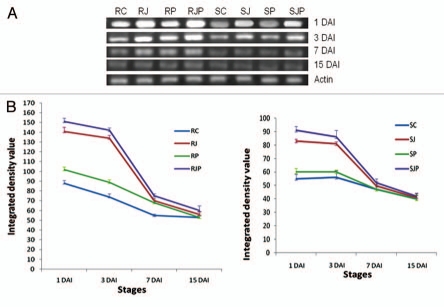
At 1 DAI which is boot emergence stage, the expression of all three cystatins viz. WC2, WC3 and WCMD genes was found to be high under all treatments applied when compared with those at 3, 7 and 15 DAI, including the control (RC/SC) (Figs. 3–5A and B). Under the influence of JA hormone, expression of all wheat cystain transcripts increased substantially (p < 0.01) in both the varieties, although the effect was more pronounced in R than in S variety. At 3 DAI, the cystatin levels declined and kept on decreasing till 15 DAI when none or negligible expression was observed in both varieties. The effect of pathogen infection on WC2 transcript cannot be stated decisively, as any significant difference was not observed between control and pathogen inoculated lines in R and S (p > 0.05). However, WC3 and WCMD transcript expression significantly increased in response to pathogen (p < 0.05) from 1 DAI to 7 DAI in both R and S variety with and without JA pretreatment.
Figure 3.
(A) Semiquantitative RT-PCR of WC2 on 1 DAI, 3 DAI, 7DAI and 15 DAI for different treatments as indicated in the figure. (B) Variation in the expression of WC2 under different treatments and stages. Data plotted are an average of three independent experiments. Line at points represents standard deviation of the mean (Left part and right part represent treatments on resistant and susceptible variety respectively).
Figure 5.
(A) Semiquantitative RT-PCR of WCMD on 1 DAI, 3 DAI, 7DAI and 15 DAI for different treatments as indicated in the figure. (B) Variation in the expression of WCMD under different treatments and stages. Data plotted are an average of three independent experiments. Line at points represents standard deviation of the mean. (Left part and right part represent treatments on resistant and susceptible variety respectively).
Thus cystatin might be a candidate gene whose expression may be induced during induction of defense responses to T. indica upon infection. This is based on observation that amount of cystatin transcripts is higher in R variety than in S at every stage analyzed. Since boot emergence stage of wheat is the most prone stage to KB infection, highest expression of cystatin appeared to be induced at this stage. The primary role of WCs' expression at an early stage of seed maturation is to control the endogenous CPs that may process newly synthesized proteins in the seed. However, under challenged conditions, these WCs can efficiently target the exogenous cysteine proteases secreted by pathogens. The upregulation of cystatins upon JA pretreatment at every stage further suggests that JA strengthens the induced defense response probably by preventing the cysteine protease mediated PCD. However, this effect of JA didn't last long as the difference of cystatin levels between control plants and JA treated lines decreased gradually with time. This short lived effect of JA on cystatin expression was expected because JA, being a phytohormone does not regulate any process for long. This could have an adverse effect on plant physiology as channeling its resources only in one direction may prove deleterious to plant health. Chini et al., 2007, described a negative feedback loop mechanism which provides an explanation of the pulsed hormonal response and subsequent desensitization of the cell to jasmonate.
Cysteine protease inhibitor assay.
After checking the expression of various cystatin genes at the RNA level, we wanted to see whether the changes are also manifested at the protein level. Though, the expression analysis of three members of cystatin gene family were carried out individually but the total cystain activity determined in the present study with the intention to find out the cumulative effects of all the cystatin temporally expressed under the influence of JA in relation to KB infection in developing spikes. Therefore, total specific activity of cystatin was analyzed by performing cysteine protease inhibitor assay of both varieties under different treatments and stages. The results of cysteine proteinase inhibitor assay to assess the levels of cystatins under different treatments and stages comply with the WC gene families RNA expression data.
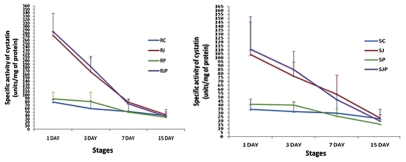
In control plants of both varieties, expression of cystatins was high at 1 DAI (p < 0.05), in boot emergence stage when the spikes are just emerging from the boot but gradually decreased thereafter as the grain formation further progressed (Fig. 6). Interestingly, this stage of spike development is the most susceptible to KB infection. This indicates that cystatin is a natural defense weapon of plant against a probable Tilletia attack and/or other floret infecting diseases. The level of cystatin in R was twice as much as in S variety. This shows that resistant wheat variety expresses greater amount of cystatins in the boot stage which provide greater resistance against KB. In pathogen inoculated R variety, cystatin expression was observed to be high at 1DAI and then steadily decreased at subsequent DAI's. However, an increase in cystatin level as compared to control (RC and SC) at 1 DAI in RP and SP treatments shows that resistant and susceptible plants upregulate cystatin expression in response to KB infection. However, the rise in S variety was less than that in R variety. On 3 DAI, the amount of cystatin expressed further increased in RP compared to control (81 vs. 60, p < 0.02). However, as the grain formation slowly progressed the expression of cystatin decreased in RP & SP with more decline in S variety as compared to the R variety. On 15 DAI, the cystatin level was less than half in S as compared to R (35 in RP vs. 16 in SP).
Figure 6.
Summary of variation in specific activity of cystatins (in Units/mg of protein) determined spectrophotometrically in developing wheat spikes subjected to various treatments on 1 DAI, 3 DAI, 7 DAI and 15 DAI in the resistant (R) and susceptible (S) variety. RC and SC: control, RJ and SJ: JA treated, RP and SP: Pathogen inoculated, RJP and SJP: JA treatment followed by pathogen inoculation. (Left part and right part represent treatments on resistant and susceptible variety respectively).
In JA treated plants, there was sharp increase in cystatin levels both in R and S varieties. The cystatin activity increased from 79 to 274 and from 35 to 104 in resistant and susceptible variety respectively. Thus, R variety was found to be more responsive to JA than S variety. With increasing time interval, however, this difference declines rapidly. On 15 DAI, total cystatin specific activity in RJ is 42 compared to 40 in RC. In S variety, this value is 24 in SJ while 23 in SC. Thus the effect of JA and its downstream signaling is transient and cystatin expression returns to basal level. Almost a similar pattern was observed in plants treated with JA followed by pathogen inoculation i.e., RJP and SJP. One DAI, cystatin activity was more in JP treated plants than in those which were only sprayed with JA. Thus, just after inoculation there was an increase in cystatin expression. However, this difference was only transient and gradually the level of cystatin decreased in JP lines than in R/SJ lines. Nevertheless, in all the treatments and stages, cystatin activity was always more in R variety than in S variety.
One can easily deduce that JA did boost up cystatin expression levels. The total cystatin content of the developing spikes markedly increase in the RJ, RJP, SJ and SJP. The increment was more in R than in S variety. Again R variety registered a greater cystatin activity at every stage monitored and treatment given than the S variety. In response to pathogen treatment also R and S lines showed an increase in cystatin activity on 1 DAI and 3 DAI. However, after that levels of cystatins were less than the control. This decrease in the cystatin expression at the protein level on 7 DAI and 15 DAI was not observed on the mRNA levels of any of the WCs. This can be explained by the fact that in pathogen inoculated lines development of disease took place. During pathogenesis T. indica secreted cysteine proteinases as a part of the protease cocktail for invasion into the host cells. This would also have induced PCD in the seeds, a process in which cysteine proteases/caspases are known to play an important role in plants. Cysteine proteases thus produced would have neutralized the cystatins induced by plants as a part of their defense mechanism by protein-protein interaction. This could have been the reason behind the decrease of cystatin levels in the pathogen inoculated lines in both R and S varieties as compared to control and JA treated plants.
Several genes termed senescence associated genes (SAG) in plants that show sequence similarity to cysteine proteases are induced during early senescence (Lohman et al. 1994). These plant proteases are good candidates for cell death initiation genes. The expression of a cysteine proteinase coincides with several developmental events associated with PCD in Solanum melongena (brinjal), i.e., during leaf senescence, fruit senescence, xylogenesis, nucellar cell degradation and anther senescence23,24 identified genes encoding ancestral caspase-like proteins, the metacaspases, which are present in plants, fungi and protozoa. Bozhkov et al., 2004,25 showed, for the first time that a principal caspase like activity is implicated in plant embryogenesis. This activity is increased at the early stages of embryo development, and is directly involved in the terminal differentiation and death of the embryo suspensor. Zupini et al., 2004,26 reported the presence of proteins which are structurally similar to animal caspase 9 and 12 in cytosolic extracts of soybean cells treated with CPA and are involved in ER-mediated PCD. Increase in cysteine peptidase activity and gene expression associated with environmental stress conditions and plant pathogen interaction have also been noted.27 Thus differential expression of cystatin by different wheat varieties can explain their differential responses to KB pathogen. This supports the candidature of cystatin gene as a marker to check the resistance of wheat varieties against KB.
In conclusion, the present study suggests that WC2, WC3 and WCMD cystatins are potential candidate genes involved in imparting resistance to Karnal bunt disease of wheat. It is believed that this knowledge would help in development of complete resistance to invading KB pathogen in florets if appropriate cystatin genes get introduced for overexpression and for inhibition of the fungal proteases required for pathogenesis. Since their expression is enhanced by JA, it opens up new avenues for introducing the JA-responsive cystatin genes in wheat to confer resistance against KB. Alternatively the genes of JA biosynthesis could be put under the control of pathogen inducible promotor in transgenic wheat plants for engineering resistance against KB of wheat.
Material and Methods
Source of plant material and pathogen.
Two wheat varieties, one highly susceptible (WH 542) and another resistant (HD 29) to KB based on their pathogenicity testing on several host differentials were collected from Crop Research Center, Pantnagar and Department of Genetics and Plant Breeding of Punjab Agricultural University, Ludhiana. The response of these two varieties to KB pathogen has been determined by calculating the coefficient of infection and overall response value by artificially inoculating them in different consecutive years.28 The fungal strain of T. indica (KBPN) was collected from Wheat Pathology Lab, Department of Plant Pathology, College of Agriculture, G. B. Pant University of Agriculture and Technology, Pantnagar. After 21 days growth of fungal mycelium in PDA, the sporidial suspension was extracted and diluted to 106 sporidia/ml. The suspension was aliquoted and stored at 4°C till inoculation at boot leaf stage of wheat spikes.
Pathogen inoculation and JA treatment under field conditions.
Surface sterilized wheat seeds (Triticum aestivum cv. HD 29 and WH 542) were germinated on wet paper and were planted on commercial soil mix. Plants were grown at 22°C/18°C (12 h light/12 h dark) in a glass house. The pot experiment was laid out in a randomized block design having four different treatments [control (C), JA treatment (J), Pathogen inoculation (P) and JA treatment followed by pathogen inoculation (JP)] in both susceptible and resistant wheat cultivars having five replicates and designated as SC, SJ, SP, SJP and RC, RJ, RP, RJP respectively. JA treatments were given at the rate of 10 sprays per spike with an atomizer having volume spray of 40 µl for each spray (400 µl for 10 sprays) and the concentration of JA was 1 µg/µl. The injection technique was adopted in which the inoculum is injected with a hypodermic syringe into the boot just as awns emerged.29,30 High percentage of infection can be obtained with this technique. Ear heads were artificially inoculated using hypodermic syringe with the 21-day-old sporidial cultures (106 sporidia/ml) in the month of January. Inoculated ear heads were covered with butter paper to prevent natural infection as well as to maintain moisture.
Plant material and selection of different stages of developing wheat spikes.
Based on the degree of susceptibility to pathogen invasion, different stages of developing wheat spikes were selected for expression kinetics of cystatin genes. For the expression profiling of cystatins both at the transcriptional and translational levels, sampling was done at different time intervals following the inoculation of wheat spikes, viz. 1 DAI (day after inoculation), 3 DAI, 7 DAI and 15 DAI. The collected plant material in the form of developing florets and spikes were cut off, frozen in liquid nitrogen and kept at −80°C until further use for RNA and protein extraction. For different treatments and stages, experiments were conducted in triplicate.
Disease scoring.
Disease scoring is primarily based on the percentage of infected kernels. Average percent infected grains were calculated after harvesting. As most of the bunted grains were infected partially, depending on the extent of damage to the grains the numerical values were given for calculating the coefficient of infection. Number of grains showing incipient infection, blackening extending up to ¼, ½, ¾ and infected grains were multiplied with the numerical values 0.25, 0.5, 0.75 and 1.00 divided by 100 to represent the value of coefficient of infection in percent. Overall aggressiveness of the pathogen was assigned a numerical value, which was the product of percent KB infection, coefficient of infection and number of wheat seeds with susceptible reaction.
Total RNA isolation and purification.
Total RNAs were extracted from the wheat spikes of resistant and susceptible genotypes at different time intervals using One-Step RNA Isolation Reagent MYRzol from Life Tech Inc., as instructed and quantified spectrophotometrically. Equal quantities of total RNA isolated from each of three plants randomly selected from each treatment were bulked and subjected for RT PCR analysis. Total RNA was treated with RNase free DNaseI according to manufacturer's instruction (BioBasic). Total RNA (10 µg) was separated on 1.2% agarose gel containing formaldehyde for assessing the quality of isolated RNA.31
Designing of primers.
The sequences of WC2, WC3 and multidomain (WCMD) cystatin were downloaded from NCBI for designing of primer sets. Different sets of primers were designed with the help of Primer Select tool available in DNASTAR software for PCR amplification of cystatin gene family members (WC2, WC3 and WCMD) by RT-PCR and for coamplification of wheat Actin gene. The details of forward and reverse primers, their Tm and %GC contents are given in Table 2.
Molecular cloning of different cystatin gene families.
For isolation of cystatin gene families, two-step RT-PCR was done using RevertAid H minus M-MuLV reverse transcriptase (RT) supplied by Fermentas. The amplified products were purified using QIAquick gel extraction kit (Qiagen). The purified products were checked spectrophotometrically for size confirmation and then sent directly for sequencing. Before transformation of the amplified RT-PCR products, they were purified with the QIAquick PCR purification kit (Qiagen). pGEM-T Easy vector system (Promega, Madison, WI) was used to clone the PCR amplicons which were then cloned into DH5α cells by CACl2 mediated transformation protocol (Sambrook 1989) and then screened by blue-white selection. Plasmids were isolated and checked spectrophotometrically to determine their concentration, restriction digested and checked on agarose gel (1.8%).
Semi-quantitative RT-PCR for expression profiling of cystatin gene families.
RT-PCR was used for amplification of cystatin genes which is a semi-quantitative measure of mRNA transcripts or cDNA levels, and is the method of choice when the copy number is low or small mounts of sample are available for analysis. RT-PCR was carried out to check the expression of wheat cystatins mRNA transcripts using the specific primers designed as above (see Table 2). For semi-quantitative RT-PCR of the cystatin gene families, the QIAGEN One Step RT-PCR kit was used. Actin gene was selected as endogenous internal standard, because it is a house keeping gene which is expressed at all stages and in all the tissues, i.e., its level of expression is expected to be constant in all tissues and at all times. Primers for actin gene were designed using software DNASTAR. Further, this gene has also been used as an endogenous internal standard in various RT-PCR studies.32 Thermal cycler was programmed as follows reverse transcription at 50°C for 30 min and subsequent 35 cycles of 94°C denaturation for 30's, 55°C annealing for 30's and 2 min extension at 72°C. After the completion of PCR, 6 µl of the amplicon was analyzed on 1.8% agarose gel by electrophoresis.
Densitometry analysis of gel for semi-quantitative analysis of expressed genes (cystatin and actin).
A densitometry analysis was done with the help of densitometry tool available in the Gene Profiler software, Alpha Innotech Corporation, Santa Clara, CA. Briefly, individual gels were scored by placing the curser over individual band and recording the relative densitometry values of three independent gels used for expression analysis.
Extraction of cystatin proteins.
The wheat spikes were ground with liquid nitrogen and total protein was extracted using Tris-Cl extraction buffer (0.05 M, pH 7.5) at room temperature. After centrifugation at 8,0009 g at 4°C, the supernatant was collected and supernatant (crude extract) was precipitated with 30–60% ammonium sulfate and then dialyzed against H2O and freeze-dried. Total protein content was measured by procedure of Bradford (1976) with bovine serum albumin as protein standard.
Cysteine proteinase inhibitor assay.
The quantitative estimation of cystatin activity was estimated by cysteine proteinase inhibitor assay. 30 µl of papain solution (1 mg/ml in 0.1 M phosphate buffer, pH 7.8) was taken in different test tubes (in negative control only phosphate buffer was added). In each tube 40 µl of activation solution (0.02 M EDTA, 0.05 M cysteine chloride, pH 8) followed by 40 µl of tris buffer (50 mM, pH 7.5) was added and incubated at 45°C for 10 min. In each tube 60 µl of protein fraction extracted from different samples followed by 330 µl of tris buffer (pH 7.5) was added (In control tubes no protein sample was added). All the tubes were incubated for 10 min at room temperature. After incubation, 200 µl of substrate (1% azocasein solution) was added in each tube. All the tubes were incubated for 3 hr at 37°C. After incubation, 300 µl of 10% TCA solution was added into each tube which was then centrifuged at room temperature at 10,000 g for 5 min and supernatant was taken. Immediately after supernatant was alkalinized with equal volume of 0.25 N NaOH solutions, the absorbance was taken at 440 nm to observe the change in OD.
Statistical analysis.
Three independent values of each treatment in both varieties for different cystatin gene families were taken and mean ± SE values were calculated for statistical analysis using paired t-test.
Figure 4.
(A) Semiquantitative RT-PCR of WC3 on 1 DAI, 3 DAI, 7DAI and 15 DAI for different treatments as indicated in the figure. (B) Variation in the expression of WC3 under different treatments and stages. Data plotted are an average of three independent experiments. Line at points represents standard deviation of the mean. (Left part and right part represent treatments on resistant and susceptible variety respectively).
Acknowledgements
Authors are grateful to CSIR for providing the senior research fellowship to SD during tenure of the study and to Dean, College of Basic Sciences and Humanities and Director, Experiment Station for providing all necessary help and facilities for carrying out this research.
References
- 1.Mitra M. New bunt on wheat in India. Ann Appl Biol. 1931;18:178–179. [Google Scholar]
- 2.Solomon M, Belenghi B, Delledonne M, Levine A. The Involvement of cysteine proteases and protease inhibitor genes in programmed cell death in plants. Plant Cell. 1999;11:431–444. doi: 10.1105/tpc.11.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mani S, Yadav MK, Khan GT, Singh US, Kumar A. Enhanced proteolysis leads to premature cell death under the influence of elicitor like mycelial components from Karnal bunt (Tilletia indica) pathogen in wheat callus cultures. Indian J Exp Biol. 2005;43:671–678. [PubMed] [Google Scholar]
- 4.Sethy NK, Pandey D, Singh US, Kumar A. Caspase-3 like protein in wheat—Tilletia indica dual culture system as potential biomarker for host resistance to Karnal bunt. Indian J Biotechnol. 2003;2:596–602. [Google Scholar]
- 5.Barrett AJ, Rawlings ND, Davis ME, Machleidt W, Salvesen G, Turk V. Cysteine proteinase inhibitors of the cystatin superfamily. In: Barrett AJ, Salvesen G, editors. Proteinase Inhibitors. Amsterdam: Elsevier; 1986. pp. 515–569. [Google Scholar]
- 6.Abe M, Abe K, Kuroda M, Arai S. Corn kernel cysteine proteinase inhibitor as a novel cystatin superfamily member of plant origin, molecular cloning and expression studies. Eur J Biochem. 1992;209:933–937. doi: 10.1111/j.1432-1033.1992.tb17365.x. [DOI] [PubMed] [Google Scholar]
- 7.Kondo H, Abe K, Nishimura I, Watanabe H, Emori Y, Arai S. Two distinct cystatin species in rice seeds with different specificities against cysteine proteinases: molecular cloning, expression and biochemical studies on oryzacystatin-II. J Biol Chem. 1990;265:15832–15837. [PubMed] [Google Scholar]
- 8.Abe M, Emori Y, Kondo H, Suzuki K, Arai S. Molecular cloning of cysteine proteinase inhibitor of rice (oryzacystatin) J Biol Chem. 1987;262:16793–16797. [PubMed] [Google Scholar]
- 9.Yang AH, Yeh KW. Molecular cloning, recombinant gene expression and antifungal activity of cystatin from taro (Colocasia esculanta cv. Kaosiung no. 1) Planta. 2005;221:493–501. doi: 10.1007/s00425-004-1462-8. [DOI] [PubMed] [Google Scholar]
- 10.Martinez M, Lopez-Solanilla E, Rodriguez-Panelzuela P, Carbonero P, Diaz I. Inhibition of plant pathogenic fungi by the barley cystatin Hv-CPI (gene icy) is not associated with its cysteine proteinase inhibitory properties. Mol Plant Microbe Interact. 2003;6:876–883. doi: 10.1094/MPMI.2003.16.10.876. [DOI] [PubMed] [Google Scholar]
- 11.Telang M, Srinivasan A, Patankar A, Harsulkar A, Joshi V, Damle A, et al. Bitter gourd proteinase inhibitors: potential growth inhibitors of Helicoverpa armigera and Spodoptera litura. Phytochemistry. 2003;63:643–652. doi: 10.1016/s0031-9422(03)00239-5. [DOI] [PubMed] [Google Scholar]
- 12.Hines ME, Osuala CI, Nielsen SS. Isolation and partial characterization of soybean cystatin cysteine proteinase inhibitor of coleopteran digestive proteolytic activity. J Agric Food Chem. 1991;39:1515–1520. [Google Scholar]
- 13.Kuroda M, Kiyosaki T, Matsumoto I, Misaka T, Arai S, Abe K. Molecular cloning, characterization and expression of wheat cystatins. Biosci Biotechnol Biochem. 2001;65:22–28. doi: 10.1271/bbb.65.22. [DOI] [PubMed] [Google Scholar]
- 14.Correy-Menguy F, Cejudo A, Mazubert C, Vidal J, Lelans-Briere C, Torres G, et al. Characterization of the expression of a wheat cystatin gene during caryopsis development. Plant Mol Biol. 2002;50:687–698. doi: 10.1023/a:1019906031305. [DOI] [PubMed] [Google Scholar]
- 15.Christova PK, Christov NK, Imai R. A cold inducible multidomain cystatin from winter wheat inhibits growth of the snow mold fungus, Microdochium nivale. Planta. 2006;223:1207–1218. doi: 10.1007/s00425-005-0169-9. [DOI] [PubMed] [Google Scholar]
- 16.Koiwa H, Bressan RA, Hasegawa PM. Regulation of protease inhibitors and plant defense. Trends Plant Sci. 1997;2:379–384. [Google Scholar]
- 17.Botella MA, Xu Y, Prabha TN, Zhao Y, Narasimhan ML, Wilson A, et al. Differential expression of soybean cysteine proteinase inhibitor genes during development and in response to wounding and methyl jasmonate. Plant Physiol. 1996;112:1201–1210. doi: 10.1104/pp.112.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolter CJ. Methyl jasmonate induces papain inhibitor(s) in tomato leaves. Plant Physiol. 1993;103:1347–1353. doi: 10.1104/pp.103.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandal MK, Pandey D, Purwar S, Singh US, Kumar A. Influence of jasmonic acid as potential activator of induced resistance against Karnal bunt in developing spikes of wheat. J Biosci. 2006;31:601–607. doi: 10.1007/BF02708413. [DOI] [PubMed] [Google Scholar]
- 20.Aujla SS, Sharma I, Singh BB. Method of teliospore germination and breaking of dormancy in Neovossia indica. Indian Phytopathology. 1986;39:574–577. [Google Scholar]
- 21.Warham EJ, Cashion NL. Evaluation of inoculation methods in the green house and field. Proceedings of a Conference held at Cuidad, Obregon, Sonora, CIMMYT, Mexico; 1984. pp. 11–13. [Google Scholar]
- 22.Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, et al. The JAZ family of repressors in the missing link in jasmonate signaling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- 23.Xu F, Chye M. Expression of cysteine proteinase during developmental events associated with programmed cell death in brinjal. Plant J. 1999;17:321–327. doi: 10.1046/j.1365-313x.1999.00370.x. [DOI] [PubMed] [Google Scholar]
- 24.Uren AG, O'Rourke K, Aravind L, Pissabaro MT, Sehagiri Si, Koonin EV, Dixit VM. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT-lymphoma. Mol Cell. 2000;6:961–967. doi: 10.1016/s1097-2765(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 25.Bozhkov PV, Filonova LH, Suarez MF, Humersson A, Smertenko AP, Zhivotovsky B, van Arnold S. VEIDase is a principle caspase-like activity involved in plant PCD and essential for embryonic pattern formation. Cell Death Different. 2004;11:175–182. doi: 10.1038/sj.cdd.4401330. [DOI] [PubMed] [Google Scholar]
- 26.Zuppini A, Navazio L, Mariani P. Endoplasmic reticiulum stress-induced programmed cell death in soybean cells. J Cell Sci. 2004;117:2591–2598. doi: 10.1242/jcs.01126. [DOI] [PubMed] [Google Scholar]
- 27.D'silva I, Poirier G, Heath MC. Activation of cysteine proteases in cowpea plants during HR; a form of PCD. Exp Cell Res. 1998;245:389–399. doi: 10.1006/excr.1998.4256. [DOI] [PubMed] [Google Scholar]
- 28.Purwar S, Marla SS, Singh US, Kumar A. Basal expression studies of cystatins during specific growth stages of wheat spikes for defining their role in differential and stage dependent immunity against Karnal bunt (Tilletia indica) Mol Biol Rep. 2010;37:1377–1389. doi: 10.1007/s11033-009-9520-8. [DOI] [PubMed] [Google Scholar]
- 29.Duran R, Cromarty R. Tilletia indica: A heterothallic wheat bunt fungus with multiple alleles controlling compatibility. Phytopathol. 1977;67:812–815. [Google Scholar]
- 30.Singh RA, Krishna A. Susceptible stage for inoculation and effect of Karnal bunt on viability of wheat seed. Indian Phytopathol. 1982;35:54–56. [Google Scholar]
- 31.Sambrook J, Fristch EF, Maniartis T. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Xiao S, Dai L, Liu F, Wang Z, Peng W, Xie D. COS1: An Arabidopsis coronative insensitive 1 suppressor essential for regulation of jasmonate mediated plant defense and senescence. Plant Cell. 2004;16:1132–1142. doi: 10.1105/tpc.020370. [DOI] [PMC free article] [PubMed] [Google Scholar]