Abstract
The cell wall is a protective barrier of paramount importance for the survival of plant cells. Monitoring the integrity of the cell wall allows plants to quickly activate defense pathways to minimize pathogen entry and reduce the spread of disease. Counterintuitively, however, pharmacological effects as well as genetic lesions that affect cellulose biosynthesis and content confer plants with enhanced resistance against necrotrophic fungi. These kind of pathogens target cellulose for degradation to facilitate penetration and to generate glucose units as a food source. Our results point towards the existence of a transcriptional reprogramming mechanism in genes encoding cellulose synthases (CesAs) that occurs very soon after Botrytis cinerea attack and that result in a temporary shut down of some CesA genes. Interestingly, the observed coordinated downregulation of CesA genes is more pronounced, and occurs earlier in myb46 mutant plants. In the resistant myb46 plants, pathogen infection induces transient downregulation of CesA genes that concur with a selective transcriptional reprogramming in a set of genes encoding structural cell wall proteins and extracellular remodeling enzymes. Together with previous indications, our results favor the hypothesis that CesAs are part of a surveillance system of the cell wall integrity that senses the presence of a pathogen and transduces that signal into a rapid transcriptional reprogramming of the affected cell.
Key words: necrotrophs, cellulose, cell wall, peroxidase, B. cinerea
The cell wall is a dynamic complex composite of cellulose, hemicellulose, pectin, lignin and proteins, among other constituents, that is constantly remodeled during growth and development and in response to environmental cues.1 In addition to providing structural support, it controls cell expansion and is involved in the exchange of water and substances throughout plant development. It is also the first defensive structure that many pathogens encounter before confronting intracellular plant defenses, not only as a passive barrier but also constituting a reservoir of antimicrobial compounds and an important sensory component for downstream signaling pathways. In fact, it has been proposed that in plants, cell damage may be sensed by detection of modification of polysaccharides, release of oligosaccharides, inhibition of cell wall synthesis or assembly or deformation of the plasma membrane adjacent to damaged and weakened cell walls.2–4 As cellulose is the most abundant polymer and the major load-bearing polysaccharide common to both primary and secondary cell wall, pathogens target it for degradation to facilitate penetration and to generate glucose units as a food source. How the plant cell detects a defect in cellulose synthesis and deposition is unknown, but it may be possible that plant cells have developed a surveillance mechanism to monitor the plasma membrane-localized cellulose synthase complex, the crystallinity and content of cellulose produced or even the generation and release of degraded cellulose fragments.5 Such a system might be similar to the cell wall integrity-sensing system found in yeast cells that responds to perturbations in the cell wall structural integrity through the action of stress sensors thought to act as cell surface mechanosensors.6,7
Cellulose consists of long parallel linear β-1,4-D-glucan chains that are assembled into crystalline microfibrils by hydrogen bonding by a large multimeric complex containing at least three different cellulose synthase (CesA) enzymes located at the plasma membrane.8,9 Arabidopsis genome holds 10 CesA genes, six of which encode proteins with known functions.1 So far, CesA1 (RADIAL SWELLING1 [RSW1]), CesA3 (ISOXABEN RESISTANT1 [IXR1]/CONSTITUTIVE EXPRESSION OF VSP1 [CEV1]), CesA6 (PRC1/IXR2), CesA2, CesA5 and CesA9 have been associated with the CesA complexes active during primary wall formation, while CesA4 (IRREGULAR XYLEM5 [IRX5]), CesA7 (IRX3) and CesA8 (IRX1) have been reported to be part of the CesA complex responsible for secondary wall cellulose synthesis, which takes place after the arrest of cell expansion (reviewed in ref. 10).
Cell wall synthesis is a process highly regulated at the transcriptional level. A group of transcription factors in the NAC and MYB families seems to represent a core set of master regulators of secondary cell wall formation, including SND1,11 MYB46,12,13 NST1 and NST3,14 and MYB58 and MYB63,15 among others. We have shown16 that the Arabidopsis transcription factor MYB46, previously described to regulate secondary cell wall biosynthesis in the vascular tissue of the stem, is pivotal for mediating disease susceptibility to the fungal pathogen Botrytis cinerea. Different myb46 knock-down mutants exhibit increased disease resistance to B. cinerea, a phenotype that is accompanied by selective transcriptional reprogramming of a set of genes encoding cell wall proteins and enzymes, of which extracellular type III peroxidases are conspicuous. We hypothesized that defense-related signaling pathways and cell wall integrity are interconnected, and MYB46 likely functions as a disease susceptibility modulator to B. cinerea through the integration of cell wall remodeling and downstream activation of secondary lines of defense.16
In this work we further investigated the link between cell wall modifications and heightened resistance to B. cinerea in myb46 plants, in comparison to wild type plants, by establishing a relationship between infection and a transcriptional regulatory network that controls expression of CesA genes. Although total cellulose accumulation appears uncompromised in myb46 plants,16 we wondered if at least some of the signaling caused by B. cinerea-induced cell wall disruption could be caused by modification of the cellulose network.
Previous studies reported a connection between cellulose and disease resistance to fungal pathogens. In this regard, inhibition of cellulose synthesis with the herbicide isoxaben induces jasmonic acid (JA) synthesis, a stress phytohormone that regulates response to necrotrophic pathogens as well as the concurrent activation of some defense-associated genes such as the defensine PDF1.2.17–19 Similarly, mutations in CesAs for either the primary or secondary cell wall induce a number of stress and defense-like responses and also enhance disease resistance to pathogens. For example, cellulose deficiency in the primary cell wall mutant cesa3 constitutively elicits ethylene (ET) and JA signaling and enhances disease resistance to fungi and aphids.20,21 Also, disruption of the secondary cell wall (e.g., cesa4, cesa7 and cesa8 mutants) causes an increase of the disease resistance to the necrotrophic fungi B. cinerea and Plectosphaerella cucumerina.22 However, we were not aware of any study reporting on the transcriptional modulation of CesA genes following fungal infection. Therefore, we measured transcript abundance for representative members of the CesA gene family by RT-qPCR during a 72-h time course following inoculation of Col-0 with B. cinerea. These studies were comparatively performed with myb46-2 plants (Fig. 1). Our results demonstrated that for all CesA genes analyzed, there was a general downregulation in gene expression following fungal inoculation that become apparent at 24 h.p.i. Exclusive of CesA3, downregulation was temporary as transcript abundance recovered, and in some cases even exceeded normal levels at 72 h.p.i. The induced repression of CesA3 remained even at 72 h.p.i. The effect on repression of the CesA gene was also reproduced in myb46-2 plants, but the mutant plants demonstrated a reproducible tendency to heighten the degree of repression observed in Col-0. This was most evident in CesA3 and CesA4, the two genes that become most rapidly and abruptly repressed following fungal infection. In addition, CesA3 and CesA4 transcripts were nearly undetectable in myb46-2 plants at 24–48 h.p.i. CesA8, and to a lesser extend CesA7, were also notably repressed following inoculation with B. cinerea. myb46-2 plants showed constitutive reduced transcript levels for CesA8. CesA8 exhibited marked expression recovery at 72 h.p.i.; a recovery that was slowed in myb46-2 plants. As a control in these RT-qPCR experiments we used the defensin PDF1.2a and the basic chitinase PR-3 marker genes whose expression patterns were opposed to those of CesA genes and became highly activated during the infection process.
Figure 1.
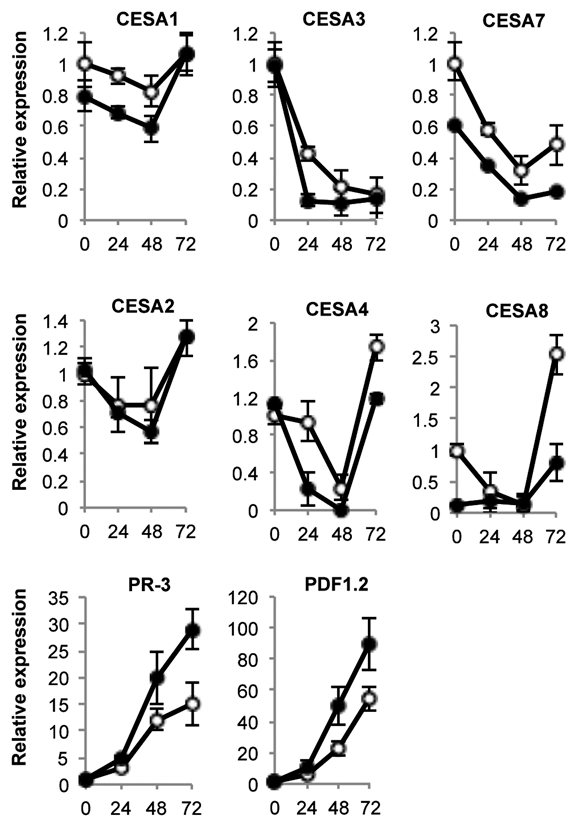
Expression of CesA genes in leaves at early stages of B. cinerea infection. Relative expression was assayed over a 72-h time course by RT-qPCR on total RNA from leaves of Col-0 (open circles) or myb46-2 (filled circles) plants after inoculation with a B. cinerea spore suspension. Expression was normalized to actine2 (ACT2) gene expression. For ease of comparison, an arbitrary relative expression value of 1 was assigned to each CesA gene and refers to the expression level attained in col-0 plants at 0 h.p.i. with B. cinerea. Error bars represent standard deviation of three independent replicates.
Several fungi use topographical cues on the plant surface to guide them towards a suitable entry point, and it may be that the cell wall composition could constitute one of these fingerprints that pathogens read to find the appropriate penetration point. Accordingly, alterations in the cellulose disposition, composition or proprieties (e.g., in cesa mutants) could change the topographic code and in turn impacting on the disease susceptibility. Our data, together with results indicating that genetic defects in cellulose biosynthesis of primary and secondary cell walls provide plants with increased disease resistance to fungal pathogens, favor the hypothesis that CesAs are part of a surveillance system of the cell wall integrity that senses the presence of a pathogen and transduce that signal into a rapid transcriptional reprogramming of the affected cell.23,24 If MYB46 is functioning as a modulator of the cell wall assembly, it is plausible that myb46 mutant plants become more sensitive to the presence of B. cinerea and in turn respond with a more efficient defense response that is primarily activated at the cell wall level. Recognition of the intruder at the cell wall sets in motion an early and very precise transcriptional reprogramming; for Arabidopsis, one of its own constituents, the CESA genes, that may help adjust the cell wall network to this new extracellular scenario. The existence of such a surveillance system for plant cell wall integrity following pathogen disruption has been previously proposed,24,25 and seems to be a component of early signaling events that must subsequently be interconnected with known plant defense networks.
Acknowledgements
This was work supported by Spanish Ministry of Science and Technology (Grants BFU2009-09771 and Consolider-TRANSPLANTA to P.V.).
References
- 1.Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, et al. Toward a systems approach to understanding plant cell walls. Science. 2004;306:2206–2211. doi: 10.1126/science.1102765. [DOI] [PubMed] [Google Scholar]
- 2.Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell AL. Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci. 2008;13:610–617. doi: 10.1016/j.tplants.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Hematy K, Cherk C, Somerville S. Host-pathogen warfare at the plant cell wall. Curr Opin Plant Biol. 2009;12:406–413. doi: 10.1016/j.pbi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Pilling E, Hofte H. Feedback from the wall. Curr Opin Plant Biol. 2003;6:611–616. doi: 10.1016/j.pbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Hematy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, et al. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol. 2007;17:922–931. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Dupres V, Alsteens D, Wilk S, Hansen B, Heinisch JJ, Dufrene YF. The yeast Wsc1 cell surface sensor behaves like a nanospring in vivo. Nat Chem Biol. 2009;5:857–862. doi: 10.1038/nchembio.220. [DOI] [PubMed] [Google Scholar]
- 7.Philip B, Levin DE. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol Cell Biol. 2001;21:271–280. doi: 10.1128/MCB.21.1.271-280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpita NC. Update on mechanisms of plant cell wall biosynthesis: how plants make cellulose and other (1→4)-{beta}-D-glycans. Plant Physiol. 2011;155:171–184. doi: 10.1104/pp.110.163360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerriero G, Fugelstad J, Bulone V. What do we really know about cellulose biosynthesis in higher plants? J Integr Plant Biol. 2010;52:161–175. doi: 10.1111/j.1744-7909.2010.00935.x. [DOI] [PubMed] [Google Scholar]
- 10.Endler A, Persson S. Cellulose synthases and synthesis in Arabidopsis. Mol Plant. 2011;4:199–211. doi: 10.1093/mp/ssq079. [DOI] [PubMed] [Google Scholar]
- 11.Zhong R, Demura T, Ye ZH. SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell. 2006;18:3158–3170. doi: 10.1105/tpc.106.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong R, Richardson EA, Ye ZH. The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell. 2007;19:2776–2792. doi: 10.1105/tpc.107.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko JH, Kim WC, Han KH. Ectopic expression of MYB46 identifies transcriptional regulatory genes involved in secondary wall biosynthesis in Arabidopsis. Plant J. 2009;60:649–665. doi: 10.1111/j.1365-313X.2009.03989.x. [DOI] [PubMed] [Google Scholar]
- 14.Mitsuda N, Iwase A, Yamamoto H, Yoshida M, Seki M, Shinozaki K, et al. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell. 2007;19:270–280. doi: 10.1105/tpc.106.047043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J, Lee C, Zhong R, Ye ZH. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell. 2009;21:248–266. doi: 10.1105/tpc.108.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramírez V, Agorio A, Coego A, García-Andrade, Hernández MJ, Balaguer B, et al. MYB46 modulates disease susceptibility to Botrytis cinerea in Arabidopsis. Plant Physiol. 2011;155:1–16. doi: 10.1104/pp.110.171843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamann T, Bennett M, Mansfield J, Somerville C. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J. 2009;57:1015–1026. doi: 10.1111/j.1365-313X.2008.03744.x. [DOI] [PubMed] [Google Scholar]
- 18.Thomma BP, Eggermont K, Penninckx IA, Mauch-Mani B, Vogelsang R, Cammue BP, et al. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. Plant Cell. 2002;14:153–164. doi: 10.1105/tpc.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis C, Karafyllidis I, Turner JG. Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae and Myzus persicae. Mol Plant Microbe Interact. 2002;15:1025–1030. doi: 10.1094/MPMI.2002.15.10.1025. [DOI] [PubMed] [Google Scholar]
- 21.Ellis C, Karafyllidis I, Wasternack C, Turner JG. The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell. 2002;14:1557–1566. doi: 10.1105/tpc.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Blanco C, Feng DX, Hu J, Sanchez-Vallet A, Deslandes L, Llorente F, et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell. 2007;19:890–903. doi: 10.1105/tpc.106.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vorwerk S, Somerville S, Somerville C. The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 2004;9:203–209. doi: 10.1016/j.tplants.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Schulze-Lefert P. Knocking on the heaven's wall: pathogenesis of and resistance to biotrophic fungi at the cell wall. Curr Opin Plant Biol. 2004;7:377–383. doi: 10.1016/j.pbi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Gus-Mayer S, Naton B, Hahlbrock K, Schmelzer E. Local mechanical stimulation induces components of the pathogen defense response in parsley. Proc Natl Acad Sci USA. 1998;95:8398–8403. doi: 10.1073/pnas.95.14.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]


