Abstract
The plant mitochondrial respiratory system changes in its activity in response to light. This response has been thought to be important for ensuring cooperative function with the photosynthetic system. A recent study addressing light responses of the respiratory chain in Arabidopsis thaliana provided further insight into the role of mitochondria in illuminated leaves. Notably, the nonphosphorylating alternative oxidase is rapidly induced when plants are exposed to the high light stress, and appears to play a key role in keeping cellular redox balance.
Key words: alternative oxidase, Arabidopsis thaliana, light acclimation, mitochondria, respiratory chain, organellar crosstalk
Response of the Respiratory Chain to Light
Plants flexibly acclimate to the surrounding light environments. In addition to the chloroplast, whose light responses have been well-characterized, the mitochondrial respiratory system also shows light-dependent changes in the activity.1,2 For example, it was reported that the activity of tricarboxylic acid (TCA) cycle is suppressed during day-period.3 We have been studying light responses of the mitochondrial respiratory system, focusing on the electron transport chain (respiratory chain). The respiratory chain in plant mitochondria possesses novel components that are not associated with ATP production. The alternative oxidase (AOX) is the most well-known example of such energy-wasteful respiratory components.4,5 Although the physiological significance of AOX is still controversial, several studies have clarified that leaf AOX level is enhanced in response to high light (HL).6–8 It was also shown that, in a shade species Alocasia odora, leaf AOX is present in the activated state under HL growth conditions, but not under low light.9 These findings imply some beneficial roles for leaf AOX in the acclimation to HL.
We recently examined responses of the respiratory chain to two distinct HL treatments, long-and short-term HL, in Arabidopsis thaliana.10 The results indicate that responses of the respiratory chain to long-and short-term HL are vastly different, and that AOX induction is important when plants are suddenly exposed to HL (hereafter, referred to as the light stress).
AOX Prevents the Over-Reduction of Respiratory Chain under Light Stress
Using the aox1a mutant in A. thaliana, we assessed physiological roles of AOX in illuminated leaves.10 In the wild-type, AOX was upregulated by the light stress treatment, which in turn stabilized the redox state of ubiquinone (UQ) pool to the modest level. On the other hand, UQ reduction level of aox1a remained high during the light stress treatment. These observations clearly indicate that AOX serves to prevent the respiratory chain from highly reduced state, when plants are subjected to the light stress. It was previously demonstrated that, in tobacco cultured cells, AOX lowers the reactive oxygen species (ROS) level possibly through functioning as a safety valve of the respiratory chain to avoid its over-reduction.11 It is now established that this AOX role is relevant in A. thaliana leaves under the light stress.
Giraud et al. pointed out the importance of AOX under the combined drought and light stress.12 We also investigated AOX protein level, UQ redox state and their interrelationship in drought-treated A. thaliana wild-type and aox1a mutant (Fig. 1). Although the drought treatment did not result in clear phenotypic difference between the wild-type and aox1a (Fig. 1A), it obviously elevated AOX protein level in the wild-type (Fig. 1B). Addition of the light stress to drought further enhanced AOX expression. Interestingly, even in the aox1a mutant, weak signals became recognizable by the drought treatment (arrows in Fig. 1B). It was reported that, under certain conditions, aox1a shows the complementary expression of other AOX genes (especially AOX1d).13,14 Therefore, it is likely that the signals observed in the drought-treated aox1a were derived from the AOX isoforms other than AOX1a, which may partly compensate for the lack of AOX1a. Nevertheless, UQ reduction level in aox1a was elevated to a quite high level by the combined drought and light stress (Fig. 1C), indicating that AOX1a plays a central role in adjusting UQ redox state in A. thaliana leaves.
Figure 1.
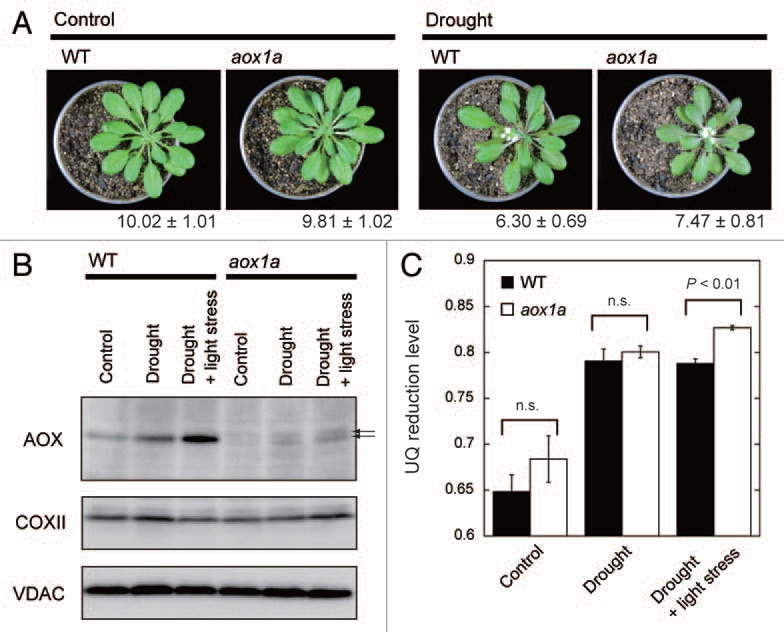
Effects of drought and its combination with the light stress on leaf respiratory property in Arabidopsis thaliana wild-type (WT) and aox1a mutant. (A) Images of plants. Plants were cultivated in a controlled growth chamber (light intensity; 80 µmol photons m−1s−1, temperature; 22–24°C, day/night; 12 h/12 h) for seven weeks. For the drought treatment, plants were not watered for two weeks before the sampling. Value below each image indicates the leaf water content, estimated by following equation; (fresh weight - dry weight)/dry weight (mean ± SE, n = 5–7). For the light stress, light at the intensity of 650 µmol photons m−2s−1 was given for 6 h. (B) Immunoblotting analysis of mitochondrial proteins, alternative oxidase (AOX), cytochrome c oxidase subunit II (COXII) and voltage-dependent anion channel (VDAC). Same amount of protein extracted from the leaves was loaded into each lane. Arrows indicate the potential AOX isoforms except for AOX1A. (C) UQ reduction level expressed as the ratio of content of the reduced form to the total. Each value represents the mean ± SE (n = 4–7). Statistical analysis was conducted using Student's t-test.
Does AOX Have an Impact on Photosynthesis?
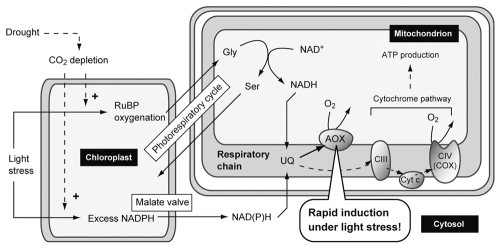
The light-dependent reduction of the respiratory chain is thought to occur via the organellar crosstalk between chloroplasts and mitochondria, including the photorespiratory cycle and redox-shuttling using the malate valve (Fig. 2).2 It should be noted that these metabolic pathways are further activated in conditions where drought is combined with the light stress; drought promotes closure of stomata and thereby CO2 depletion within the leaf, which in turn accelerates the photorespiratory cycle and redox-shuttling, finally reducing the respiratory chain. Given that AOX stabilizes UQ redox state and is therefore a key component of the organellar crosstalk, it is likely that AOX contributes to efficient photosynthetic reaction. In fact, the impairment of AOX by specific inhibitors or genetic techniques exerted negative impacts on photosynthesis.12,13,15,16 Furthermore, using a simultaneous determination system of the in vivo redox state of plastoquinone (PQ) and UQ,17 we indicated that the photosynthetic electron transport in the chloroplast is liable to be repressed when the respiratory chain is highly reduced.10 Despite these reports, it still remains unclear whether AOX is required for efficient photosynthetic performance and, if so, to what extent AOX contributes to photosynthesis. Future studies focusing on the photosynthetic properties and other cellular metabolic pathways in the AOX-impaired plants will broaden and deepen our understanding of the AOX role.
Figure 2.
Simplified model of the mitochondrial role in the light stress. See main text for details. AOX, alternative oxidase; CIII, complex III; CIV, complex IV; COX, cytochrome c oxidase; Cyt c, cytrochrome c; Gly, glycine; RuBP, ribulose 1,5-bisphosphate; Ser, serine; UQ, ubiquinone.
References
- 1.Rasmusson AG, Escobar MA. Light and diurnal regulation of plant respiratory gene expression. Physiol Plant. 2007;129:57–67. [Google Scholar]
- 2.Yoshida K, Noguchi K. Interaction between chloroplasts and mitochondria: activity, function and regulation of the mitochondrial respiratory system during photosynthesis. In: Kempken F, editor. Plant Mitochondria: Advances in Plant Biology 1. NY: Springer; 2010. pp. 383–410. [Google Scholar]
- 3.Tcherkez G, Cornic G, Bligny R, Gout E, Ghashghaie J. In vivo respiratory metabolism of illuminated leaves. Plant Physiol. 2005;138:1596–1606. doi: 10.1104/pp.105.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siedow JN, Umbach AL. The mitochondrial cyanide-resistant oxidase: structural conservation amid regulatory diversity. Biochim Biophys Acta. 2000;1459:432–439. doi: 10.1016/s0005-2728(00)00181-x. [DOI] [PubMed] [Google Scholar]
- 5.Finnega PM, Sool KL, Umbac AL. Alternative mitochondrial electron transport proteins in higher plants. In: Day DA, Millar AH, Whelan J, editors. Plant Mitochondria: From Genome to Function. London: Kluwer Academic Publishers; 2004. pp. 163–230. [Google Scholar]
- 6.Yoshida K, Terashima I, Noguchi K. Upregulation of mitochondrial alternative oxidase concomitant with chloroplast over-reduction by excess light. Plant Cell Physiol. 2007;48:606–614. doi: 10.1093/pcp/pcm033. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida K, Watanabe C, Kato Y, Sakamoto W, Noguchi K. Influence of chloroplastic photo-oxidative stress on mitochondrial alternative oxidase capacity and respiratory properties: a case study with Arabidopsis yellow variegated 2. Plant Cell Physiol. 2008;49:592–603. doi: 10.1093/pcp/pcn031. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida K, Noguchi K. Differential gene expression profiles of the mitochondrial respiratory components in illuminated Arabidopsis leaves. Plant Cell Physiol. 2009;50:1449–1462. doi: 10.1093/pcp/pcp090. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi K, Taylor NL, Millar AH, Lambers H, Day DA. Response of mitochondria to light intensity in the leaves of sun and shade species. Plant Cell Environ. 2005;28:760–771. [Google Scholar]
- 10.Yoshida K, Watanabe CK, Hachiya T, Tholen D, Shibata M, Terashima I, et al. Distinct responses of the mitochondrial respiratory chain to long- and short-term high light environments in Arabidopsis thaliana. Plant Cell Environ. 2011;34:618–628. doi: 10.1111/j.1365-3040.2010.02267.x. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giraud E, Ho LHM, Clifton R, Carroll A, Estavillo G, Tan YF, et al. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008;147:595–610. doi: 10.1104/pp.107.115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strodtkötter I, Padmasree K, Dinakar C, Speth B, Niazi PS, Wojtera J, et al. Induction of the AOX1D isoform of alternative oxidase in A. thaliana T-DNA insertion lines lacking isoform AOX1A is insufficient to optimize photosynthesis when treated with antimycin A. Mol Plant. 2009;2:284–297. doi: 10.1093/mp/ssn089. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe CK, Hachiya T, Takahara K, Kawai-Yamada M, Uchimiya H, Uesono Y, et al. Effects of AOX1a deficiency on plant growth, gene expression of respiratory components and metabolic profile under low-nitrogen stress in Arabidopsis thaliana. Plant Cell Physiol. 2010;51:810–822. doi: 10.1093/pcp/pcq033. [DOI] [PubMed] [Google Scholar]
- 15.Padmasree K, Raghavendra AS. Importance of oxidative electron transport over oxidative phosphorylation in optimizing photosynthesis in mesophyll protoplasts of pea (Pisum sativum L.) Physiol Plant. 1999;105:546–553. [Google Scholar]
- 16.Yoshida K, Terashima I, Noguchi K. Distinct roles of the cytochrome pathway and alternative oxidase in leaf photosynthesis. Plant Cell Physiol. 2006;47:22–31. doi: 10.1093/pcp/pci219. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida K, Shibata M, Terashima I, Noguchi K. Simultaneous determination of in vivo plastoquinone and ubiquinone redox states by HPLC-based analysis. Plant Cell Physiol. 2010;51:836–841. doi: 10.1093/pcp/pcq044. [DOI] [PubMed] [Google Scholar]