Abstract
Barley is an alternative host for the rice blast fungus Magnaporthe oryzae but is resistant to Magnaporthe species associated with the grass genera Pennisetum and Digitaria. The latter cases are examples for nonhost resistance which confers effective and durable protection to plants against a broad spectrum of pathogens. Comparative transcript profiling of host and nonhost interaction revealed an early and pronounced change in gene expression in epidermal tissue of barley infected with a Magnaporthe nonhost isolate. Interestingly, this set of genes did not overlap considerably with the transcriptional response of barley against nonhost rust or powdery mildew isolates. For a functional testing of candidate genes a combined approach of virus-induced gene silencing (VIGS) and subsequent pathogen challenge was established. As anticipated, VIGS-mediated downregulation of Mlo-transcripts led to higher resistance against Blumeria graminis f.sp. hordei and enhanced susceptibility against M. oryzae.
Key words: Blumeria graminis, Magnaporthe, macroarray, mlo, nonhost resistance, VIGS
Nonhost resistance (NHR) of a plant species operates against all races of a given pathogen species for which the plant is not considered a host.1 Sustainability and broad-spectrum resistance under field conditions make NHR a promising resource for crop improvement.2,3 Interrogating for a common mechanism of NHR in barley against different pathogens, we analyzed the transcriptional response of one particular barley genotype against three pairs of adapted and non-adapted Magnaporthe, Blumeria and Puccinia isolates, respectively.4 The study showed that NHR of barley against each pathogen is associated with the regulation of distinct sets of genes which, however, are involved in similar metabolic or signaling pathways. We chose the interaction between barley and fungi of the genus Magnaporthe as a model to study the mechanisms underlying NHR in more detail.5 Isolates of the species M. oryzae, best-known as the causal agent of “rice blast,” are pathogenic on rice and other cultivated grasses, such as millet, wheat and barley while other Magnaporthe species isolated from Digitaria or Pennisetum are not able to infect barley.5,6 Mechanistically this nonhost type of resistance appears to be based on a more efficient execution of different defense strategies, i.e., formation of papillae and onset of the hypersensitive response, also known from attacked epidermal cells in the host interaction.7,8 Here, we summarize our efforts to characterize the NHR of barley against Magnaporthe at the molecular level using transcriptome profiling and VIGS.
Transcriptional Response of Barley against Magnaporthe
To elucidate determinants of the barley NHR repertoire a global transcript profiling approach was conducted comparing barley plants inoculated with either host or nonhost Magnaporthe species (Fig. 1A). The analysis was restricted to the epidermis because this tissue primarily gets attacked by the pathogen and in case of a nonhost interaction the pathogen gets locked in this tissue. Therefore RNA was isolated from peeled epidermis of barley harvested 6, 12 or 24 h post inoculation (h p.i.) and analyzed using the barleyPGRC1 macroarray at IPK Gatersleben.4 As a result 250 genes could be identified, which were either up or downregulated during the nonhost interaction. Expression level of 180 of these genes was not altered during the host interaction. Looking at the kinetics of transcriptional changes it was remarkable that they arose as early as 6 h p.i. during the nonhost interaction (Fig. 1B). In contrast, generally fewer genes were regulated during the host interaction and the detected changes peaked rather late at 24 h p.i. This confirms the hypothesis, that defense reactions against Magnaporthe are triggered faster in the nonhost situation than in the host situation and therefore operate more efficiently.5,9 This is in agreement with results of time-course analyses of the barley transcriptome during host and nonhost interactions with powdery mildew.10 Among the genes that were specifically upregulated in the Magnaporthe nonhost interaction and therefore might play a crucial role in NHR, several lipid transfer proteins, a cytochrome P450 and an ascorbate peroxidase were listed.4 Functional characterization of these genes could be achieved by generating stable RNAi transformants, however, this is difficult and time-consuming in barley. To circumvent this drawback we decided to adopt a VIGS approach using the rod-shaped hordeivirus BSMV as a vector which was the first to be used among monocotyledonous plants.11 Gene fragments of interest can be placed into the viral γ-subunit of the tripartite BSMV genome using a multiple cloning site (MCS).12 After infecting plants with the transformed BSMV the plant's natural antiviral defense system leads to a transient knockdown of the corresponding plant gene (reviewed in ref. 13).
Figure 1.
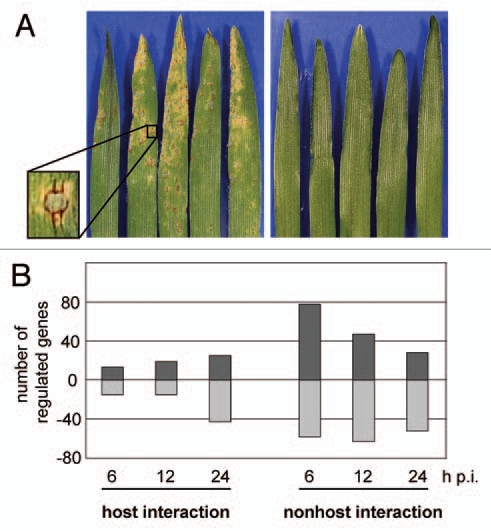
Characterization of host and nonhost interactions between barley and different Magnaporthe isolates at the macroscopical and transcriptional level. (A) Primary leaves of barley 5 days after inoculation with Magnaporthe oryzae isolate TH6772 (host interaction, left side) and a Magnaporthe species isolated from Pennisetum (CD 180, nonhost interaction, right side). A typical M. oryzae disease symptom on barley is shown in larger scale (inset). (B) Number of genes up (positive values) or downregulated (negative values) in barley/Magnaporthe interactions was identified using the barleyPGRC 1 macroarray. Only genes with a ≥2-fold differential regulation relative to the control treatment (FDR ≤5%) in four independent experiments are considered.
Validation of BSMV-IGS using Mlo-Silencing as a Case Study
Prior to an analysis of candidate genes, the BSMV-IGS system was validated in our lab using Mlo as a test gene. Barley plants carrying a loss of function mutation at the Mlo locus are completely resistant to all known isolates of Bgh but behave hypersusceptible to M. oryzae and Bipolaris sorokiniana.14–16 It has already been shown that silencing of Mlo using transient-induced gene silencing based on biolistic transgene delivery phenocopied the resistance of mlo-mutant plants against Bgh.17 But to our knowledge this approach hasn't been addressed for barley using VIGS so far. A 251 bp gene fragment of the barley Mlo gene was amplified by PCR using primers Mlofor: GCA TTT TGT GTG GAC AGT GG and Mlorev: CCG TGT CTC GGA CTT TCT TC and cloned in antisense-orientation into BamHI restriction site of pT7-BSMV-γMCS to form pT7-BSMV-γMlo. Inoculation of barley plants cv. Morex with viral RNAs was done as described in reference 12. Infection of barley plants with BSMV containing the Mlo silencing construct against the Mlo gene resulted in transcriptionally downregulation of the target gene as confirmed by qPCR (data not shown). However, we found an upregulation of Mlo transcripts in response to inoculation with the unmodified virus which is in accordance with the known responsiveness of Mlo to biotic and abiotic stresses.18 This BSMV-related increase in Mlo transcript abundance was reduced by 60% in average due to the presence of the Mlo silencing construct in the modified BSMV-γMlo (data not shown). Plants from this experiment showing viral disease symptoms on secondary leaves were selected and inoculated on detached third leaves with Bgh. This resulted in heavily infected control Mlo-plants whereas mlo11-plants showed no mildew symptoms, thus confirming the suitability of the assay (Fig. 2A). Microscopic inspection of infection sites verified, that fungal penetration in the mlo11 genotype was counterattacked to an extent of 100% by the formation of cell wall appositions (papillae), which couldn't be penetrated by Bgh (Fig. 2B and see also ref. 19). Plants inoculated with unmodified BSMV showed more disease symptoms as compared to untreated Mlo-plants (Fig. 2A) which is in agreement with higher Mlo-transcript abundance detected in these plants. Plants infected with BSMV-γMlo showed less Bgh-pustules and, at the microscopic level, a higher frequency of effective papillae compared to control Mlo-plants and BSMV-γMCS infected plants (Fig. 2A and B). First results in an analogous experiment but with M. oryzae as challenging pathogen indicate the anticipated higher susceptibility of Mlo-silenced plants (data not shown). In sum our results confirmed that BSMV-mediated silencing in combination with Blumeria or Magnaporthe infections as a reliable system in barley to test candidate genes for their involvement in NHR.
Figure 2.

Macroscopical and microscopical analysis of Bgh inoculated barley leaves after BSMV-IGS of the Mlo gene. Third leaves of barley cv. Morex (control Mlo), Grannenlose Zweizeilige (mlo11), Morex infected with unmodified BSMV (BSMV-γMCS) and Morex infected with BSMV carrying a Mlo silencing construct (BSMV-γMlo), respectively, were inoculated with Bgh. (A) Powdery mildew disease symptoms 8 days after inoculation. (B) For quantitative cytological analysis leaves were harvested at 48 h p.i., cleared and stained with blue ink. Only sites with a non-penetrated papilla beneath the appressorium were counted. The micrograph shows an example of these interaction sites (sp = spore, gt = germ tube, pap = papilla). Results presented in the bar chart are means and standard errors from 4 leaves with 100 interaction sites inspected per leaf. Significant differences (α = 5%) were determined using OneWayAnova and indicated by different letters. The experiment was repeated twice with similar results.
Acknowledgements
The authors would like to thank Dr. Merete Albrechtsen, University of Aarhus, for providing the BSMV cDNA clones, and Dr. Patrick Schweizer, IPK Gatersleben, and Dr. Roger Wise, Iowa State University, for assistance with the VIGS system. This work was supported by the Deutsche Forschungsgesellschaft (grant to R.D.) and by the Peter und Traudl Engelhorn-Stiftung (grant to N.Z.). Present address of N.Z.: Botanical Institute, University of Cologne, Albertus-Magnus-Platz, 50923 Cologne, Germany.
References
- 1.Heath MC. Non-host resistance to plant pathogens: Nonspecific defense or the result of specific recognition events? Physiol Mol Plant Pathol. 2001;58:53–54. [Google Scholar]
- 2.Ellis J. Insights into nonhost disease resistance: Can they assist disease control in agriculture? Plant Cell. 2006;18:523–528. doi: 10.1105/tpc.105.040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thordal-Christensen H. Fresh insights into processes of nonhost resistance. Curr Opin Plant Biol. 2003;6:351–357. doi: 10.1016/s1369-5266(03)00063-3. [DOI] [PubMed] [Google Scholar]
- 4.Zellerhoff N, Himmelbach A, Dong W, Bieri S, Schaffrath U, Schweizer P. Nonhost resistance of barley to different fungal pathogens is associated with largely distinct, quantitative transcriptional responses. Plant Physiol. 2010;152:2053–2066. doi: 10.1104/pp.109.151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zellerhoff N, Jarosch B, Groenewald JZ, Crous PW, Schaffrath U. Nonhost resistance of barley is successfully manifested against Magnaporthe grisea and a closely related Pennisetum-infecting lineage but is overcome by Magnaporthe oryzae. Mol Plant-Microbe Interact. 2006;19:1012–1022. doi: 10.1094/MPMI-19-1014. [DOI] [PubMed] [Google Scholar]
- 6.Couch BC, Kohn LM. A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia. 2002;94:683–693. doi: 10.1080/15572536.2003.11833196. [DOI] [PubMed] [Google Scholar]
- 7.Jarosch B, Collins NC, Zellerhoff N, Schaffrath U. RAR1, ROR1 and the actin cytoskeleton contribute to basal resistance to Magnaporthe grisea in barley. Mol Plant-Microbe Interact. 2005;18:397–404. doi: 10.1094/MPMI-18-0397. [DOI] [PubMed] [Google Scholar]
- 8.Zellerhoff N, Jansen M, Schaffrath U. Barley Rom1 antagonizes Rar1 function in Magnaporthe oryzae-infected leaves by enhancing epidermal and diminishing mesophyll defence. New Phytologist. 2008;180:702–710. doi: 10.1111/j.1469-8137.2008.02597.x. [DOI] [PubMed] [Google Scholar]
- 9.Faivre-Rampant O, Thomas J, Allegre M, Morel JB, Tharreau D, Notteghem JL, et al. Characterization of the model system rice-Magnaporthe for the study of nonhost resistance in cereals. New Phytologist. 2008;180:899–910. doi: 10.1111/j.1469-8137.2008.02621.x. [DOI] [PubMed] [Google Scholar]
- 10.Eichmann R, Biemelt S, Schafer P, Scholz U, Jansen C, Felk A, et al. Macroarray expression analysis of barley susceptibility and nonhost resistance to Blumeria graminis. J Plant Physiol. 2006;163:657–670. doi: 10.1016/j.jplph.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Holzberg S, Brosio P, Gross C, Pogue GP. Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J. 2002;30:315–327. doi: 10.1046/j.1365-313x.2002.01291.x. [DOI] [PubMed] [Google Scholar]
- 12.Bruun-Rasmussen M, Madsen CT, Jessing S, Albrechtsen M. Stability of barley stripe mosaic virus-induced gene silencing in barley. Mol Plant-Microbe Interact. 2007;20:1323–1331. doi: 10.1094/MPMI-20-11-1323. [DOI] [PubMed] [Google Scholar]
- 13.Vance V, Vaucheret H. RNA silencing in plants—Defense and counterdefense. Science. 2001;292:2277–2280. doi: 10.1126/science.1061334. [DOI] [PubMed] [Google Scholar]
- 14.Jarosch B, Kogel KH, Schaffrath U. The ambivalence of the barley Mlo locus: Mutations conferring resistance against powdery mildew (Blumeria graminis f. sp. hordei) enhance susceptibility to the rice blast fungus Magnaporthe grisea. Mol Plant-Microbe Interact. 1999;12:508–514. [Google Scholar]
- 15.Jørgensen JH. Discovery, characterzation and exploitation of Mlo powdery mildew resistance in barley. Euphytica. 1992;63:141–152. [Google Scholar]
- 16.Kumar J, Hückelhoven R, Beckhove U, Nagarajan S, Kogel KH. A compromised Mlo pathway affects the response of barley to the necrotrophic fungus Bipolaris sorokiniana (Teleomorph: Cochliobolus sativus) and its toxins. Phytopathology. 2001;91:127–133. doi: 10.1094/PHYTO.2001.91.2.127. [DOI] [PubMed] [Google Scholar]
- 17.Douchkov D, Nowara D, Zierold U, Schweizer P. A high-throughput gene-silencing system for the functional assessment of defense-related genes in barley epidermal cells. Mol Plant-Microbe Interact. 2005;18:755–761. doi: 10.1094/MPMI-18-0755. [DOI] [PubMed] [Google Scholar]
- 18.Piffanelli P, Zhou FS, Casais C, Orme J, Jarosch B, Schaffrath U, et al. The barley MLO modulator of defense and cell death is responsive to biotic and abiotic stress stimuli. Plant Physiol. 2002;129:1076–1085. doi: 10.1104/pp.010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piffanelli P, Ramsay L, Waugh R, Benabdelmouna A, D'Hont A, Hollricher K, et al. A barley cultivation-associated polymorphism conveys resistance to powdery mildew. Nature. 2004;430:887–891. doi: 10.1038/nature02781. [DOI] [PubMed] [Google Scholar]


