Abstract
UV-B radiation (280–315 nm) is an integral part of solar radiation and has many harmful effects on plant growth and development. However, the molecular mechanism for the inhibition of plant growth by UV-B remains largely unknown. UV-B radiation induces various responses such as growth inhibition, DNA damage and changes of gene expression. Recently, by using synchronous root tip culture, we found that UV-B modulates the expression of cell cycle regulatory genes through DNA damage. Western blotting analysis revealed that UV-B induced G1-to-S arrest did not correlate with the protein abundance of CDKB1;1 and CYCD3;1 gene regulating proteins, but may with the posttranslational control. We extended the expression analysis of cell cycle related genes based on the published microarray data and the results strengthen our assumption that cell cycle arrest could occur in plant under solar UV-B radiation. Further study is needed to elucidate the relationship between cell cycle regulation and protective pathway induced by low dose of UV-B radiation and the fundamental molecular mechanism for how plants respond to solar UV-B radiation.
Key words: Arabidopsis root tips, CDK, CPD, cyclin D, cell cycle, G1-to-S transition, UV-B
Plants use the energy in sunlight for photosynthesis, but as a consequence are exposed to the harmful effects of UV-B radiation, resulting in decreased growth and productivity.1 DNA is one of the major targets of UV damage and UV-B radiation is capable of directly altering its structure. The majority of DNA damage consists of cyclobutane pyrimidine dimers (CPDs, approx. 75%), while the (6-4) photoproducts (6,4-PPs) account for most of the remainder.2 Plants have various mechanisms for coping with such UV-B-induced DNA damage, including photoreactivation (photorepair) and nucleotide excision repair (dark repair).2 However, the molecular mechanism for the relationship between UV-B induced growth inhibition and DNA damage is largely unknown.
Recently, we demonstrated that UV-B radiation resulted in the delay of G1-to-S transition,3 which may be a protective mechanism that prevents cells with damaged DNA from dividing and may explain the plant growth inhibition under UV-B radiation. In a variety of eukaryotic cells, signals induced by UV or ionizing radiation act at pre-replication (G1/S) and premitosis (G2/M) check-points to inhibit progression through the eukaryotic cell cycle.4,5 In plants, little is known about cell cycle arrest in response to UV-B radiation.
We found that the expression of Histone H4 and E2Fa, which are the marker genes of G1-to-S transition, were downregulated by higher level of UV-B (0.45 W m−2) in synchronous wild type root-tip cells. This phenomenon occured in the uvh1 mutant even under medium level of UV-B (0.25 W m−2), suggesting that the UV-B mediated expression of cell cycle genes resulted from DNA damage.3 The expressions of other cell cycle related genes, such as CDKA;1, CDKB1;1 and CYCD3;1, were also suppressed by UV-B.3 Because most of the cell cycle regulators are kinases and phosphatases, it is of great interest to assay the protein levels after UV-B treatment. However, only CDKA;1 was decreased after prolonged UV-B treatment in synchronous root tips, but CYCD3;1 did not show any significant changes (Fig. 1). Though CDKB1;1 protein showed a mild increase in the UV-A control, its abundance was still lower after UV-B treatment, except there was a dramatic decline at 2 h (Fig. 1). These results suggest that the delay of G1-to-S transition by UV-B is not always correlated with the protein abundance of CDKs and CYCD3;1, but may due to posttranslational control. A similar protein pattern of cell cycle regulators was verified in the salt stressed Arabidopsis roots.12
Figure 1.
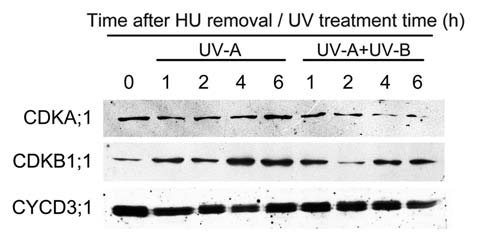
The protein profiles of CDKA;1, CDKB1;1 and CYCD3;1 after UV-B radiation. One-week-old Arabidopsis root tips were synchronized by hydroxyurea (2.5 mM) for 18 h and then washed by MS liquid medium, followed by UV-B treatment (0.45 W m−2) for indicated times. (There was little UV-A in UV-B treatment that cannot be filtered out, so the same level of UV-A was used as control). The protein profiles were detected by western blotting with CDKA;1-, CDKB1;1- and CYCD3;1-specific antibodies, respectively.
Despite the recognized importance of UV-B radiation in limiting plant growth and cause inhibition of biomass accumulation,1 little is known about the underlying molecular mechanisms. Here, we propose that plants reduce their growth as a primary adaptation response to UV-B rather than as a secondary consequence of resource limitation. Use of DNA damage repair systems by plants to minimize the DNA damage induced by UV-B radiation is closely coordinated with cell cycle regulators. Atypical E2F transcription factor DP-E2F-like1 (E2Fe/DEL1), an important regulator of endocycle onset, is identified as a transcriptional repressor of the type-II CPD photolyase DNA repair gene PHR1.6 Surprisingly, E2Fe/DEL1 knockout plants had improved DNA repair abilities under UV-B.6 Thus, it could be proposed that cell cycle regulation is the primary adaptation response to UV-B induced DNA damage.
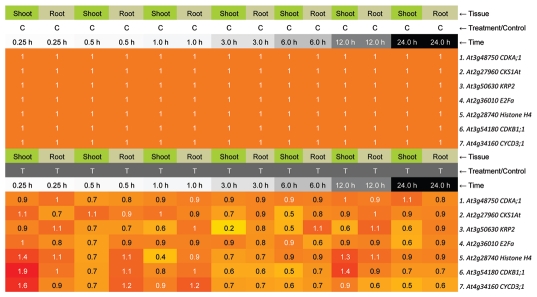
Plants are inevitably exposed to solar UV-B radiation. Because of the importance of cell cycle regulation in UV-B responses, it is intriguing to ask whether cell cycle arrest occurs in plants under solar UV. We do not observe any expression changes of tested genes in wild type synchronous root tip under medium level of UV-B for 6 h, which is a realistic UV irradiance in Guangzhou (23.1°N, 113.25°E; it should be noted that we used roots as a model to study cell cycle, though roots do not receive UV-B in nature). However, after performing an in silico expression of these genes from the published microarray data,7 we found that most of genes were downregulated in synchronous Arabidopsis after 15 min UV-B treatment (Fig. 2). The UV-B dose used for microarray analysis7 was 1.062 kJ m−2, while the UV-B dose in our previous research3 was 5.4 kJ m−2 (for comparison the daily UV-B summer dose in Guangzhou is about 4–6 kJ m−2). All these UV-B dose were weighted by Caldwell's “Generalized Plant Action Spectrum.”8 Under even lower UV-B dose (0.378 kJ m−2),9 genes putatively encoding cell cycle regulators were still suppressed (Table 1). However, whether such low dose of UV-B suppressed cell cycle-related genes expression is correlated with DNA damage is still unknown. But another study, using sun simulators, revealed that elevated solar UV-B doses increase the frequency of somatic homologous DNA rearrangements in Arabidopsis and tobacco plants, which was highly correlated with DNA damage repair pathway.10 This implies that cell cycle regulation may occur in plant under some area, where a higher dose of solar UV-B radiation is identified. Microarray analysis using field UV-B treated maize further confirmed that cell cycle regulation indeed occurs under natural condition.11
Figure 2.
In silico expression of cell cycle related genes from published micorarray data.7 Time indicated represents 15-min UV-B treatment and the time left in standard chamber without UV-B. The figure was generated by Bio-Array Resource for Plant Biology website (bbc.botany.utoronto.ca/).
Table 1.
Cell cycle related genes that downregulated by 15 min UV-B treatment
| Time after 15 min UV-B treatment (0.42 Wm−2) | Accession No. | Annotation |
| 1 h | At1g51060 | Histone H2A, putative |
| At3g45930 | Histone H4 | |
| At1g06760 | Histone H1, putative | |
| At5g06270 | B-type cyclin-related | |
| At4g27230 | Histone H2A, putative | |
| At3g53730 | Histone H4 | |
| At5g54640 | Histone H2A | |
| 6 h | At1g76540 | Cell division control protein, putative |
| At3g25980 | Mitotic checkpoint protein related | |
| At1g34460 | Cyclin, putative | |
| At1g44110 | Cyclin, putative |
Data are extracted from Ulm et al.9
Therefore, cell cycle regulation plays an important role in protecting the plant from solar UV-B radiation and might be one of the primary responses to solar UV-B radiation. But the molecular mechanisms of UV-B induced cell cycle regulation need to be established. Further research on how plants sense the DNA damage induced by UV-B, and how the cell cycle regulation coordinates the following protective pathway are necessary to elucidate the whole responsive mechanisms.
Acknowledgements
We thank Prof. Dirk Inzé (Gent University) for kindly providing CDKA;1 and CDKB1;1 antibodies. This work was supported by the grants to Prof. Shaoshan Li from the National Natural Science Foundation of China (NSFC, 31070242) and the Research Fund for the Doctoral Program of Higher Education of China (20070574003).
Abbreviations
- 6,4-PPs
(6-4) photoproducts
- CDKs
cyclin-dependent kinases
- CPDs
cyclobutane pyrimidine dimers
References
- 1.Kakani VG, Reddy KR, Zhao D, Sailaja K. Field crop response to ultraviolet-B radiation: a review. Agr Forest Meteorol. 2003;120:191–218. [Google Scholar]
- 2.Frohnmeyer H, Staiger D. Ultraviolet-B radiation-mediated responses in plants: Balancing damage and protection. Plant Physiol. 2003;133:1420–1428. doi: 10.1104/pp.103.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang L, Wang Y, Björn L, Li S. UV-B-induced DNA damage mediates expression changes of cell cycle regulatory genes in Arabidopsis root tips. Planta. 2011;233:831–841. doi: 10.1007/s00425-010-1340-5. [DOI] [PubMed] [Google Scholar]
- 4.Draper CK, Hays JB. Replication of chloroplast, mitochondrial and nuclear DNA during growth of unirradiated and UVB-irradiated Arabidopsis leaves. Plant J. 2000;23:255–265. doi: 10.1046/j.1365-313x.2000.00776.x. [DOI] [PubMed] [Google Scholar]
- 5.Murray A, Hunt T. The Cell Cycle: an Introduction. New York: W.H. Freeman & Co.; 1993. [Google Scholar]
- 6.Radziejwoski A, Vlieghe K, Lammens T, Berckmans B, Maes S, Jansen MA, et al. Atypical E2F activity coordinates PHR1 photolyase gene transcription with endoreduplication onset. EMBO J. 2010;30:355–363. doi: 10.1038/emboj.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, et al. The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007;50:347–363. doi: 10.1111/j.1365-313X.2007.03052.x. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell MM. Solar UV-B irradiation and the growth and development of higher plant. In: Giese AC, editor. Photophysiology. New York: Academic Press; 1971. pp. 131–171. [Google Scholar]
- 9.Ulm R, Baumann A, Oravecz A, Mate Z, Adam E, Oakeley EJ, et al. Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc Natl Acad Sci USA. 2004;101:1397–1402. doi: 10.1073/pnas.0308044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ries G, Heller W, Puchta H, Sandermann H, Seidlitz HK, Hohn B. Elevated UV-B radiation reduces genome stability in plants. Nature. 2000;406:98–101. doi: 10.1038/35017595. [DOI] [PubMed] [Google Scholar]
- 11.Casati P, Walbot V. Gene expression profiling in response to ultraviolet radiation in maize genotypes with varying flavonoid content. Plant Physiol. 2003;132:1739–1754. doi: 10.1104/pp.103.022871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West G, Inze D, Beemster GT. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004;135:1050–1058. doi: 10.1104/pp.104.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]