Abstract
Mechanical irritation of trigger hairs and subsequent generation of action potentials have significant impact on photosynthesis and respiration in carnivorous Venus flytrap (Dionaea muscipula). Action potential-mediated inhibition of photosynthesis and stimulation of respiration is confined only to the trap and was not recorded in adjacent photosynthetic lamina. We showed that the main primary target of electrical signals on assimilation is in the dark enzymatic reaction of photosynthesis. Without doubt, the electrical signaling is costly, and the possible co-existence of such type of signals and photosynthesis in plant cell is discussed.
Key words: action potential, carnivorous plant, Dionaea muscipula, electrical signaling, photosynthesis, respiration, Venus flytrap
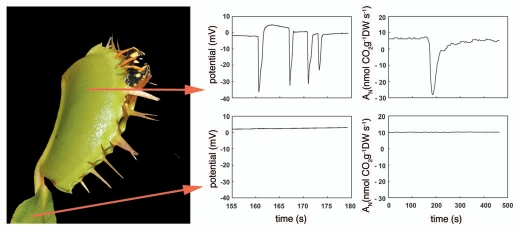
Trap closure of the Venus flytrap (Dionaea muscipula) is one of the fastest movements in plant kingdom. Mechanical irritation of trigger hairs protruding from upper leaf epidermis results in generation of action potential. At room temperature, two touches generate two action potentials and activate the trap snap shut in a fraction of second.1 After the rapid movement secures the prey, struggling results in generation of further action potentials which cease to occur when the prey stops moving.2 We documented that trigger hair irritation and subsequent generation of action potentials have significant effect on photosynthesis and respiration. Action potentials propagate in the trap and were not recorded in adjacent lamina (Fig. 1). This is in accordance with the observation that no changes of photosynthetic and respiration rate as well as effective quantum yield of photosystem II photochemistry were recorded in lamina. Detailed analysis of chlorophyll fluorescence kinetics revealed that the main primary target of action potentials is in the dark enzymatic reaction of photosynthesis and changes in quantum yield of primary photochemistry are just a consequence of decreased CO2 fixation. However, electrical signals have probably also small effect on excitation energy trapping, charge stabilization and recombination reaction in photosystem II as measurements of fast chlorophyll a fluorescence transient indicates. This effect may be explained by repulsion of charges in reaction center of photosystem II.3,4 The changes of photosynthesis upon impact of electrical signals probably have no benefit for plant and are only a negative consequences caused by the changes of the ionic environment.
Figure 1.
Dionaea muscipula with entrapped wasp of the genus Polistes. Action potentials and rate of net assimilation at irradiance 80 µmol m−2s− PAR (An) in response to 15 s mechanical trigger hair irritation (between 160–175 s) in trap (upper row) and photosynthetic lamina (lower row).
These findings may have more consequences for plants in general. The electrical activity of plant cell was for the first time described by Burdon-Sanderson in 1873.5 Hence electrical signals do not belong exclusively to animal kingdom however they never develop the same degree of complexity as in animal nerves. Electrical signals are capable of transmitting signals more quickly over long distances when compared with chemical signals (e.g., hormones).6,7 They are not confined only to the sensitive plants (e.g., Mimosa, Dionaea), but play also an important role in every non-sensitive plants and in both groups have significant effect on photosynthesis and respiration.8–14 It is not surprising, that if electrical signals are costly in term of consumption of ATP and increased respiration with concurrent inhibition of photosynthesis, the same degree of complexity as in animals could not be developed. If plant growth depends on photosynthesis, this raises the question whether electrical signals and photosynthesis may co-exist together. The continuous electrical activity would inhibit the main source of energy for plants—photosynthetic assimilation. This may also explain why the plants are sessile organisms. For rapid coordinated movements, electrical activity plays an important role in animals. Unlike animals, plants usually rely on slow movements in which the role of plant hormones is indispensable. In this concept, it is not surprising that the more complex electrical activity was recorded in root transition zone—the heterotrophic part of plant body.15,16 And this may also explain why the more evident electrical activity in the plant world has evolved in the traps of carnivorous plants like Dionaea, Aldrovanda or Drosera.17–19 In general the traps of carnivorous plants are considered to be less efficient in photosynthesis.20 Any of the action potentials produced by Drosera tentacles or Dionaea trap do not spread to photosynthetic active lamina, thus the main side of CO2 fixation is protected.21 It is possible that such temporal carbon costs associated with insect trapping and retention may be outweighed by the benefits gained later from the prey—increased nitrogen concentration in the leaves stimulates photosynthetic assimilation.22 The possible ecophysiological impact of electrical signals on daily carbon gain in sensitive plants remains to be elucidated. We still do not completely understand the electrical signals in plants, and further research in this area is necessary to understand the full meaning of electrical activity in plants.
Acknowledgements
This work was supported by grant VEGA 1/0040/09 from the Scientific Grant Agency of the Ministry of Education of the Slovak Republic.
References
- 1.Juniper BE, Robins RJ, Joel DM. The carnivorous plants. London: Academic Press; 1989. [Google Scholar]
- 2.Affolter JM, Olivo RF. Action potentials in Venus's-flytraps: long term observations following the capture of prey. Am Midl Nat. 1975;93:443–445. [Google Scholar]
- 3.Pavlovič A, Demko V, Hudák J. Trap closure and prey retention in Venus flytrap (Dionaea muscipula) temporarily reduces photosynthesis and stimulates respiration. Ann Bot. 2010;105:37–44. doi: 10.1093/aob/mcp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlovič A, Slováková L, Pandolfi C, Mancuso S. On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis) J Exp Bot. 2011;62:1991–2000. doi: 10.1093/jxb/erq404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdon Sanderson JS. Note on the electrical phenomena which accompany stimulation of the leaf of Dionaea muscipula. Proc R Soc. 1873;21:495–496. [Google Scholar]
- 6.Fromm J, Lautner S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2007;30:249–257. doi: 10.1111/j.1365-3040.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 7.Yan X, Wang Z, Huang L, Wang C, Hou R, Xu Z, Qiao X. Research progress onelectrical signals in higher plants. Prog Nat Sci. 2009;19:531–541. [Google Scholar]
- 8.Mancuso S. Hydraulic and electrical transmission of wound-induced signals in Vitis vinifera. Aust J Plant Physiol. 1999;26:55–61. [Google Scholar]
- 9.Bulychev AA, Kamzolkina NA. Differential effects of plasma membrane electric excitation on H+ fluxes and photosynthesis in characean cells. Bioelectrochemistry. 2006;69:209–215. doi: 10.1016/j.bioelechem.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Dziubinska H, Trebacz K, Zawadzki T. The effect of excitation on the rate of respiration in the liverwort Conocephalum conicum. Physiol Plant. 1989;75:417–423. [Google Scholar]
- 11.Hlaváčková V, Krchnák P, Nauš J, Novák O, Špundová M, Strnad M. Electrical and chemical signals involved in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta. 2006;225:235–244. doi: 10.1007/s00425-006-0325-x. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser H, Grams TEE. Rapid hydropassive opening and subsequent active stomatal closure follow heat-induced electrical signals in Mimosa pudica. J Exp Bot. 2006;57:2087–2092. doi: 10.1093/jxb/erj165. [DOI] [PubMed] [Google Scholar]
- 13.Koziolek C, Grams TEE, Schreiber U, Matyssek R, Fromm J. Transient knockout of photosynthesis mediated by electrical signals. New Phytol. 2003;161:715–722. doi: 10.1111/j.1469-8137.2004.00985.x. [DOI] [PubMed] [Google Scholar]
- 14.Lautne S, Grams TEE, Matyssek R, Fromm J. Characteristics of electrical signals in poplar and responses in photosynthesis. Plant Physiol. 2005;138:2200–2209. doi: 10.1104/pp.105.064196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner ED, Stahlberg R, Mancuso S, Vivanco J, Baluška F, Van Volkenburgh E. Plant neurobiology: an intergrated view of plant signaling. Trends Plant Sci. 2006;11:413–419. doi: 10.1016/j.tplants.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Masi E, Ciszak M, Stefano G, Renna L, Azzarello E, Pandolfi C, et al. Spatiotemporal dynamics of the elctrical network activity in the root apex. Proc Natl Acad Sci USA. 2009;106:4048–4053. doi: 10.1073/pnas.0804640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams SE, Pickard BG. Receptor potentials and action potentials in Drosera tentacles. Planta. 1972;103:193–221. doi: 10.1007/BF00386844. [DOI] [PubMed] [Google Scholar]
- 18.Williams SE, Pickard BG. Properties of action potentials in Drosera tentacles. Planta. 1972;103:222–240. doi: 10.1007/BF00386845. [DOI] [PubMed] [Google Scholar]
- 19.Sibaoka T. Rapid plant movements triggered by action potentials. Bot Mag Tokyo. 1991;104:73–95. [Google Scholar]
- 20.Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. Carnivory in the bromeliad Brocchinia reducta with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient poor habitats. Am Nat. 1984;124:479–497. [Google Scholar]
- 21.Pavlovič A. Spatio-temporal changes of photosynthesis in carnivorous plants in response to prey capture, retention and digestion. Plant Signal Behav. 2010;5:1325–1329. doi: 10.4161/psb.5.11.11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavlovič A, Singerová L, Demko V, Hudák J. Feeding enhances photosynthetic efficiency in the carnivorous pitcher plant Nepenthes talangensis. Ann Bot. 2009;104:307–314. doi: 10.1093/aob/mcp121. [DOI] [PMC free article] [PubMed] [Google Scholar]