Abstract
Novel compounds with significant medicinal properties have gained much interest in therapeutic approaches for treating various inflammatory disorders like arthritis, odema and snake bites and the post-envenom (impregnating with venom) consequences. Inflammation is caused by the increased concentration of secretory Phospholipases A2 (sPLA2s) at the site of envenom. A novel compound Tris(2,4-di-tert-butylphenyl) phosphate (TDTBPP) was isolated from the leaves of Vitex negundo and the crystal structure was reported recently. The acute anti-inflammatory activity of TDTBPP was assessed by Carrageenan-induced rat paw odema method. TDTBPP reduced the raw paw odema volume significantly at the tested doses of 50 mg/kg and 70 mg/kg body weight. Molecular docking studies were carried out with the X-ray crystal structures of Daboia russelli pulchella's (Vipera russelli, Indian Russell's viper) venom sPLA2 and Human non-pancreatic secretory PLA2 (Hnps PLA2) as targets to illustrate the antiinflammatory and antidote activities of TDTBPP. Docking results showed hydrogen bond (H-bond) interaction with Lys69 residue lying in the anti-coagulant loop of D. russelli's venom PLA2, which is essential in the catalytic activity of the enzyme and hydrophobic interactions with the residues at the binding site (His48, Asp49). Docking of TDTBPP with Hnps PLA2 structure showed coordination with calcium ion directly as well as through the catalytically important water molecule (HOH1260) located at the binding site.
Keywords: Vitex negundo; Tris(2,4-di-tert-butylphenyl) phosphate; Anti-inflammatory; Carrageenan; Antidote; PLA2; Induced Fit Docking
Background:
The importance of natural products in modern medicine has been well recognized. Natural compounds isolated from plant sources have an extensive past and present use in the treatment of diverse diseases. They also serve as compounds of interest both in their natural form and also templates for synthesizing derivatives with higher activity and lower toxicity. More than 20 new drugs, launched worldwide between 2000 and 2005, originate from natural products. Scrutiny of medical indications by source of compounds has demonstrated that natural products and related drugs are used to treat 87% of all categorized infectious and non-infectious human diseases [1]. Vitex negundo (verbenaceae) is an important Indian medicinal plant, which is a source of such natural compounds. It is a medicinal herb and various parts of the plant have been employed in the folklore systems of medicine in Asia including India, China and Malaysia for various diseases. Many ethnobotanical and pharmacological activities of V. negundo have been reported such as: analgesic and anti-inflammatory activity [2, 3], antioxidant activity [4], enzyme inhibitions [5], nitric oxide scavenging activity [6], antiradical and antilipoperoxidative activity [7], CNS activity [8], hepatoprotective activity [9], anti-bacterial activity [10], antifungal activity [11], larvicidal activity [12], antiandrogenic effects [13] and mosquito repellent activity [14]. V. negundo leaves were found to have NSAIDs like activity and fresh leaves have been suggested to possess anti-inflammatory and pain suppressing activities possibly mediated via prostaglandin (PG) synthesis inhibition [15]. The leaf extract was also used as adjuvant to standard anti-inflammatory drug [16]. The methanolic extract of roots was reported to have snake venom neutralizing property [17]. Many biologically active compounds have been isolated from this plant in the past. Recently we isolated and purified a Polyphenyl compound TDTBPP from the ethyl acetate extract of V. negundo leaves and reported its crystal structure (Figure 1) [18]. Phospholipases A2 (PLA2s; EC 3.1.1.4) are enzymes that play a major role in the formation of pro-inflammatory and inflammatory mediators such as prostaglandins, leukotrienes, platelet-aggregating factor and lysophospholipids [19, 20]. These enzymes catalyse the hydrolysis of sn-2 acyl bond of phospholipids to produce unsaturated fatty acids and lysophospholipids. Arachidonic acid, one of the fatty acids released by the hydrolysis can lead to the biosynthesis of eicosanoids and hydrolysis of cellular phospholipids of the activated inflammatory cells and therefore acts as a very important inflammatory precursor. Increased concentrations of eicosanoids are found in the state of inflammation [21]. PLA2s are classified as intracellular and extracellular. Intracellular PLA2s are of high molecular weight and involved in phospholipid metabolism, signal transduction and other cellular processes [22]. Extracellular PLA2/secretory PLA2 (sPLA2) are of low molecular weight and found in mammalian pancreatic juice, snake and insect venoms [23–25]. The release of arachidonic acid by sPLA2 is followed by the eicosanoid production. Hence, sPLA2s are considered as “the inflammatory PLA2s” and act as target for anti-inflammatory drugs. Thus, inhibition of PLA2 by various biologically active substances has gained therapeutic importance. Most of the natural compounds and their derivatives interfere directly or indirectly with specific molecules or mechanisms, such as various inflammatory mediators (arachidonic acid metabolites, peptides, cytokines etc.), production or action of second messengers (cGMP, cAMP, various protein kinases) and in release of pro-inflammatory molecules [26]. The present study was undertaken to illustrate the anti-inflammatory activity of TDTBPP by carrageenan-induced rat paw odema model and to carry out Molecular docking studies (in silico) carried out with the X-ray crystal structures of PLA2-inhibitor complexes available in the Protein Data Bank (PDB; http://www.pdb.org/pdb) to illustrate the interactions exhibited by TDTBPP with the target protein. D. russelli venom sPLA2 (PDB ID: 3H1X) [27] and Hnps PLA2 (PDB ID: 1KVO) [28] structures were chosen for docking studies. The favorable interactions of TDTBPP with the active site residues of the sPLA2 could be correlated with the potency and the compound is proved to be an antiinflammatory agent as well as an antidote. TDTBPP therefore could be co-crystallized with sPLA2 and the in vitro binding mode can be studied.
Figure 1.
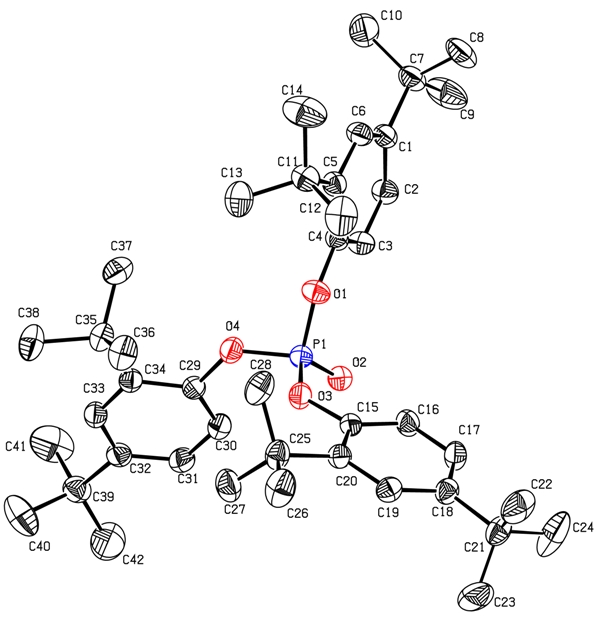
Crystal structure of TDTBPP (displacement ellipsoids drawn at 30% probability level. Hydrogen atoms are omitted for clarity
Methodology:
Animal Model Studies:
Chemicals & drugs:
The Carrageenan and Sodium carboxymethyl cellulose were purchased from Himedia Laboratories, Mumbai and the standard Non-Steroid Anti-Inflammatory Drug (NSAID) Indomethacin was purchased from Sigma Aldrich, Germany.
Animals:
The experiments were carried out in the animal house of Center of Advanced Study in Marine Biology, Annamalai University, Chidambaram. Albino rats of Wistar strains of either sex weighing between 120-150 g were chosen for study. The animals were kept on diet and allowed food and water ad libitum. They were housed in polypropylene cages maintained under standard conditions.
Preparation of Sample:
TDTBPP was separated and purified as mentioned in our previous studies [18]. The crystals of TDTBPP obtained from the ethyl acetate extract of V. negundo L. were crushed in to fine powder, lyophilized and suspended in 0.1% sodium carboxymethyl cellulose in 1M phosphate buffer pH 7.2 (vehicle) and used for the study. It is one of the most commonly used vehicles and has no effect on the activity.
Evaluation of anti-inflammatory activity:
Acute anti-inflammatory activity was evaluated by carrageenan-induced rat paw odema method. The ethical clearance was obtained by the Institutional Animal Ethics (Registration No: 4620/1A/2011/CPCSEA) before carrying out the experiment.
Statistical Analysis:
The experimental data were expressed as the mean + standard error (SE). The standard error of the mean (SEM) is the standard deviation of the sample mean estimate of a population mean. SEM is estimated by the sample estimate of the population standard deviation (sample deviation) divided by the square root of the sample size. Statistical analysis was carried out using one-way analysis of variance followed by Dunnet's Multiple Comparison Test and p values implied significance (p<0.01) [31].
Molecular Modeling Studies:
Molecular modeling studies have been carried out using GLIDE (Grid-based Ligand Docking with Energetics) [30] software v5.5 developed by Schrödinger running on Red Hat Enterprise Linux 5 (RHEL5) workstation. Maestro v9.0 Graphical User Interface (GUI) workspace was used for all the steps involved in ligand preparation, protein preparation and Induced Fit Docking (IFD).
Ligand Preparation:
The ligands used in this study were prepared using LigPrep [35] module of v2.3 of Schrödinger Suite 2009. LigPrep follows OPLS-AA (Optimized Potential Liquid Simulations for All Atoms) force fields for energy minimization. In this study we used the crystal coordinates of the ligands Indomethacin (Figure 2a), OAP (4-(s)-[(1-oxo-7-phenylheptyl) amino]-5-[4- (phenylmethyl)phenylthio] pentanoic acid) (Figure 2b) retrieved from their corresponding PDB complex structures and the crystal coordinates of TDTBPP obtained from the crystal data collected using Bruker's Smart Apex II CCD Single-crystal X-ray diffractometer available in the department [18]. The crystal structure retrieved from PDB lack hydrogen atoms and hence hydrogen atoms were added to Indomethacin and OAP structures and energy minimized.
Figure 2.
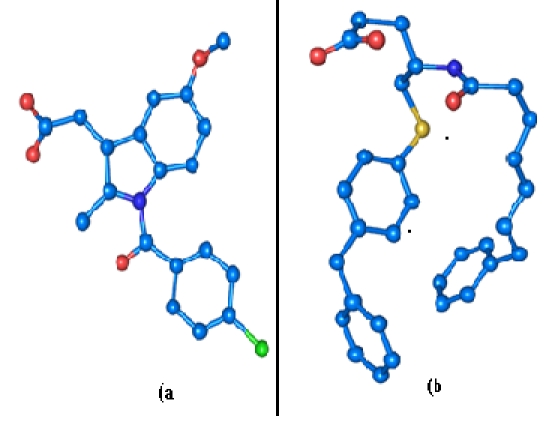
(a) 3D crystal structure of Indomethacin obtained from 3H1X. (b) 3D crystal structure of OAP obtained from 1KVO
Protein Preparation:
The X-ray crystal structures retrieved from PDB database as raw could not be suitable for molecular docking studies. A typical PDB structure consists only of heavy atoms, waters, cofactors, metal ions and can be of multimeric. These structures do not have the information about bond orders, topologies or formal atomic charges. The terminal amide groups may be misaligned because the X-ray structure analysis cannot distinguish between O and NH2. Ionization and tautomeric states are also unassigned. So, the raw PDB structure retrieved from PDB should be prepared in a suitable manner for docking. Protein Preparation Wizard of GLIDE software was used to process and prepare the protein. This Wizard allows one to properly prepare a protein for docking studies. This also follows the Optimized Potential for Liquid Simulations-All Atoms (OPLS-AA) force fields for energy minimization. Preparation of Daboia russelli's venom sPLA2: The X-ray crystal structure of D. russelli's venom sPLA2 (PDB id: 3H1X) retrieved from PDB is a monomeric structure. It consists of 120 amino acids with one inhibitor molecule Indomethacin, 243 water molecules and 3 sulphate ions. The protein was prepared by removing all the water molecules and sulphate ions present in the structure. Since the raw data do not contain any hydrogen in it, the implicit hydrogen atoms were added to the atoms to satisfy their appropriate valancies. Then the structure was optimized by assigning the bond orders, bond angles and topology. The formal atomic charges were fixed for the amino acid residues. The optimized structure was then energy minimized to remove the steric clashes between the atoms. The energy minimization was done till it reached a Root Mean Square Deviation (RMSD) cutoff of 0.18 Å and the resulting structure was used for docking.
Preparation of Hnps PLA2:
The X-ray crystal structure of Hnps PLA2 (PDB id: 1KVO) was retrieved from the PDB database. 1KVO is a hexameric structure with six identical chains. A monomeric unit consists of one OAP (co-crystallized inhibitor), 2 calcium metals (CA191 and CA192) and 96 water molecules. Since all the 6 chains were identical, a single chain with OAP, calcium metal (CA191 chelated to OAP and active site residues) and one water molecule (HOH1260, a significant metabolic water) were retained and used for further preparation steps. The remaining 95 water molecules of the retained chain and the other five monomeric units (124 × 5 amino acid residues, 2 × 5 calcium metals and 96 × 5 water molecules) were removed. The implicit hydrogen atoms were added to the structure so as to satisfy their valancies. Then the bond orders, bond angles, topology and the formal atomic charges for the amino acid residues were assigned. The calcium metal in the structure was treated and ionized to Ca2+ ionic state. The structure was then energy minimized until it reached a RMSD of 0.18 Å and used for docking.
Induced Fit Docking (IFD):
IFD of the prepared ligands with the prepared proteins was performed using Induced Fit Docking protocol of GLIDE v5.5 from Schrödinger Suite 2009 [31]. It is based on GLIDE and Prime Refinement module. Prime accurately predicts the ligand binding modes and concomitant structural changes in the receptor. In IFD, both the ligand and the receptor are flexible which enables to dock the ligand at the receptor's binding site to generate multiple poses of the receptor-ligand complex, each including unique structural conformations of the receptor to fit the ligand pose and ranks them by Glide score (G-score) to find the best structure of the docked complex. G-score takes into account a number of parameters like hydrogen bonds (H-bond), hydrophobic contacts (Lipo), van der-Waals (vdW), columbic (Coul), polar interactions in the binding site (Site), metalbinding term (Metal) and penalty for buried polar group (BuryP) and freezing rotatable bonds (RotB) G-score = H bond + Lipo + Metal + Site + 0.130 Coul + 0.065 vdW – BuryP – RotB. The prepared structures of 3H1X and 1KVO were used for induced fit docking simulations. Initially a receptor grid, where the ligand has to be docked with the receptor was set by picking the centroid of the co-crystallized inhibitor (Indomethacin in 3H1X and OAP in 1KVO) present at the active site. It creates a grid box and the size of the grid box was limited to 20 Å. The generation of different conformations of the docked complexes (poses) was set to a maximum of 20. Then TDTBPP was docked at the active site of 3H1X and 1KVO individually. The poses generated were ranked based on G-score. The pose that made the maximum hydrogen bond (H-bond) interactions from TDTBPP-3H1X and TDTBPP-1KVO docked complexes were considered for further analysis and the results are compared.
Visualization and Analysis:
The PyMol Molecular Graphics System [37] was used to analyze the hydrogen bond interactions and preparation of high resolution images. The hydrophobic interactions were obtained as Ligplot diagram by submitting the docked complexes to the online PDBsum server (http://www.ebi.ac.uk/pdbsum).
Results and Discussion:
Acute anti-inflammatory Activity:
TDTBPP at the dose of both 50 mg/kg and 70 mg/kg exhibited significant anti-inflammatory activity in carrageenan induced paw odema model (p<0.01) (Table 1, see supplementary material). No mortality of rats was observed and hence it is non-toxic in the administered dosages. It is well known that carrageenan induced paw edema model is commonly used as an experimental model for evaluating anti inflammatory activity of natural products [32] and is believed to be biphasic, of which the first phase is mediated by the release of histamines and 5 hydroxy tryptamin in the early stage followed by kinin release and then prostaglandin in the later stage. Prostaglandins and bradykinins were suggested to play important role in carrageenan induced edema [33, 34]. Both steroidal and non steroidal anti-inflammatory drugs can be tested by the carrageenan-induced paw inflammation test. The edema induced in the rat paw by the injection of 1% carrageenan is brought about by autocoids, histamine and 5-hydroxy tryptamine (5-HT) during the first one hour, after which kinins act, to increase the vascular permeability upto two and a half hours. The maximum inflammation is seen approximately three hours post the carrageenan injection, after which it begins to decline. Following that the prostaglandins act from two and a half hours to six hours, which results in the migration of leucocytes into the inflamed site [35, 36].
IFD of TDTBPP with 3H1X:
3H1X is a low molecular weight protein (13.6 kDa) and the structure of PLA2 consists of N-terminal helix, H1 (residues: 2- 12), a calcium binding loop (residues: 25-35), a second α-helix, H2 (residues: 40-55), a short two stranded antiparallel β-sheet/β wing (residues: 75-84) and a third α-helix, H3 (residues 90-108) [28]. The anti-coagulation region of the protein lies between the residues 54 and 77 and it is positively charged with the residues such as Lys69, Arg72, Arg74, Lys76 and Arg77 [37]. The opening and closure of Trp31 was observed in substrate/inhibitor bound and unbound form of the protein [38] (Figure 3). During the IFD of TDTBPP at the active site of 3H1X, the Trp31 residue remains in open conformation, but the side chain of the residue found to be displaced to a distance of 2.6 Å by 95.5°down through the C(γ) atom when compared with the IFD results of Indomethacin with 3H1X. The side chain of Lys69 was bent 71.2° down to a distance of 1.9Å through the C(δ) atom when compared to Indomethacin-3H1X complex, which favored the oxygen atom O(2) bonded to the central phosphorus atom of TDTBPP to make a H-bond interaction with the N(ε) atom at a distance of 2.83Å. The tert-butyl group of the phenyl ring was inserted in between His 48 and Asp 49 and makes hydrophobic contact with them. In addition to that, all the three tert-butylphenyl ring system of the molecule formed an array of hydrophobic interactions with the residues Phe5, Tyr22, Ser23, Tyr28, Gly30, Gly32, Tyr52, Gly53, Pro56, Cys61 and Pro68 lining the binding region (Figure 4a). The Glide score of TDTBPP is –5.0 and Glide energy is –50.9 Kcal/mol (Table 2, see supplementary material). IFD of Indomethacin into the active site of 3H1X has not influenced any significant structural changes in the overall conformation of the protein backbone. The docked orientation of Indomethacin was similar to the orientation found in the PDB complex. Trp31 residue was in open conformation. Indomethacin exhibited three H-bonds with the active site residues. The O(3) of the carbonyl group of Indomethacin formed H-bonds with the N(δ1) atom of His48 at a distance of 2.99 Å and O(δ1) atom of Asp49 residue at a distance of 2.48Å, respectively. The O(2) of the carbonyl group is involved in H-bond with the N(ε) atom of the Lys69 at a distance of 2.93Å (Figure 4b). The G-score of is –5.2 and Glide energy is –41.1 Kcal/mol. The overall binding of TDTBPP was in such a way that the O(2) atom of phosphate group maintains the interaction with Lys69 of the anticoagulant region as exhibited by Indomethacin. The G-score was also almost same and the Glide energy (directly related with the binding energy) was higher than Indomethacin, which indicated that TDTBPP has higher binding energy when compared to Indomethacin. The tert-butylphenyl ring makes hydrophobic contact with His48 and Asp49. These residues form a dyad, and along with other determinants stabilize the transition state of the catalytic assembly of the sPLA2 [39]. The molecule was well positioned within the hydrophobic channel of the newly identified binding site, which is effective in blocking the exposure of the amino acid residues present in the anti-coagulant region and also the catalytic region to bind the substrate, thereby arresting the enzymatic activity of PLA2.
Figure 3.
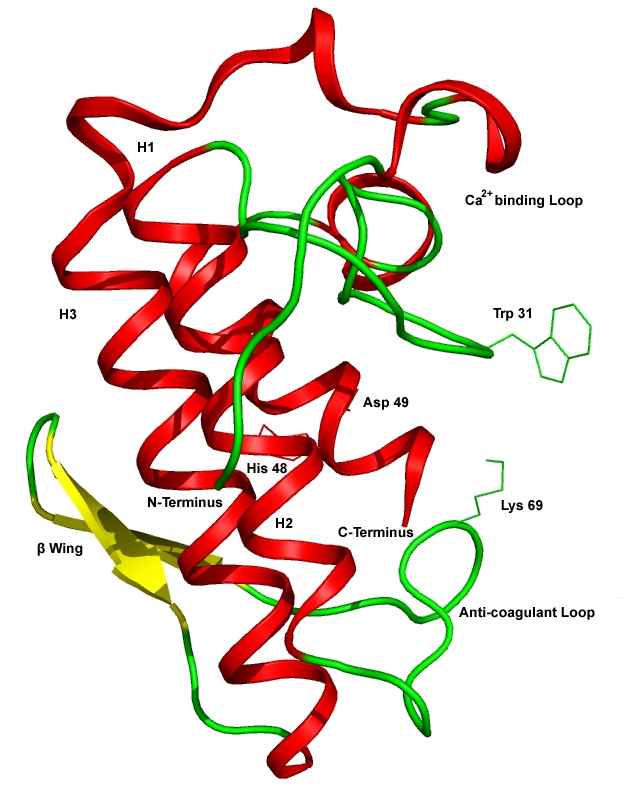
Cartoon representation of native form of Daboia russelli's venom sPLA2 (PDB id: 2PYC) showing the H1, H2, H3 α-helices, β-wing, Ca2+ binding loop, anti-coagulant loop and the key residues Trp31, His48, Asp49 and Lys69.
Figure 4.
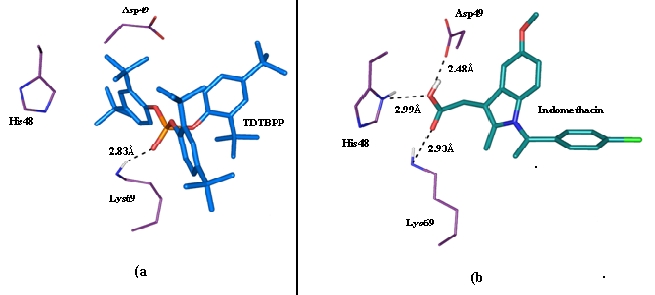
(a) H-bond and Hydrophobic interactions exhibited by TDTBPP with 3H1X. The O(2) atom of central phosphate group formed an H-bond with Lys69 (represented as black dotted lines with corresponding bond distance) in the anti-coagulant loop of the protein and the other active site residues His48 and Asp49 surrounding the tert-butylphenyl ring (b) H-bonds exhibited by Indomethacin with the active site residues of 3H1X.
IFD of TDTBPP with Hnps PLA2:
1KVO is also a low molecular weight protein (14 kDa protein) found in both inflammatory cells [40, 41] and a variety of inflammatory exudates fluids in soluble form [42]. The chemistry and catalytic activity of this protein is as similar to 3H1X, but with some modifications in the residues forming hydrophobic channel and their modulations during the substrate binding. The O(2) of the central phosphate group of TDTBPP was chelated to the calcium ion at a distance of 2.28Å (Figure 5b). This is similar to the distances exhibited by OAP with calcium ion and other reported complexes. The calcium ion in this complex was also has pentagonal bipyramidal geometry. As in the PDB complex Gly30, Gly32, His28 and Asp49 were also chelated to the calcium ion. The water molecule HOH1260 was chelated to the calcium ion and makes a H-bond interaction with the oxygen atom O(1) of the central phosphate group of TDTBPP). The carbonyl group oxygen atom of Asp42 displaced about 0.4 Å distance has an H-bond with the main chain N atom of His28, which was not found in the redocked pose of OAP-1KVO complex. This bond may hold the residues in the calcium binding loop of the protein to remain stable in the TDTBPP bound form. The angle between the calcium ion and the chelating oxygen atoms of water molecule and TDTBPP is 49.8°. The angle between the chelating oxygen atoms of His28 and TDTBPP with the calcium ion is 134.1°. The corresponding angle exhibited by sn-3 phosphate group of other classical inhibitors of PLA2 was about 164°. This is significant in the effective binding of TDTBPP with single phosphate group at the center of three phenyl rings. The angle between the chelating oxygen atoms of Asp49 and O(2) of TDTBPP with calcium ion is about 92.9°. The oxygen atoms of Asp49, Gly30 and Gly32 involved in the chelation with calcium ion are found to be near equatorial as reported in the PDB complex. This is much similar to that of the re-docked pose of OAP with 1KVO. The phenyl rings with substituted tert-butyl groups are well positioned within the hydrophobic channel of the protein and have hydrophobic interactions with Tyr22, Ala18, Ile9, His6, Phe5, Phe24, Gly23, Val31, Leu2, Try52 and Cys44 lining in the hydrophobic channel within a distance of 4Å. This is much similar to the hydrophobic contacts exhibited by OAP. The Glide score is –6.8 and the Glide energy is –47.0 Kcal/mol. This is also comparable to that of the co-crystallized ligand (OAP). The OAP was re-docked nearby the active site and the orientation was such that, the C(11) atom was displaced to a distance of 1.9 Å away, the phenyl ring attached to the S(11) was twisted about 72.9° down and the O(21) atom was displaced by 1.6Å away. The calcium ion in this re-docked complex was in the characteristic pentagonal bipyramidal configuration as that of the PDB complex. The OAP was bound near the binding site and coordinated with the calcium ion at a distance of 2.28Å. The oxygen atoms of His28, Gly30, Gly32, and Asp49 (bifurcated) are also involved in chelation with calcium ion. In addition to the direct coordination with calcium, the carbonyl oxygen O(32) atom of amide group of OAP is also involved in a water (HOH1260) mediated interaction with Ca2+ ion, which was not found in the original PDB complex (Figure 5b). This water molecule was found to be displaced about 1.2 Å towards the calcium ion which has made it to make chelation with the calcium ion and H-bond interaction with the O(32) atom of OAP. The angle between the calcium ion and coordinating oxygen atoms of OAP and water molecule is about 68.8°. The angle between the calcium ion and the chelating oxygen atoms of His28 and OAP was 145.4°. The corresponding angle was found to be 177° in the PDB complex. The difference in this angle in the re-docked complex may be due to the inclusion of water molecule within the channel and the interactive force exerted by it made the oxygen atom of His28 to significantly move about 0.7 Å when compared to the PDB complex. The chelation exhibited by the O(21) was not found in the re-docked complex due to the significant displacement, and it only had an H-bond with the N atom of the His48 as that of the PDB complex. The coordinating oxygen atoms of the amide carbonyl group of OAP and Gly30, Gly32 and Asp49 were on a near equatorial plane. O(32) atom also had an H-bond interaction with the N atom of Gly32. The O(31) atom of the carbonyl group was also found to have H-bond interaction with the N(ε) atom of Lys69. The re-docked pose of OAP also maintained the array of hydrophobic contacts with Cys27, Tyr22, Ala18, Ile9, His6, Phe5, Phe24, Gly23, Val31, Leu2, Try52 and Cys44 like that of the PDB complex. The Glide energy and G-score of the re-docked complex were –65.6 Kcal/mol and –8.2, respectively (Table 3, see supplementary material).
Figure 5.
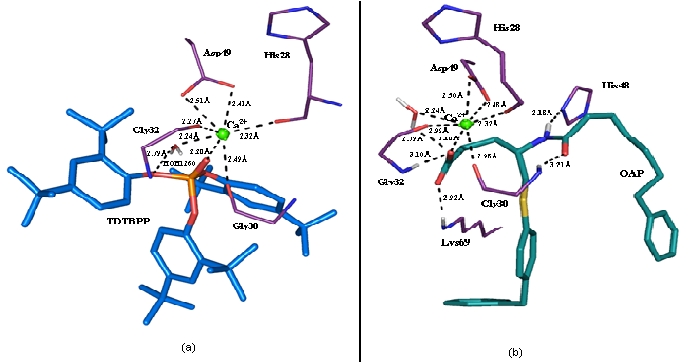
(a) The co-ordinations formed by Ca2+ ion with the oxygen atom of TDTBPP and other active site residues of 1KVO are represented in black dotted lines with their corresponding bond distances (b) The co-ordinations formed by Ca2+ ion with OAP and the active site residues of 1KVO.
Conclusion:
Literature survey shows the anti-inflammatory property of various parts of V. negundo. Recent literature survey [43] indicates that V. negundo is an antidote for D. russelli bites. The molecular docking with 3H1X and 1KVO confirms that TDTBPP can bind both the calcium independent as well as calcium dependent forms of sPLA2s. Hence Tris (2, 4-di-tert-butylphenyl) phosphate (TDTBPP) can be co-crystallized with the sPLA2 and the in vitro binding mode and energy with the protein could be revealed. It may be a good start to use novel class of compounds from natural sources in treating inflammatory disorders and snake bites.
Supplementary material
Acknowledgments
This work is supported by Indian council of Medical Research (ICMR), Ministry of Health and Human Welfare, Government of India. The authors thank UGC, Government of India for providing financial support under SAP to CAS in Crystallography and Biophysics, CAS in Marine Biology, Annamalai University for carrying out Animal model studies and Bioinformatics Infrastructure Facility (DBT-BIF), University of Madras for providing computational facilities.
Footnotes
Citation:Vinuchakkaravarthy et al, Bioinformation 7(4): 199-206 (2011)
References
- 1.YW Chin, et al. APPS J. 2006;8:E239. [Google Scholar]
- 2.MG Dharmasiri, et al. J Ethnopharmacol. 2003;87:199. doi: 10.1016/s0378-8741(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 3.Amritpal Singh, et al. Rev International J Integrative Biology. 2008;3(1):57. [Google Scholar]
- 4.RR Kulkarni, et al. Indian J Pharm Sci. 2008;70:838. doi: 10.4103/0250-474X.49140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azar-Ul-Haq, et al. Phytomedicine. 2006;13:255. doi: 10.1016/j.phymed.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.GC Jagetia, MS Baliga. J Med Food. 2004;7:343. doi: 10.1089/jmf.2004.7.343. [DOI] [PubMed] [Google Scholar]
- 7.TC J Munasinghe, et al. Phytother Res. 2001;15:519. doi: 10.1002/ptr.994. [DOI] [PubMed] [Google Scholar]
- 8.M Gupta, et al. Indian J Exp-Biol. 1999;37:143. [PubMed] [Google Scholar]
- 9.Y Avadhoot, AC Rana. Arch Pharma Res. 1991;14:96. [Google Scholar]
- 10.R Perumal Samy, et al. J Ethnopharmacol. 1998;62:173. [Google Scholar]
- 11.M Damayanti, et al. Cytobios. 1996;86:155. [PubMed] [Google Scholar]
- 12.E Pushpalatha, J Muthukrishnan. Indian J Malariol. 1995;32:14. [PubMed] [Google Scholar]
- 13.SK Bhargava. J Ethnopharmacol. 1989;27:327. doi: 10.1016/0378-8741(89)90007-x. [DOI] [PubMed] [Google Scholar]
- 14.DS Hebbalkar, et al. Indian J Med Res. 1992;95:200. [PubMed] [Google Scholar]
- 15.RS Telang, et al. Indian J Pharmacol. 1999;31:363. [Google Scholar]
- 16.VR Tandon, RK Gupta. Indian J Med Res. 2006;124:447. [PubMed] [Google Scholar]
- 17.MI Alam, A Gomes. J Ethnopharmacol. 2003;86:75. doi: 10.1016/s0378-8741(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 18.Vinuchakkaravarthy, et al. Acta Cryst E. 2010;66:o2207. doi: 10.1107/S1600536810029673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EA Dennis, et al. FASEB J. 1991;5:2068. [Google Scholar]
- 20.W Pruzanski, P Vadas. Immunol Today. 1991;12:143. doi: 10.1016/S0167-5699(05)80042-8. [DOI] [PubMed] [Google Scholar]
- 21.P Needleman, et al. Annu Rev Biochem. 1986;55:69. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 22.AB Mukherjee, et al. Biochem Pharmacol. 1994;48:1. [Google Scholar]
- 23.HM Verheij, et al. Rev Physiol Biochem Pharmacol. 1981;91:91. [Google Scholar]
- 24.CJ Van den Bergh, et al. J Cell Biochem. 1989;39:379. doi: 10.1002/jcb.240390404. [DOI] [PubMed] [Google Scholar]
- 25.RM Kini, et al. J. Biol. Chem. 1987;262:14402. [PubMed] [Google Scholar]
- 26.A Huwiler, et al. Biochim Biophys Acta. 1997;1348:257. doi: 10.1016/s0005-2760(97)00073-8. [DOI] [PubMed] [Google Scholar]
- 27.Nagendra Singh, et al. J Mol Recognit. 2009;22:437. doi: 10.1002/jmr.960. [DOI] [PubMed] [Google Scholar]
- 28.SS Cha, et al. J Med Chem. 1996;39:3878. doi: 10.1021/jm960502g. [DOI] [PubMed] [Google Scholar]
- 29.CW Dunnet. Biometrics. 1964;20:482. [Google Scholar]
- 30.RA Friesner, et al. J Med Chem. 2004;47:1739. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 31.W Sherman, et al. J Med Chem. 2006;49:534. [Google Scholar]
- 32.CA Winter, et al. Proc Soc Exp Biol. 1962;111:544. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 33.R Vinegar, et al. J Pharmacol Exp Ther. 1969;166:96. [PubMed] [Google Scholar]
- 34.A Dray, M Perkin. Trends Neurosci. 1993;16:99. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- 35.J Castro, et al. Life Sci. 1968;7:129. [Google Scholar]
- 36.M Di-Rosa, et al. J Pathol. 1971;104:15. [Google Scholar]
- 37.RM Kini, HJ Evans. J Biol Chem. 1987;262:14402. [PubMed] [Google Scholar]
- 38.V Chandra, et al. Acta Crystallogr D Biol Crystallogr. 2001;57:1793. doi: 10.1107/s0907444901014524. [DOI] [PubMed] [Google Scholar]
- 39.MJW Janssen, et al. Protein Eng. 1999;12:497. doi: 10.1093/protein/12.6.497. [DOI] [PubMed] [Google Scholar]
- 40.RM Kramer, et al. J Biol Chem. 1989;264:5768. [Google Scholar]
- 41.JJ Seilhamer, et al. J Biol Chem. 1989;264:5335. [Google Scholar]
- 42.T Nevalainen. Clin Chem. 1993;39:2453. [PubMed] [Google Scholar]
- 43.EE Sánchez, A Rodríguez-Acosta. Immunopharmacol Immunotoxicol. 2008;30:647. doi: 10.1080/08923970802279019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


