Abstract
Increased expression of an Arabidopsis vacuolar pyrophosphatase gene, AVP1, leads to increased drought and salt tolerance in transgenic plants, which has been demonstrated in laboratory and field conditions. The molecular mechanism of AVP1-mediated drought resistance is likely due to increased proton pump activity of the vacuolar pyrophosphatase, which generates a higher proton electrochemical gradient across the vacuolar membrane, leading to lower water potential in the plant vacuole and higher secondary transporter activities that prevent ion accumulation to toxic levels in the cytoplasm. Additionally, overexpression of AVP1 appears to stimulate auxin polar transport, which in turn stimulates root development. The larger root system allows AVP1-overexpressing plants to absorb water more efficiently under drought and saline conditions, resulting in stress tolerance and increased yields. Multi-year field-trial data indicate that overexpression of AVP1 in cotton leads to at least 20% more fiber yield than wild-type control plants in dry-land conditions, which highlights the potential use of AVP1 in improving drought tolerance in crops in arid and semiarid areas of the world.
Key words: drought tolerance, proton pump, salt tolerance, transgenic cotton, vacuolar membrane
Drought and salinity are major environmental factors that limit agricultural productivity in most parts of the world.1 Climate change will likely make many places worse in terms of water availability and soil salinization,2 which will have negative impacts on food production in world agriculture. Yet, the demand for more food will continue to rise because of the growing world population that may reach 9 billon people by 2050.3 Therefore, the primary challenge we face during this century is the production of more food under the constraints of limited water and fertilizer on marginal soils.
Many genes that respond to abiotic stresses have been identified in the model plant Arabidopsis,4 and some of them were shown to play important roles in protecting plants under abiotic stress conditions.5 The Arabidopsis vacuolar pyrophosphatase gene AVP1 appears to be one of the most promising genes that may be used to improve drought- and salt-tolerance in crops.6 Roberto Gaxiola's group first demonstrated that overexpression of AVP1 could lead to significantly improved drought- and salt-tolerance in transgenic Arabidopsis plants.7 Later when this gene was introduced into tomato8 and rice,9 similar tolerance phenotypes were observed. Overexpression of AVP1 in cotton, not only improved drought- and salt-tolerance in greenhouse conditions, but also increased fiber yield in dryland field conditions.6 AVP1-expressing cotton plants produced larger root systems and bigger shoot biomass than controls when grown under hydroponic conditions in the presence of up to 200 mM NaCl.6 In the greenhouse, AVP1-expressing cotton plants also produced more root and shoot biomass than controls when grown under saline conditions or reduced irrigation.6 The increased yield by AVP1-expressing cotton plants is due to more bolls produced, which in turn is due to larger shoot system that AVP1-expressing cotton plants develop under saline or drought conditions.6
The larger root systems of AVP1-expressing cotton plants under saline and water-deficit conditions allow transgenic plants access to more of the soil profile and available soil water resulting in increased biomass production and yield. Li et al. showed that the larger root systems of AVP1-overexpressing Arabidopsis is caused by increased auxin polar transport in the root, which stimulates root development in AVP1-overexpressing Arabidopsis plants.10 Furthermore, a recent comparative study of transgenic Arabidopsis lines that produce enlarged leaves showed that auxin levels were increased by 50% in AVP1-overexpressing plants.11 To test if altered auxin level is responsible for the observed larger root systems in AVP1-expressing cotton plants, we germinated wild-type and AVP1-expressing cotton plants in the absence or presence of the auxin polar transport inhibitor Naphthylphthalamic acid (NPA). Both wild-type and AVP1-expressing cotton plants developed robust lateral root systems in the absence of NPA (Fig. 1A). The presence of 50 µM NPA resulted in nearly complete inhibition of lateral root development in wild-type plants, while lateral root development in AVP1-expressing plants was reduced, it was significantly greater than wild-type (Fig. 1B). These data indicate that AVP1-overexpression could overcome the inhibitory effects of NPA on root development in AVP1-expressing cotton plants, suggesting that either increased auxin transport or higher auxin concentration in the root systems of AVP1-expressing cotton plants is responsible for the observed larger root systems, and eventually for the increased boll numbers and fiber yields under dryland field conditions.
Figure 1.
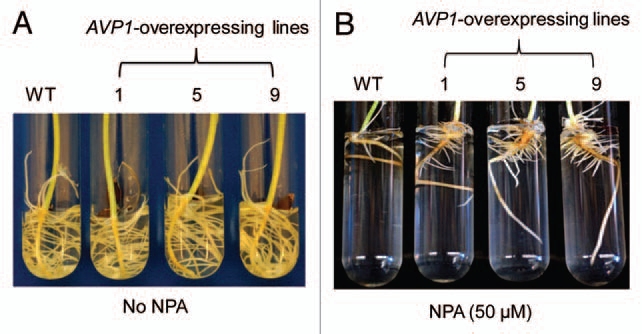
Root development of wild-type and AVP1-expressing cotton plants in the absence and presence of auxin transport inhibitor NPA. (A) Phenotype of cotton roots after 10 days of growth in the absence of NPA. WT, Wild-type; 1, 5, 9, three independent AVP1-overexpressing cotton lines. (B) Phenotype of cotton roots after 10 days of growth in the presence of 50 µm NPA.
Many genes that may play important roles under water-deficit conditions have been tested in laboratory conditions,4,5 but very few have been tested vigorously in field conditions. A bacterial cold shock protein gene was shown to improve drought tolerance in maize based on multi-year and multi-place field trial experiments,12 and it appears that this gene will likely gain approval for commercial release and become the first genetically engineered product that demonstrates improved drought tolerance in a major crop in the U.S. Another example of increased drought tolerance supported by multiple field trial experiments is through downregulation of farnesylation in transgenic canola plants.13 Downregulation of farnesyltransferase by antisense or RNAi techniques in transgenic canola leads to increased sensitivity to abscisic acid, consequently resulting in smaller guard cell aperture under drought conditions. These transgenic canola plants lose less water through transpiration and are more drought resistant. Data from more than 5 years of field studies in Canada consistently proved that this approach can indeed increase drought tolerance in transgenic canola. Our study with AVP1-expressing cotton over the last several years in field conditions is another example that genetic engineering approach can be an efficient tool in generating drought-tolerant crops. AVP1-expressing cotton plants can establish a larger shoot mass in dryland conditions (Fig. 2), which results in increased boll numbers and fiber production. Our approach is likely applicable to other major crops as well.
Figure 2.

Wild-type and AVP1-expressing cotton plants grown in the dryland field condition. Plants were planted in the middle of may 2009 and the picture was taken in the middle of July 2009 at the USDA experimental Farm in Lubbock, Texas.
Acknowledgements
The work described in this addendum was supported by a grant from USDA National Institute of Food and Agriculture (Grant No. 2007-35100-18382) and from Texas Tech International Cotton Research Center.
References
- 1.Bartels D, Sunkar R. Drought and salt tolerance in plants. Cri Rev Plant Sci. 2005;24:23–58. [Google Scholar]
- 2.Battisti DS, Naylor RL. Historical warnings of future food insecurity with unprecedented seasonal heat. Science. 2009;323:240–244. doi: 10.1126/science.1164363. [DOI] [PubMed] [Google Scholar]
- 3.FAOUN, author. Food Security statistics 2010. Available at: www.fao.org/economic/ess/food-security-statistics/en/. [Google Scholar]
- 4.Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J Exp Bot. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- 5.Vinocur B, Altman A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol. 2005;16:123–132. doi: 10.1016/j.copbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Pasapula V, Shen G, Kuppu S, Paez-Valencia J, Mendoza M, Hou P, et al. Expression of an Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) in cotton improves drought- and salt-tolerance and increases fiber yield in the field conditions. Plant Biotech J. 2011;9:88–99. doi: 10.1111/j.1467-7652.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- 7.Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, et al. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA. 2001;98:11444–11449. doi: 10.1073/pnas.191389398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, et al. Upregulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci USA. 2005;102:18830–18835. doi: 10.1073/pnas.0509512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao FY, Zhang XJ, Li PH, Zhao YX, Zhang H. Co-expression of the Suaeda salsa SsNHX1 and Arabidopsis AVP1 confer greater salt tolerance to transgenic rice than the single SsNHX1. Mol Breeding. 2006;17:341–353. [Google Scholar]
- 10.Li J, Yang H, Peer WA, Richter G, Blakeslee J, Bandyopadhyay A, et al. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science. 2005;310:121–125. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez N, De Bodt S, Sulpice R, Jikumaru Y, Chae E, Dhondt S, et al. Increased leaf size: different means to an end. Plant Physiol. 2010;153:1261–1279. doi: 10.1104/pp.110.156018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castiglioni P, Warner D, Bensen RJ, Anstrom DC, Harrison J, Stoecker M, et al. Bacterial RNA chaperones confer abioticstress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiol. 2008;147:446–455. doi: 10.1104/pp.108.118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Vanderbeld B, Wan J, Huang Y. Narrowing down the targets: towards successful genetic engineering of drought-tolerant crops. Mol Plant. 2010;3:469–490. doi: 10.1093/mp/ssq016. [DOI] [PubMed] [Google Scholar]


