Abstract
VLCFAs are the main components of cuticular wax, which covers and protects plants from physical and biological stresses. However, the effect of fatty acid composition or the physiological role of VLCFAs on plant development under normal growth conditions is not well understood. We analyzed loss-of-function mutants of ONION1 (ONI1) which encodes fatty acid elongase (β-ketoacyl CoA synthase) catalyzing an elongation reaction of a carbon chain of VLCFAs. We showed that oni1 shoot contained a reduced amount of VLCFAs, and differentiation and functionality of an outermost cell layer (L1) were highly perturbed in oni1 shoot. In spite of the L1-specific expression of ONI1, the effects of the oni1 mutation were not restricted to L1, but expanded to inner cells, so that the entire shoot development was impaired including failure of the maintenance of the SAM and ectopic expression of SAM-specific KNOX genes in leaf. Thus, ONI1 function is cell non-autonomous, and signaling from L1 to inner cells may support proper development of inner cells. Here we report that expression of auxin-related genes was affected in oni1 shoot, and we speculate the existence of improper auxin distribution due to a lack of normal L1 in oni1 shoot.
Key words: fatty acid elongase, very-long-chain fatty acid, auxin, shoot development, rice
Altered Expression of PIN Genes in oni1
Since L1 is known to be a preferential pathway for PAT in shoot apex of rice, we examined the expression of PIN genes which encode efflux carrier proteins for PAT.1 We carried out qRT-PCR analysis of two PIN genes, OsPIN3a and OsPIN5a, using RNAs isolated from 1-week-old shoot apex of oni1-1 and wild type seedlings, since these two genes were reported to be expressed in the entire shoot apex with preference in L1.1 The results showed that the expression of OsPIN3a was upregulated in oni1-1, whereas the expression of OsPIN5a was downregulated (Fig. 1A). This indicates that the expression level or expression pattern of the PIN genes was altered in oni1 shoot, which suggests that PAT is perturbed in oni1 shoot.
Figure 1.
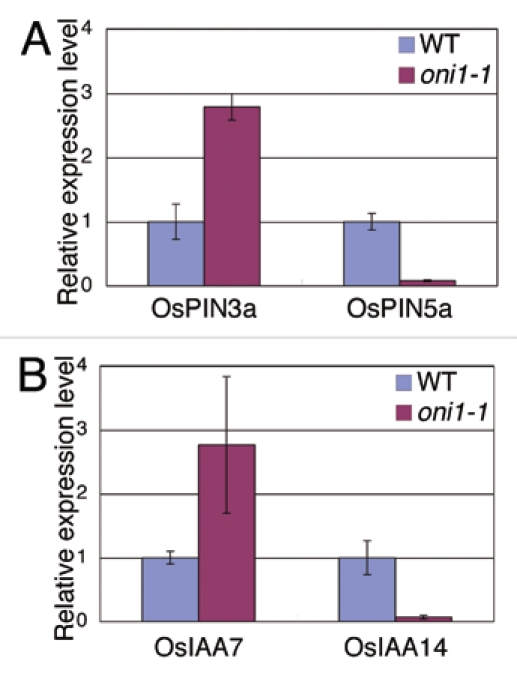
Expression of auxin-related genes in oni1 shoot. (A) Expression of OsPIN3a and OsPIN5a. (B) Expression of OsIAA7 and OsIAA14. RNAs were isolated from shoot of 1-week-old seedlings grown in a growth chamber at 30°C with continuous light and subjected to qRT-PCR. Actin was used as a reference.
Altered Expression of IAA Genes in oni1
If PAT is perturbed in oni1 shoot, auxin distribution will be changed and this change will result in altered expression of IAA genes. The expression of IAA genes is generally known to be induced by auxin, although the regulatory mechanisms of their expression in rice have not been well understood yet.2 We carried out qRT-PCR analysis of two IAA genes, OsIAA7 and OsIAA14, using RNAs again isolated from 1-week-old shoot apex of oni1-1 and wild type seedlings. The results showed that the expression of OsIAA7 was upregulated in oni1-1, whereas the expression of OsIAA14 was downregulated (Fig. 1B). This suggests a change of auxin distribution in oni1 shoot.
Speculations
We showed altered expression of OsPIN genes and OsIAA genes in oni1 shoot, which lacked normal L1, a preferential pathway of PAT. Since OsPIN genes potentially encode an efflux carrier protein of PAT and OsIAA genes are homologues of auxin-inducible IAA genes,1,2 our results suggest that oni1 has altered auxin distribution in its shoot apex. It was reported that VLCFAs are necessary for proper subcellular localization of PIN proteins, which is essential for PAT.3 Auxin is known to regulate various aspects of plant growth and development, and improper distribution of auxin is expected to alter plant morphologies.4 Thus, it could be speculated that altered auxin distribution is associated with abnormal shoot development in oni1. In addition, auxin was shown to negatively regulate KNOX gene expression.4 Ectopic expression of KNOX genes in oni1 leaf might also be associated with the altered auxin distribution. Our speculation is that a lack of normal L1 in oni1 shoot perturbed proper PAT, which resulted in abnormal auxin distribution and finally affected entire shoot development. It remains elusive, however, as to whether auxin distribution is actually altered in oni1 shoot. Further studies of auxin distribution in oni1 and the relationship between auxin and oni1 morphologies should help with better understanding the physiological role of auxin and its polar transport on rice shoot development and interaction between L1 and inner cells during rice shoot development.
Acknowledgements
We thank Yoko Shiroto for her technical assistance. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (Plant Genome Barrier, 18075012) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- L1
layer 1
- PAT
polar auxin transport
- qRT-PCR
quantitative RT-PCR
- SAM
shoot apical meristem
- VLCFA
very-long-chain fatty acid
References
- 1.Miyashita Y, Takasugi T, Ito Y. Identification and expression analysis of PIN genes in rice. Plant Sci. 2010;178:424–428. [Google Scholar]
- 2.Jain M, Kaur N, Garg R, Thakur JK, Tyagi AK, Khurana JP. Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sativa) Funct Integr Genomics. 2006;6:47–59. doi: 10.1007/s10142-005-0005-0. [DOI] [PubMed] [Google Scholar]
- 3.Roudier F, Gissot L, Beaudoin F, Haslam R, Michaelson L, Marion J, et al. Very-long-chain fatty acids are involved in polar auxin transport and developmental patterning in Arabidopsis. Plant Cell. 2010;22:364–375. doi: 10.1105/tpc.109.071209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shani E, Yanai O, Ori N. The role of hormones in shoot apical meristem function. Curr Opin Plant Biol. 2006;9:484–489. doi: 10.1016/j.pbi.2006.07.008. [DOI] [PubMed] [Google Scholar]


