Abstract
Nitrate reductase is a central enzyme of nitrogen assimilation in plants. In a recent work, we have revealed MPK6 could phosphorylate Arabidopsis NIA2 at the serine 627 in hinge 2 region, this phosphorylation may represent a rapid activation mechnism when plant need excessive nitrate reduction. Interestingly, all eukaryotic NRs have conserved docking sequence in their FAD domains, and many plant NR proteins have the conserved MAPK phosphorylation site. Those indicated that phosphorylation of NR protein by MAP kinase cascade may be conserved in different species. We noticed that the phosphorylation of S627 residue by MPK6 have a specially influence on the NO generation. Although no homology of mammalian NOS has been identified in high plants, NR may still share a similar regulation mechanism with mammalian NOS.
Key words: nitrate reductase, nitric oxide, MPK6, reactive oxygen species, post-transcriptional modification
Nitrogen assimilation is a vital process controlling plant growth and development. In high plants, nitrate is the major nitrogen source, after taken up into plant cells, it must be reduced to ammonia for further usage. As the first enzyme in nitrate reduction pathway, the nitrate reductase (NR, NIA) is critical for regulation of the nitrogen assimilation.1 It is well documented that the amount and activity of NR is tightly controlled at transcriptional and pos-transcriptional levels by nitrate, light and CO2 levels, circadian rhythms, nitrogen and carbon metabolites, phytohomones, etc. Post-translational mechanisms could reversibly modulate NR activity within minutes and permit quick responses to environmental and cellular metabolism changes, which is the dominant regulation mechanism of NR activity.
Generally, plant NR protein contains three catalytic domains: Mo-molybdopterin (Mo-MPT) and interface domain, cytochrome b (Cyt b) domain, and FAD and NADH domain. Hinge 1 and hinge 2 regions are localized between those domains and joined them together (Fig. 1A).1 Emerging evidences have indicated that the phosphorylation of hinge 1 and hinge 2 regions have dominant influences on the NR activity. For example, phosphorylation of hinge 1 region at serine residue in Arabidopsis (S534), spinach (S543) or tobacco (S521) inhibited NR activities.2–4 The phosphorylation may coresponding to rapidly inactivate NR in response to several signals, including dark, decrease in CO2 levels or increase in cytosolic pH.1,3 On the contrary, phosphorylation at hinge 2 region showed a positive effect on NR activity, site-directed mutagenesis of the serine (S627) to aspartic acid, which mimic the phosphyorylation form of NIA2, caused the increase of NR activities about 2.5-fold.5 Interestingly, application of exgenous reactive oxygen species (ROS),5 or accumulation endogenous ROS in some condition, cause the rapid activation of NR via phosphorylation at this site. For example, during light-to-dark transitions, release of single oxygen is coupled with activation of NR.6,7 Application of exogenous salicylic acid or accumulation of endogenous salicylic acid in rcd1, induced the ROS generation, also could activate MPK6,8 and increase total NR activity.9 Phosphorylation at S627 of NR by MPK6 may represent a rapid activation mechnism when plant need excessive nitrate reduction.
Figure 1.
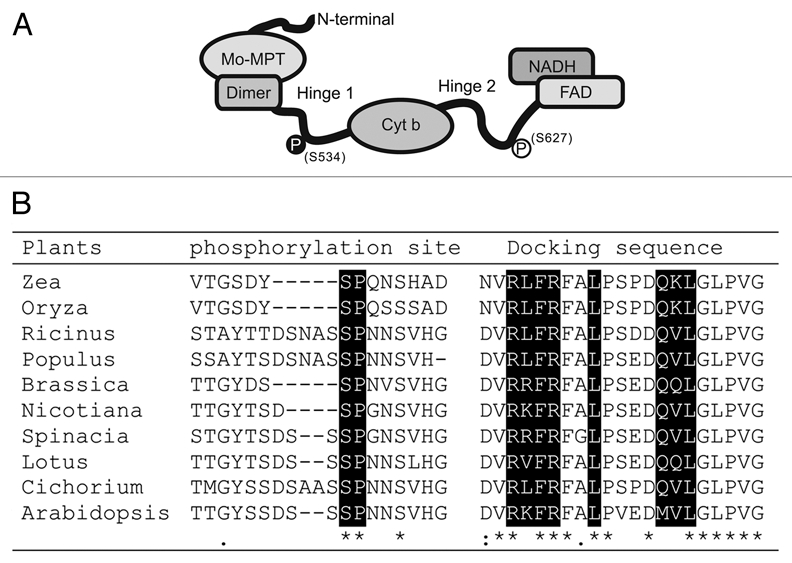
MAPK phosphorylation site and docking sequence are highly conserved in NR proteins. (A) Sequence model of the Arabidopsis NIA2 protein, phosphorylation sites S534 and S627 were marked. (B) Amino acid sequence comparison around the putative MAPK phosphorylation site and docking sequence (high lighted) of NR in high plants.
Interestingly, phosphorylation by MPK6 might be a conserved modification mechanism of NR. All eukaryotic NRs have conserved docking sequence in their FAD domain, which may be necessary for recognition of MAP kinase (Fig. 1B).10 NR proteins in some dicots and monocots plant species, including Barssica, tobacco, Lotus, rice, Maize, Cichorium and spinach, have the conserved MAPK phosphorylation site (SP residue, high lighted in Fig. 1B) at hinge 2 region. It has been reported that the hinge 2 evolved fastest in all domains in NR protein.11 High conservation of MAPK recognition and phosphorylation site in this region indicates the importance of the post-transcriptional regulation of NR activation, and the modulation at hinge 2 region by MAP kinase may be responsible for the accurate control of NR activity under certain internal or environmental conditions.
MAP kinase cascades are conserved signal transduction cascade that transduce extracellular stimuli into intracellular responses in yeast and animal cells. In plant, MAP kinase also modulates various biological progresses. MPK6, one of well-characterized MAPK in plant, mediated innate immunity,12,13 ethylene and jasminate signaling,14–17 abiotic stresses,18 leaf senescence,19 stomotal,20 anther,21 ovule22 and root development.23 The diverse function of MPK6 suggested the central role of MAPK cascade in intracellular signaling network. Our work indicated the conserved signaling cascades also involved in lateral root development, by modulation of NR phosphorylation and NO synthesis.
The primary function of NR to is reduce nitrate to nitrite, somehow, it also catalyzes the nitric oxide (NO) production.24,25 NO generation induced by auxins, abscisic acid or stresses are dependent on NR activity.26–28 Recent works have addressed the relationship between mitogen-activated protein kinase (MAPK) cascades and NO generation in tobacco and Arabidopsis.5,29 So far, details about the biochemical progresses and regulatory mechanisms of NR dependent NO generation are still largely unknown. However, phosphorylation of NIA2 also increased the NO generation dramatically in transgenic plants, but this modulation have different effects on NR activity and NO generation.5 For example, overexpression of NIA2 induced the NR activity more than eight-fold, but it only stimulated the NO generation slightly. On the contrary, application of exogenous H2O2 has a stronger effect on NO generation than NR activity in both wild-type and NIA2WT transgenic plants, but not in NIA2D and NIA2A plants. A possibility is that the phosphorylation of S627 by MPK6 have a specially influence on the NO generation activity of NIA2. In mammalian, a serine/threonine protein kinase Akt (protein kinase B) can directly phosphorylate endothelial nitric oxide synthase (eNOS) on serine 1179 and activate the enzyme, leading to NO production.30,31 According to this, mpk6 mutant showed lower NO accumulation and enhanced lateral root development under the application of NO donor sodium nitroprusside (SNP) or H2O2. Although no homology of mammalian NOS has been identified in high plants,32 NR may still share a similar regulation mechanism with mammalian NOS.
Acknowledgements
The work was supported by grants from the National Natural Science Foundation of China (91017001 and 30900100).
References
- 1.Campbell WH. Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Ann Rev Plant Physiol Plant Molec Biol. 1999;50:277–303. doi: 10.1146/annurev.arplant.50.1.277. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann M, Shiraishi N, Campbell WH, Yoo BC, Harmon AC, Huber SC. Identification of Ser-543 as the major regulatory phosphorylation site in spinach leaf nitrate reductase. Plant Cell. 1996;8:505–517. doi: 10.1105/tpc.8.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su W, Huber SC, Crawford NM. Identification in vitro of a post-translational regulatory site in the hinge 1 region of Arabidopsis nitrate reductase. Plant Cell. 1996;8:519–527. doi: 10.1105/tpc.8.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lillo C, Lea US, Leydecker MT, Meyer C. Mutation of the regulatory phosphorylation site of tobacco nitrate reductase results in constitutive activation of the enzyme in vivo and nitrite accumulation. Plant J. 2003;35:566–573. doi: 10.1046/j.1365-313x.2003.01828.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang P, Du Y, Li Y, Ren D, Song CP. Hydrogen peroxide-mediated activation of MAP Kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cel. 2010:2981–2998. doi: 10.1105/tpc.109.072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.op den Camp RGL, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cookson SJ, Williams LE, Miller AJ. Light-dark changes in cytosolic nitrate pools depend on nitrate reductase activity in Arabidopsis leaf cells. Plant Physiol. 2005;138:1097–1105. doi: 10.1104/pp.105.062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Jr, et al. Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umebese CE, Olatimilehin TO, Ogunsusi TA. Salicylic acid protects nitrate reductase activity, growth and proline in amaranth and tomato plants during water deficit. Am J Agri Biol Sci. 2009;4:224–229. [Google Scholar]
- 10.Stolz JF, Basu P. Evolution of nitrate reductase: molecular and structural variations on a common function. ChemBioChem. 2002;3:198–206. doi: 10.1002/1439-7633(20020301)3:2/3<198::AID-CBIC198>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Kleinhofs A. Molecular evolution of nitrate reductase genes. J Molec Evol. 1996;42:432–442. doi: 10.1007/BF02498637. [DOI] [PubMed] [Google Scholar]
- 12.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 13.Ren D, Liu Y, Yang KY, Han L, Mao G, Glazebrook J, et al. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA. 2008;105:5638–5643. doi: 10.1073/pnas.0711301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi F, Yoshida R, Ichimura K, Mizoguchi T, Seo S, Yonezawa M, et al. The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell. 2007;19:805–818. doi: 10.1105/tpc.106.046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Zhang S. Phosphorylation of 1-aminocyclo-propane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo SD, Cho Y, Sheen J. Emerging connections in the ethylene signaling network. Trends Plant Sci. 2009;14:270–279. doi: 10.1016/j.tplants.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo SD, Cho YH, Tena G, Xiong Y, Sheen J. Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signalling. Nature. 2008;451:789–795. doi: 10.1038/nature06543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, et al. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell. 2004;15:141–152. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Cai Z, Guo Y, Gan S. An Arabidopsis mitogen-activated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol. 2009;150:167–177. doi: 10.1104/pp.108.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hord CLH, Sun YJ, Pillitteri LJ, Torii KU, Wang H, Zhang S, et al. Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases. Mol Plant. 2008;1:645–658. doi: 10.1093/mp/ssn029. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Liu Y, Bruffett K, Lee J, Hause G, Walker JC, et al. Haplo-insufficiency of MPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell. 2008;20:602–613. doi: 10.1105/tpc.108.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller J, Beck M, Mettbach U, Komis G, Hause G, Menzel D, et al. Arabidopsis MPK6 is involved in cell division plane control during early root development and localizes to pre-prophase band, phragmoplast, trans-Golgi network and plasma membrane. Plant J. 2009;61:234–248. doi: 10.1111/j.1365-313X.2009.04046.x. [DOI] [PubMed] [Google Scholar]
- 24.Desikan R, Griffiths R, Hancock J, Neill S. A new role for an old enzyme: Nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2002;99:16314–16318. doi: 10.1073/pnas.252461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean JV, Harper JE. The conversion of nitrite to nitrogen oxide(s) by the constitutive NAD(P) H-nitrate reductase enzyme from Soybean. Plant Physiol. 1988;88:389–395. doi: 10.1104/pp.88.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto-Katou A, Katou S, Yoshioka H, Doke N, Kawakita K. Nitrate reductase is responsible for elicitin-induced nitric oxide production in Nicotiana benthamiana. Plant Cell Physiol. 2006;47:726–735. doi: 10.1093/pcp/pcj044. [DOI] [PubMed] [Google Scholar]
- 28.Kolbert Z, Bartha B, Erdei Ls. Exogenous auxin-induced NO synthesis is nitrate reductase-associated in Arabidopsis thaliana root primordia. J Plant Physiol. 2008;165:967–975. doi: 10.1016/j.jplph.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Asai S, Ohta K, Yoshioka H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell. 2008;20:1390–1406. doi: 10.1105/tpc.107.055855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fulton D, Gratton J, McCabe T, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher A. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 32.Crawford NM. Mechanisms for nitric oxide synthesis in plants. J Exp Bot. 2006;57:471–478. doi: 10.1093/jxb/erj050. [DOI] [PubMed] [Google Scholar]


