Abstract
The use of monoclonal antibodies (mAbs) has become a general approach for specifically targeting and treating human disease. In oncology, the therapeutic utility of mAbs is usually evaluated in the context of treatment with standard of care, as well as other small molecule targeted therapies. Many anti-cancer antibody modalities have achieved validation, including the targeting of growth factor and angiogenesis pathways, the induction of tumor cell killing or apoptosis and the blocking of immune inhibitory mechanisms to stimulate anti-tumor responses. But, as with other targeted therapies, few antibodies are curative because of biological complexities that underlie tumor formation and redundancies in molecular pathways that enable tumors to adapt and show resistance to treatment. This review discusses the combinations of antibody therapeutics that are emerging to improve efficacy and durability within a specific biological mechanism (e.g., immunomodulation or the inhibition of angiogenesis) and across multiple biological pathways (e.g., inhibition of tumor growth and induction of tumor cell apoptosis).
Key words: antibody combination, receptor tyrosine kinase, angiogenesis, immunomodulation, apoptosis, CD20
Introduction
For the past two decades, most antibody therapeutic programs have focused on the generation and development of single monoclonal antibodies (mAbs) for various disease indications. The ability to robustly produce single mAbs has become widespread across the industry, resulting in >150 mAbs in clinical trials in 2010 for various indications.1 To date, there are fewer than a dozen approved mAbs for cancer, but many of these have been exceptionally successful commercially despite the fact that most provide modest average long-term improvements in the progression-free survival of cancer patients. The limited efficacy of many directed therapeutics, including small molecules and proteins/mAbs, presents an overarching challenge to academic and industrial scientists to identify novel therapeutics with enhanced potency and improved durability—particularly in oncology.
While targeted therapies have incredible potential for modifying specific disease mechanisms, they often fall short of their goal of being truly disease modifying because of redundancies and checkpoints that exist naturally within our cellular and physiological systems. Knowledge of tumor biology, including the many mechanisms of tumor cell growth, survival, immune evasion, angiogenesis and metastasis has grown substantially over the past 20 years and has led researchers to integrate combinations of targeted therapeutics to bridge mechanistic or synergistic opportunities that may bring enhanced or more durable efficacy to patients. Figure 1 illustrates many of the most validated antibody targets in oncology that are being considered for combination therapy.
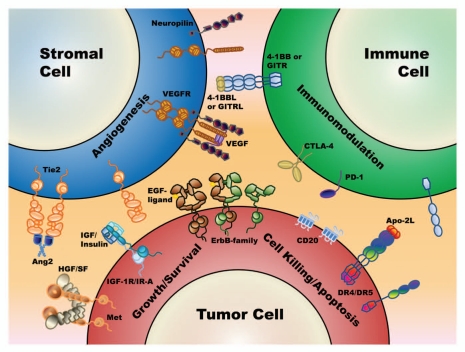
Figure 1.
A schematic diagram of the major antigens and cell types where mAb combinations are being evaluated. These include the direct targeting of tumor cell antigens for reducing tumor growth/survival (receptor tyrosine kinases such as cMet, IGF-1R and the ErbB family members) and the direct targeting of tumor cell antigens for inducing intrinsic (death receptors, CD20) and extrinsic (CD20) mechanisms of tumor cell killing. Also included is the targeting of the tumor microenvironment and tumor stroma, such as the VEGF/VEGFR and the Ang2/Tie2 pathways for halting tumor angiogenesis. Finally, also illustrated is the targeting of cell surface antigens (e.g., CTLA-4, PD-1) on lymphocytes to enable a patient to overcome or reverse tumor-induced suppression of their own natural immune surveillance for abnormal cell growth (also known as immunomodulatory approaches).
mAb therapeutics now represent a large proportion of new investigational drugs; however, they are still relatively new, with most having entered the clinic only in the last decade. Thus, even with the dramatic increase in the clinical evaluation of mAb therapeutics, the use of combinations of mAbs to treat disease has not, until recently, been widely reported. However, the number of publications describing mAb combinations, particularly in oncology, has increased substantially over the past two years (Fig. 2). While many other drug combinations that represent both new and old paradigms are also being evaluated, this article will focus strictly on mAb combinations that are currently under investigation in oncology. These combinations commonly target cell-surface receptors involved in tumor cell growth, angiogenesis, apoptosis or cell killing, or immunomodulation, and may include mAbs that target the same or different antigens. Rationale for selection of the various mAb combinations is discussed in each case.
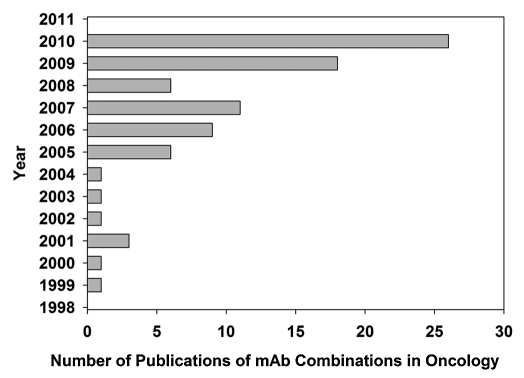
Figure 2.
Bar diagram of the escalation in mAb combination publications over the last decade. The publication numbers came directly from our bibliography and not from specific key word searches within PubMed.
mAb Combinations Targeting Receptor Tyrosine Kinases
Receptor tyrosine kinases (RTKs) are cell-surface proteins with intrinsic kinase activity that respond to extracellular signals via ligand binding and influence intracellular signaling cascades. They regulate a variety of cellular processes such as cell growth, differentiation, metabolism and migration. Many RTKs are growth factor receptors that play critical roles in the development and progression of human cancers and, therefore, are attractive targets for intervention in cancer therapy using either small molecule kinase inhibitors or antagonistic mAbs. Several mAbs (cetuximab, panitumumab, trastuzumab) and small molecule kinase inhibitors (erlotinib, gefinitib, lapatinib) targeting the epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) are approved in the US and other countries for treating breast, colorectal (CRC), non-small cell lung (NSCLC) and head and neck cancers. RTK cross-talk or co-activation is a process by which cancer cells simultaneously activate two or more RTKs to attain network robustness and increase the diversity of signaling outcomes that can be achieved using a limited repertoire of intracellular signaling components.2 Such crosstalk often limits the effectiveness of agents targeting a single RTK and results in tumor resistance to therapy. One approach being pursued to improve the efficacy of cancer therapy is the rational combination of mAbs specific for different RTKs.
HER/ErbB family.
Both EGFR and HER2 belong to the HER/ErbB family of RTKs, which also includes HER3 and HER4. HER activation upon ligand binding coupled with receptor dimerization affects multiple downstream signaling transduction pathways (e.g., PI3K/Akt, Ras/Raf/MAPK, JAK/STAT) that regulate cell proliferation, survival and apoptosis. These proteins are often expressed (or overexpressed) in various tumor types and help mediate the tumorigenic phenotype.3 For example, EGFR and HER2 are expressed in a significant percentage of pancreatic carcinoma cases (45–95% for EGFR and 43–69% for HER2), although the levels of HER2 are low in the majority of tumors. Marketed therapeutics targeting EGFR (e.g., cetuximab, panitumumab) or HER2 (e.g., trastuzumab) are often efficacious in a fraction of cancer patients, and many patient tumors eventually acquire resistance to treatment. Preclinical studies indicate that tumor resistance to an inhibitor of one HER2 family member is often attributable to compensatory upregulation of other HER members.4 For example, human breast cancer BT-474 cells selected for resistance to the anti-HER2 trastuzumab in vivo were found to overexpress EGFR and ErbB ligands and exhibit higher levels of EGFR/HER2 heterodimers and increased sensitivity to EGFR inhibitors.5 HER3 plays a central role in HER2-overexpressing breast tumors and can be involved in resistance to trastuzumab.6,7
Combination mAb therapies consisting of inhibitors of multiple HER family members are being explored. In preclinical studies, the combination of inhibitory EGFR and HER2 mAbs reduced in vitro cell proliferation or survival to a greater extent than single mAbs in ovarian,8 esophageal squamous cell carcinoma,9 CRC10 and cervical cancer cell lines.11 Long-term trastuzumab exposure of human breast carcinoma cell lines induced HER reprogramming, increased expression of EGFR and HER3 and sensitized these cells to EGFR inhibitors gefitinib and cetuximab or an inhibitory HER3 mAb.12 Tumor cells developing acquired resistance to cetuximab have exhibited strong activation of HER2, HER3 and cMET and increased EGFR dimerization with HER2 and HER3 leading to their transactivation. Inhibition of HER2 with mAb 2C4 or HER3 knockdown both sensitized these cells to cetuximab.4 In vivo therapeutic synergism of trastuzumab and matuzumab (an inhibitory anti-EGFR mAb) was observed in one ovarian and two pancreatic xenograft models.13 A de novo lung cancer model induced by the activating EGFR T790M-L858R mutations rapidly became resistant to cetuximab accompanied by the activation of HER3. Concomitant cetuximab treatment with the anti-HER3 mAb MM-121 resulted in a sustained and durable response in this model.14 Taken together, these findings suggest a rationale for the clinical evaluation of combinatorial anti-HER targeting approaches in tumors resistant to single receptor targeting.
Currently, the efficacy and safety of combined therapies with two mAbs targeting EGFR, HER2 or HER3 are being evaluated in patients with pancreatic cancer, CRC and breast cancers in Phase 1 or 2 studies (Table 1).
Table 1.
RTK, angiogenesis and RTK/angiogenesis mAb combinations in clinical trials
| mAb pair | Targets | Indication | Study phase |
| RTK/RTK | |||
| Cetuximab/Trastuzumaba | EGFR/HER2 | Metastatic pancreatic cancer | 1/2 |
| Cetuximab/Trastuzumab | EGFR/HER2 | HER2+ metastatic breast cancer | 1 |
| Cetuximab/Pertuzumab | EGFR/HER2 | Advanced or metastatic CRC | 1/2 |
| Trastuzumab/MM-111 | HER2/HER3 | Advanced HER2+ breast cancer | 1/2 |
| Cetuximab/IMC-A12 | EGFR/IGF-1R | Metastatic CRC | 2 |
| Cetuximab/IMC-A12 | EGFR/IGF-1R | Metastatic HNSCC | 2 |
| Panitumumab/AMG479 | EGFR/IGF-1R | KRAS wild-type metastatic CRC | 2 |
| Panitumumab/AMG102 | EGFR/cMet | KRAS wild-type metastatic CRC | 2 |
| Angiogenesis/Angiogenesis | |||
| Bevacizumab/MNRP1685Aa | VEGF/-Neuropilin-1 | Advanced or metastatic solid tumors | 1b |
| Bevacizumab/AMG102 | VEGF/HGF | Recurrent malignant glioma | 2 |
| Bevacizumab/Etaracizumab | VEGF/αvβ3 | Unresectable or metastatic kidney cancer | 1/2 |
| Bevacizumab/CNTO95 | VEGF/αvβ5 | Advanced solid tumors | 1 |
| Bevacizumab/AMG386 | VEGF/Ang1/2 | Metastatic CRC | 2 |
| Bevacizumab/AMG386 | VEGF/Ang1/2 | HER2− breast cancer | 2 |
| Bevacizumab/AMG386 | VEGF/Ang1/2 | Recurrent glioblastoma | 2 |
| Angiogenesis/RTK | |||
| Bevacizumab/Cetuximaba,b | VEGF/EGFR | Many trials in several indications | 1/2/3 |
| Bevacizumab/Trastuzumaba,b | VEGF/HER2 | Primarily HER2+ metastatic breast cancer | 1/2/3 |
| Bevacizumab/Pertuzumaba | VEGF/HER2 | Neuroendocrine cancer | 2 |
| Bevacizumab/MetMAb | VEGF/cMet | Advanced or metastatic solid tumors | 1 |
| Combinations Against Single Target | |||
| Trastuzumab/Pertuzumab | HER2/HER2 | HER2+ metastatic breast cancer | 3 |
| Trastuzumab/Pertuzumaba | HER2/HER2 | HER2+ Metastatic breast cancer | 3 |
| Trastuzumab/Pertuzumab | HER2/HER2 | HER2+ advanced or metastatic CRC | 1/2 |
| Trastuzumab/Pertuzumaba | HER2/HER2 | HER2+ breast cancer | 2 |
| Sym004 | EGFR/EGFR | Advanced solid tumors | 1 |
Combination with standard of care, chemotherapy or small molecule agent.
Over a dozen ongoing trials in various oncology indications. Note: As of April 2011, bevacizumab, cetuximab, panitumumab and trastuzumab were approved for marketing.
The type I insulin-like growth factor receptor (IGF-1R).
The IGF/IGF-1R axis has been known to affect tumor growth, survival and metastases of various tumor types for many years, and levels of the ligand and its binding proteins have been correlated with enhanced risk factors for neoplastic development.15,16 The most advanced inhibitory anti-IGF-1R mAbs in the clinic are figitumumab17 and IMC-A12;18 however, a host of additional inhibitory mAbs with varying mechanisms of action (MOAs) have joined these entities in clinical oncology trials.15,19 EGFR and IGF-1R pathways can crosstalk with each other at different levels through association at the membrane surface, induced ligand expression and downstream feedback mechanisms, and they often cooperate to promote tumor growth and progression.20,21 Preclinical studies indicate that IGF-1R and HER signaling may mediate resistance to one another in tumor models and numerous preclinical studies have reported that dual inhibition of a HER family member and IGF-1R can improve anti-tumor activity and overcome this resistance.22,23 Most preclinical drug combination studies in this area have focused on small molecule ErbB inhibitors. However, IGF-1R and EGFR mAb inhibitors in combination have been shown to significantly decrease tumor growth and survival in various in vivo models of NSCLC, cutaneous squamous cell carcinoma, pancreatic and CRC compared with the single molecules alone.24–26
Clinical trials investigating the safety and efficacy of anti-EGFR and anti-IGF-1R mAb combinations are ongoing (Table 1). Recently, a randomized Phase 2 study of IMC-A12 (IGF-1R) with cetuximab (EGFR) in patients with metastatic CRC resulted in no improvements in anti-tumor activity in this patient population,27 suggesting that patient selection and selective therapy may be critical to the clinical success of these combinations. Indeed, a recent study reported results from a patient with head and neck squamous cell carcinoma (HNSCC) who progressed on multiple cytotoxic agents, cetuximab and the anti-IGF-1R mAb AMG-479, but achieved a complete response to methotrexate, suggesting that the combination of cetuximab and AMG479 may have sensitized the tumor.28 The results of ongoing and future trials will be important to determine the relevance of treatments combining HER-family and IGF-1R inhibitors.
MET/RON.
MET and RON belong to the c-MET RTK family. Both the binding of the macrophage-stimulating protein to RON and hepatocyte growth factor (HGF) to MET promote cell proliferation, survival, migration and morphogenesis—collectively known as invasive growth. Altered expression or activation of MET or RON have been well-documented in several human cancers. Both receptor pathways have been implicated in the progression and metastasis of tumors. Various approaches have been taken to target MET, and to a lesser extent RON, for cancer therapy. Small molecules inhibiting MET kinase activity and mAbs blocking receptor-ligand interaction, including a one-armed anti-MET antibody (MetMAb), two HGF antagonistic mAbs (AMG102, TK701), and a RON neutralizing mAb (IMC-41A10) have shown promising anti-tumor activity in preclinical studies, and several of these therapeutic candidates have progressed into clinical studies.29
There are many compelling reasons to co-target the MET and HER pathway because overexpression or ligand-mediated activation of the cMET pathway is a well-documented mechanism of resistance to HER member inhibition.4 Concomitantly, the inhibitory anti-RON mAb IMC-41A10 in combination with the anti-EGFR cetuximab induced pancreatic tumor regression while each single agent could only slow tumor growth in preclinical studies.30 Numerous preclinical reports have indicated that treatment with MET or HGF inhibitors (both mAb and small molecule) can overcome resistance to small molecule tyrosine kinase inhibitors (TKIs) directed at EGFR.31–34 Conversely, EGFR or HER3 activation has been implicated in resistance to a MET TKI in gastric cancer cells, providing additional rationale for combined targeting of both receptor pathways.35 One ongoing clinical study is testing the efficacy of co-administration of a mAb combination (anti-EGFR panitumumab and anti-HGF AMG102) targeting these pathways (Table 1).
mAb Combinations Targeting Angiogenesis
Angiogenesis, the formation of new blood vessels sprouting from preexisting ones, is essential for the growth and metastasis of most tumors. Anti-angiogenic therapy is a widely pursued and validated strategy for treating human cancers. Vascular endothelial growth factor (VEGF-A, also known as VEGF) and its receptor VEGFR2 play a central role in promoting angiogenesis, and VEGF is often overexpressed in many tumors and correlated with advanced disease and poor prognosis.36,37 Three VEGF pathway inhibitors, bevacizumab, a humanized mAb specific for VEGF-A, and sunitinib and sorafinib, small molecule inhibitors with activity against VEGFR2, have been approved as therapy for advanced cancers. Bevacizumab is approved for treatment of mCRC, NSCLC and recurrent glioblastoma. Clinical trials of bevacizumab are underway in many other indications. There is growing interest in elucidating the mechanisms of resistance to anti-VEGF therapies and developing other anti-angiogenic targeted therapeutics alone or in combination with anti-VEGF agents.
VEGF pathway.
VEGF-A belongs to a family of growth factors that also includes VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF). VEGF-A binds to two RTKs, VEGFR1 (Flt-1) and VEGFR-2 (KDR/Flk-1). VEGFR2 is predominantly expressed on endothelial cells where it is the main mediator of VEGF effects, including cell survival, proliferation, migration and permeability. The roles of VEGFR1 and its additional ligands VEGF-B and PlGF in angiogenesis are complex, but they are reported to have both pro-oncogenic and pro-metastatic functionality.38 VEGF-C and -D predominantly bind and activate VEGFR3, which plays a key role in lymphoangiogenesis.39 VEGF ligands also interact with neuropilin-1 and neuropilin-2, which were originally discovered as axon guidance molecules mediating neuronal development and later shown to play critical roles in vascular development, serving as co-receptors for VEGFRs and modulating their signaling. The neuropilins may also have VEGFR-independent effects on tumor angiogenesis/lymphangiogenesis.40 In addition to targeting VEGF with bevacizumab, many other molecules that intervene in the pathway are being tested for their efficacy in various cancers. MAbs inhibiting VEGFR2 (IMC-1121B/ramucirumab) and VEGFR1 (IMC-18F1), VEGFR3 (IMC-3C5), neuropilin-1 (MNRP1685A), PlGF (TB-403) have all progressed into clinical studies.
Combinations of VEGF-pathway inhibitors are now being evaluated. Preclinical studies have shown that mAb combinations against VEGFR2 and VEGFR3 resulted in additive inhibition of angiogenesis and tumor growth in multiple models, and the combination appeared to more potently decrease lymph node and lung metastasis.41,42 Some anti-PlGF mAbs have shown activity in blocking angiogenesis and slowing tumor growth, and resulted in additive anti-tumor activity in combination with an anti-VEGFR2 mAb,43,44 while others have shown no additive effects by combining the two agents.45 Anti-neuropilin-1 mAbs demonstrated an additive effect with anti-VEGF therapy in reducing vascular density and tumor growth. The neuropilin-1 mAbs reduced vessel remodeling and pericyte association in tumors rendering them more susceptible to anti-VEGF therapy.46 A Phase 1b study of the safety and pharmacology of the anti-neuropilin-1 antibody, MNRP1685A, in combination with bevacizumab with or without paclitaxel in patients with locally advanced or metastatic solid tumors was initiated recently (Table 1).
Combinations inhibiting both VEGF-pathway and non-VEGF-pathway angiogenesis.
Angiogenic pathways that do not rely on VEGF may develop as tumors progress; thus tumors that are initially sensitive can develop resistance to anti-VGEF therapy. Some of the targets in these alternative angiogenic pathways and the relevant mAb combinations with VEGF-pathway inhibitors that are being assessed are discussed below.
The human angiopoietin family consists of Ang1, Ang2 and Ang4, all of which bind to the endothelial receptor tyrosine kinase Tie-2. Ang1 is tightly regulated, and either overexpression or inactivity can have deleterious effects on vascular physiology.47 Ang2 is a positive regulator of neovessel formation, is less widely expressed, and is often found upregulated in tumors, making it a much more tractable oncology target.47,48 At least six Ang2 antagonists are in the clinic, including human Ang2 inhibitory mAbs REGN910 and MEDI-3617, Ang1/2 neutralizing peptibodies AMG386 and AMG780, CovX-body CVX-60 and CVX-241, which also targets VEGF.48 Ang-2 is known to enhance the sensitivity of vessels to VEGF and stimulate new vessel sprouting in the presence of VEGF while promoting vessel regression if the activity of VEGF is inhibited.47 The combination of Ang2 inhibitors (AMG386, CVX-60, 3.19.3) with VEGF inhibitors (bevacizumab, DC101, TKIs) led to greater efficacy than each single inhibitor alone in preclinical models.48–50 Currently, the safety and efficacy of the most advanced Ang1/2 inhibitor AMG386 in combination with bevacizumab is being investigated in clinical trials in patients with mCRC, breast cancer and glioma.
Integrins are a large family of heterodimeric receptors consisting of α- and β-subunits that bind extracellular matrix proteins whose roles in regulating tumor cell adhesion, survival, proliferation, migration and invasion, and angiogenesis have been reviewed in references 51 and 52. The αvβ3, αvβ5 and α5β1 integrins, in particular, can also be expressed in tumor vasculature and play an important role in angiogenesis. Three mAbs, etaracizumab (MEDI-522, Vitaxin, anti-αvβ3), CNTO95 (anti-αvβ5) and volociximab (anti-α5β1 mAb) have all demonstrated anti-angiogenic and anti-tumor activity in preclinical models, and all three are in various clinical trials in oncology.52,53 In transgenic mice lacking the β3 gene, tumor angiogenesis can be compensated by VEGF-pathway activation.52 The combination of etaracizumab (anti-αvβ3) and bevacizumab (anti-VEGF) was evaluated in preclinical studies and more effectively inhibited tumor growth than either mAb alone in an orthotopic ovarian cancer model.54 Combinations of bevacizumab with CNTO95 or etaracizumab are being evaluated in early-stage clinical studies (Table 1).
In addition to its contribution to invasive tumor growth, HGF/MET signaling is also known to promote angiogenesis.55 Co-administration of HGF and VEGF synergistically promote new blood vessel formation, and HGF induces VEGF expression while downregulating tumor cell expression of thrombospondin-1 (TSP1), a negative regulator of angiogenesis.56 The combination of anti-HGF AMG102 with bevacizumab was well-tolerated in patients with advanced solid tumors, and an encouraging proportion of patients achieved stable disease lasting ≥16 weeks in a Phase 1b study.57 Currently, a Phase 2 study is ongoing to evaluate the efficacy and safety of AMG102 and bevacizumab in subjects with recurrent malignant glioma (Table 1).
Platelet-derived growth factors (PDGFs) are mitogens for mesenchymally-derived cells. They signal through two RTKs, PDGFRα and PDGFRβ. PDGFRβ, which is normally expressed on connective tissue cells, is upregulated in most solid tumors, and the paracrine signaling between endothelial cell-derived PDGF-B (ligand) and PDGFRβ expressed on pericytes plays a key role in tumor angiogenesis by enhancing pericyte recruitment and vessel maturation. Human mAbs directed against PGFRβ have been reported to enhance the anti-angiogenic and anti-tumor activity of the anti-VEGFR2 mAb DC101 in several human xenograft models.58,59 There are currently no clinical trials testing inhibitory anti-PDGFRβ mAbs either alone or in combination with anti-VEGF-pathway mAbs. However, the clinical success of sunitinib is largely accredited to its multi-specificity and potent inhibitory activity of both VEGFR and PDGFR because a more specific VEGFR inhibitor was not effective, validating the use of dual inhibition of VEGFR and PDGFRβ in anti-angiogenesis therapy.
Both the TAM and Eph family receptors have been shown to play important roles in tumor progression, metastasis and angiogenesis.60,61 Among them, Axl (TAMR) and EphB4 play critical roles in tumorigenesis and angiogenesis.61,62 In A549 and MDA-MB-231 tumor models, an anti-Axl mAb was shown to inhibit tumor xenograft growth and reduce tumor vascular density.63 The combination of anti-Axl and anti-VEGF reduced tumor growth and vascular density to a greater extent than either antibody alone. mAbs against EphB4 have also shown enhanced antitumor activity and induction of tumor regression in combination with bevacizumab in preclinical studies.64
Activin receptor-like kinase 1 (ALK1) is a type-I endothelial cell-specific member of the TGFβ receptor superfamily. It plays a critical role in vascular development, remodeling and pathologic angiogenesis. Recently, it was reported that an inhibitory mAb (PF03446962) inhibited tumor growth by attenuating vessel angiogenesis and demonstrated anti-tumor activity in a melanoma model with acquired resistance to a VEGFR kinase inhibitor. The combination of anti-ALK1 and bevacizumab displayed enhanced anti-tumor activity in a human/mouse chimera model, suggesting that anti-ALK1 may represent a novel approach complementary to anti-VEGF therapy.65
Delta-like 4 (DLL4) is an endothelial-selective Notch ligand and is required for embryonic vascular development and arteriogenesis.66 It is overexpressed on tumor vasculature and inhibition of DLL4 leads to hyperproliferation, increased non-productive tumor vascularization and reduced tumor growth in several models.67 The VEGF pathway interplays at several levels with DLL4/Notch signaling in tumor angiogenesis.68,69 DLL4 also contributes to stem cell renewal, and anti-DLL4 may reduce tumor-initiating cell frequency in breast and colorectal tumors.70,71 The combination of anti-DLL4 and anti-VEGF mAbs yielded a more potent anti-tumor response than either antibody alone.72 Anti-DLL4 mAbs have entered clinical studies; however, chronic DLL4-blockade can abnormally activate endothelial cells causing pathological changes in multiple organs, which has raised safety concerns in ongoing trials targeting DLL4.73
Anti-RTK and Anti-Angiogenesis mAb Combinations
The most intensely investigated targeted therapy combinations have been directed towards the VEGF(R)/EGFR pathways particularly in NSCLC, breast cancer and CRC—with such extensive activity in this area that the combinations themselves have been reviewed in references 74–77. In preclinical studies, simultaneous blockade of EGFR and VEGFR2 with C225 and DC101, respectively, demonstrated enhanced anti-tumor activity in xenograft models of CRC, gastric cancer, glioma, pancreatic cancer and squamous cell carcinoma.78–83 Despite the promising results from dual inhibition of VEGF pathway and EGFR in preclinical models, many of the clinical trials have yielded disappointing results that indicate no added benefit or increased toxicity.84–86 In particular, two Phase 3 trials (PACEE and CAIRO2) that evaluated the combination of panitumumab or cetuximab with chemotherapy plus bevacizumab both showed a detrimental effect when the combination was added to first-line treatment for mCRC.87,88 Despite these setbacks, evaluations of the combination of cetuximab and bevacizumab are still underway in over a dozen clinical studies in patients with various stages of HNSCC, CRC, NSCLC, liver cancer and glioma to determine if the combination may provide benefits in specific patient populations and indications (Table 1).
Other RTK combinations with bevacizumab could include anti-HER2 and anti-IGF-1R inhibitors. Currently, trastuzumab is the only anti-RTK antibody approved for HER-2-positive breast cancer. Little has been published regarding the combination with a VEGF pathway inhibitor such as bevacizumab in this patient population.89,90 Still, the combination of trastuzumab and bevacizumab is under evaluation in several clinical trials (Table 1). IGF-1R activation can upregulate VEGF expression and promote vascular formation in tumors. The combination of adenoviral-induced dominant-negative IGF-1R and bevacizumab was highly effective in treating gastric tumor cell lines.91 No IGF-1R/VEGF pathway mAb combinations are being evaluated in clinical studies.
mAb Combinations Targeting a Single RTK
There is a perception that therapeutic mAbs (and small molecules) fully neutralize their targets when given as single agents. Historical studies with polyclonal antibodies against various oncology targets such as fibroblast-like growth factor 2 (FGF2, also known as basic FGF),92,93 have shown that there is merit to the idea that mimicking the natural human immune response, which generates multiple antibodies that recognize numerous epitopes on foreign targets, may be a more effective approach than single mAb therapy. New studies with mAb combinations that utilize multiple MOAs to potentiate or attenuate the activity of a single target are now challenging the perception that single mAbs always provide a maximal effect on a single target.
EGFR.
Cetuximab and panitumumab are anti-EGFR mAbs approved for use in metastatic colorectal cancer patients with the wild-type KRAS gene. The mAbs bind overlapping epitopes on domain III of the receptor extracellular domain, enabling each of them to block ligand binding and ligand-mediated activation of EGFR.94 The existence of multiple inhibitory epitopes on EGFR that could be used to synergistically inhibit the receptor was first described 20 years ago,95 but was not extensively explored. Two inhibitory anti-EGFR antibodies, matuzumab and ch806, that inactivate the receptor using alternate molecular mechanisms and recognize different epitopes than cetuximab and panitumumab are being evaluated for their anti-tumor activities in clinical trials. While not a competitive EGF blocker, matuzumab binds domain III of EGFR near the EGF binding site and traps the receptor in its inactive conformation by inhibiting dimer formation.96,97 The ch806 antibody binds an isoform of EGFR missing exons 2–7 (de2-7 EGFR), which is often overexpressed on various human tumors and particularly in malignant gliomas, and recognizes a peptidic region near the C-terminus of domain II of EGFR.96–99
Recent combinations of inhibitory anti-EGFR mAbs with varying MOAs have led to compelling increases in anti-tumor activity. The ch806 antibody was combined with a cetuximab-like antibody, 528, leading to rapid receptor degradation, decreases in the anti-proliferative marker Ki-67 and enhanced anti-tumor activity in human tumor xenografts.100 Other combinations of inhibitory anti-EGFR antibodies with non-overlapping epitopes have also demonstrated significant increases in EGFR degradation.101,102 Matuzumab and cetuximab have been shown to lead to distinct inhibitory signaling patterns in A431 epidermal carcinoma cells, and when used in combination, the two mAbs synergistically inhibit ERK1/2 and AKT phosphorylation and lead to improved EGFR degradation.103 Dechant and coworkers also showed that the combination of matuzumab and cetuximab (but not panitumumab) resulted in synergistic increases in complement-dependent cytotoxicity (CDC) of tumor cells expressing EGFR in vitro with the addition of human sera.104 Whether these effects would be transient because of the reported increase in receptor degradation is unknown. Like matuzumab, another inhibitory antibody, EMD55900, was also shown to synergistically inhibit the growth of a breast tumor cell line in combination with cetuximab.105 At this time, Sym004, which is composed of an antibody mixture, is the only combination of inhibitory anti-EGFR antibodies undergoing evaluation in humans (Table 1).
HER2.
Unlike the marketed anti-EGFR mAbs, the anti-HER2 mAb trastuzumab, approved in HER2+ metastatic breast cancer, binds domain IV of the HER2 extracellular domain.106 The molecular mechanism by which it attenuates HER2 signaling is unclear, although trastuzumab does block the cleavage of the extracellular domain that leads to p95HER2, a truncated and constitutively hyperactive form, and can block artificially homodimerized HER2 signaling.107,108 However, trastuzumab does not block dimerization with other ErbB family members, and thus additional avenues may exist for potentiating its anti-HER2 activity. As with the anti-EGFR mAbs, other anti-HER2 mAbs with epitopes that do not overlap with that of trastuzumab have been discovered. One in particular, pertuzumab, which binds the central region of domain II of HER2 and blocks receptor homo/heterodimerization,109 is now in multiple clinical studies ranging from Phase 1–3 in various forms of cancer.110
Like EGFR, the first report of anti-HER2 mAb combinations exerting synergistic antagonistic effects on HER2-expressing tumors was reported over 20 years ago.111 In these studies with HER2 oncogene-transfected NIH3T3 cell xenografts, single anti-HER2 mAbs could provide a cytostatic effect, but only mAb combinations could eradicate the tumors.111 Preclinical data support the combination of pertuzumab and trastuzumab for the treatment of various HER2-positive cancers. Individually, both trastuzumab and pertuzumab demonstrated reasonable growth inhibitory properties on NCSLC and breast tumor xenografts; however, the combination led to regression and tumor remission.112 The trastuzumab/pertuzumab combination reduces ErbB family member heterodimerization and increases the level of tumor cell apoptosis,113 and both pertuzumab and trastuzumab can induce antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro.112 The combination of trastuzumab and pertuzumab was also being studied in combination with small molecule TKIs with the goal of completely shutting down tumor cell survival and growth pathways.114 The trastuzumab/pertuzumab combination is being actively pursued by Genentech for the treatment of metastatic breast cancer (Table 1) with encouraging activity reported in a Phase 2 study.115 The risk/benefit profile of long-term administration of the combination particularly regarding cardiac function is being evaluated in Phase 3 studies.116 How this combination performs in these studies may have a substantial and general affect on the development of mAb combinations against single targets in oncology in the future.
IGF-1R.
Results for antibody inhibitors of IGF-1R with varying epitopes and MOAs have been reported in the literature for decades.117–120 Recently, a combination of mAbs that both allosterically and competitively inhibit IGF-1R ligand binding was shown to enhance tumor cell growth inhibition over what could be achieved with the single mAbs.120,121 The inhibitory mAb combination was consolidated into a single bispecific antibody to enhance the effective concentration of each of the inhibitors and ease the manufacturing and clinical development path.122
Additional anti-cancer mAb combinations against single targets.
There is a paucity of other mAb combinations targeting single receptors. The proto-oncogene protein Met and its ligand HGF/SF have both been targeted using multiple inhibitory mAbs. HGF binds cMet with both a high-affinity and low-affinity binding site. Originally, complete blockade of HGF-driven cMet activation was identified using multiple mAbs that recognized different epitopes of HGF,123 although intensive screening has yielded new HGF mAbs with potentially similar inhibitory activity compared with these original mAb combinations.124–126 Met has been more difficult to target due to antibody-mediated agonism induced by bivalent crosslinking, but the ability of two anti-Met mAbs to synergistically inhibit tumor cell growth compared with the activity of the individual mAbs was reported in 2009.127 Tvorogov and coworkers reported in 2010 that two mAbs directed at VEGFR3, one that blocks ligand blocking and one that blocks receptor dimerization (like the cetuximab/ch806 mAbs directed against EGFR), can synergistically inhibit VEGF-C-mediated cell survival and vascular sprouting.128 Similar to the inhibitory IGF-1R antibody case, the competitive ligand inhibitor loses its single activity at high ligand concentrations, but synergistically inhibits VEGF-C-mediated cell survival when combined with the dimerization blocker.128
Apoptosis-Inducing mAbs Targeting Death Receptors
Substantial research efforts have been devoted to studying the ability of specific tumor necrosis factor receptor (TNFR) family members (e.g., TNFR1, FAS, DR3, DR4 and DR5) to induce tumor cell apoptosis through associations of their Fas-associated death domains (FADDs) with intracellular components that drive apoptosis.129,130 The paradoxical tumor cell killing versus growth activities of TNFR1 and FAS, as well as systemic toxicities that arise when they are agonized, has steered researchers toward DR4 and DR5 for cancer therapy.131–133 While an abundance of preclinical data has shown that agonizing DR4 or DR5 can effectively kill tumor cells, clinical studies with agonistic mAbs directed at these receptors have yet to show strong antitumor efficacy as single agents in human patients.133 However, the safety and tolerability profiles of these mAbs indicate that they may be useful in combination with other treatments. Initial clinical combinations are ones that sensitize tumor cells to TRAIL-R-mediated apoptosis, such as histone deacetylases that induce DR4 and DR5 expression or bortezomib, which inhibits the degradation of proapoptotic mitochondrial Bcl-2 proteins that facilitate DR4 and DR5 activity.133 Relevant mAb combinations targeting death receptors are currently all in preclinical development. In HER2-positive breast tumor cell lines and in spontaneous tumors that form in HER-2 transgenic mice, anti-HER2 and anti-DR5 mAbs each slowed tumor growth; however, the combination resulted in strong tumor regression.134 DR5 expression is generally high in breast cancer, and one hypothesis for synergism is that HER-2 inhibition reduces the levels of anti-apoptotic proteins such as Akt, c-FLIP and Bcl-XL,134 thus sensitizing cells to DR5. The combination of anti-DR4 mapatumumab with anti-CD20 rituximab also led to enhanced tumor growth inhibition in rituximab-resistant B-cell lymphoma xenograft models.135,136 Combining rituximab's extrinsic ADCC and CDC activities and the intrinsic apoptotic activity of Apo-2L (DR4- and DR5-ligand) to treat B-cell lymphoma xenographs resulted in greatly enhanced efficacy in multiple tumor models.137
Few combinations of mAbs targeting multiple TNFR-family members have been reported. Co-targeting of DR4 and DR5 has been interrogated using their common ligand Apo-2L. However, Marini and coworkers demonstrated enhanced antitumor activity in colorectal tumor cell lines in vitro using a combination of anti-DR4 and anti-DR5 mAbs, particularly after the cells had been sensitized by radiation.138 Combining the apoptotic activity of mAbs directed toward DR5 and LTβR, which lacks a FADD, also resulted in more potent cell killing across a broader group of tumor cell lines.139 Another example of combining antibodies to increase the efficacy of an agonistic anti-DR5 mAb is the TriMab approach, which combines the DR5-apoptosis mechanism with stimulation of cytotoxic T cells using agonistic antibodies against CD40 and CD137 to help eradicate tumors.140
Immunomodulatory mAb Combinations
The approval of the anti-cytotoxic T-lymphocyte antigen (CTLA)-4 ipilimumab (Yervoy®, Bristol-Myers Squibb) in March 2011 as a treatment for metastatic melanoma has spurred interest in combining immunomodulatory mAbs with other mAbs for cancer therapy. CTLA-4 is a member of the immunoglobulin superfamily that plays an important immune regulatory role.141,142 Binding of CTLA-4 to CD80 and CD86 on antigen-presenting dendritic and B cells regulates T-cell stimulation and attenuates self-reactive T-cell surveillance, including tumor monitoring.141 Ipilimumab blocks the activity of CTLA-4, thereby producing an active immune response against cancer cells.
In addition to the CTLA-4/CD80/CD86 axis, many other receptor-ligand pairs have been identified that contribute to the homeostasis of T-cell activation/suppression.143 A newly discovered and important co-inhibitory axis is that of the programmed death-1 (PD-1) receptor, expressed on activated T cells, along with its ligands PD-L1 and PD-L2, which lead to peripheral T-cell tolerance through anergy, apoptosis and exhaustion.144 Although CTLA-4 and PD-1 are both co-inhibitory and both bind CD80,145 the finding that they use distinct nonredundant intracellular signaling pathways to inhibit T cells has evoked interest in targeting the PD-1/PD-ligand interaction.146–148 The combination of anti-PD-1 and anti-CTLA-4 agents synergistically improved the ratio of activated T effector cells compared with regulatory T cells and myeloid suppressor cells in implanted tumors in mice, modifying the tumor environment from a suppressive to inflammatory environment.149–151 MDX-1106, an anti-PD-1 mAb, is currently being evaluated in patients with refractory or relapsed malignancies in Phase 1/2 clinical studies; preliminary results indicate promising clinical activity in renal cell carcinoma and melanoma and better safety profile compared with ipilimumab.152 A clinical study focused on the safety and efficacy of the ipilimumab and MDX-1106 combination in melanoma is underway (Table 2).
Table 2.
Immunomodulatory and B-cell targeted mAb combinations in clinical trials
| mAb paira | Targets | Indication | Study phase |
| Immunomodulatory | |||
| Ipilimumab/MDX-1106 | CTLA-4/PD-1 | Malignant melanoma | 1 |
| B cell | |||
| Rituximab/Alemtuzumabb | CD20/CD52 | Chronic lymphocytic leukemia | 2 |
| Rituxmiab/Alemtuzumab | CD20/CD52 | Diffuse large B-cell lymphoma and Hodgkin's lymphoma | 2 |
| Rituximab/Epratuzumab | CD20/CD22 | Follicular non-Hodgkin's lymphoma | 2 |
| Rituximab/Galiximab | CD20/CD80 | Non-Hodgkin's lymphoma | 2 |
| Veltuzumab/Milatuzumab | CD20/CD74 | Non-Hodgkin's lymphoma | 1/2 |
| B cell/Others | |||
| Rituximab/SGN-40 | CD20/CD40 | Non-Hodgkin's lymphoma | 1 |
| Rituximab/SGN-40 | CD20/CD40 | Diffuse large B-cell lymphoma | 2 |
| Rituximab/CT-011 | CD20/PD-1 | Follicular lymphoma | 2 |
| Rituximab/Siplizumab | CD20/CD2 | T-cell/NK cell non-Hodgkin's lymphoma | 1 |
| Rituximab/Bevacizumab | CD20/VEGF | Diffuse large B-cell lymphoma | 3 |
| Rituximab/Bevacizumab | CD20/VEGF | Chronic lymphocytic leukemia | 2 |
| Rituximab/Bevacizumab | CD20/VEGF | Non-Hodgkin's lymphoma | 2 |
The majority of these mAb pairs are being tested in combination with other drug regimens that represent the standard of care.
Several ongoing trials in different CLL stages with various chemotherapy regimens. Note: As of April 2011, alemtuzumab, bevacizumab, ipilimumab and rituximab were approved for marketing.
Activation of the glucocorticoid-induced TNFR family-related protein (GITR) blocks T-cell suppression and enhances the tumor infiltration of activated lymphocytes.153 In preclinical models, agonistic anti-GITR mAbs block T-cell suppression, which results in tumor growth inhibition and regression. Importantly, combination of an agonistic anti-GITR mAb with an antagonistic anti-CTLA-4 mAb leads to enhanced anti-tumor activity in preclinical models by enabling T cells to more effectively attack cancer cells.154 Advancement of mAb combinations including anti-GITR antibodies depends on the results of single agent tolerability and efficacy studies, particularly a Phase 1 study of TRX518 in melanoma patients.
CD137 (4-1BB/ILA) is another TNFR-family member whose activation on T cells provides a stimulatory effect that is being pursued as an avenue for treating various cancers.155 Surprisingly, an anti-CD137 mAb appeared to reduce the autoimmune side effects of an anti-CTLA-4 mAb while synergistically combining to enhance anti-tumor activity in mice.140,156 A few clinical trials for anti-CD137 BMS663513 were initiated, including a combination therapy with ipilimumab; however, these trials were halted based on a Phase 2 monotherapy study in second-line metastatic melanoma where liver toxicity and a high incidence of hepatitis was observed.155,157
Efforts to enhance the ability of anti-CTLA-4 and anti-PD-1 to elicit endogenous anti-tumor immunity (tumor vaccines, immunostimulatory mAbs) have been reported in preclinical models.158,159 The combination of inhibitory anti-CTLA-4 and agonistic anti-CD40 mAbs apparently induces a strong circulating T-lymphocyte response to exogenous administration of leukemic cells in mice.160 Combination of anti-CTLA-4, anti-PD-1 or anti-GITR mAbs with TriMab, are also undergoing preclinical evaluation.140,158,161 Nevertheless, dosing and sequence of TriMab with other stimulatory mAbs is complex. Thus, while many T-cell co-stimulatory mAbs (including mAbs targeting CTLA-4, PD-1, GITR, CD137, CD40, OX40 and TIM3) are under investigation as single agents, their use in combination has yet to mature into clinical feasibility studies.158,159,161,162
Lastly, immunomodulatory approaches are being considered with other forms of anti-tumor therapy to combine multiple MOAs. For instance, inhibitory anti-IL23 mAbs with IL-2, which negate innate tumor suppression of immune surveillance, are being evaluated in preclinical studies with anti-HER2 regimens to evaluate possible synergies.163 Also, the combination of the immunomodulatory mAb denosumab, which suppresses bone loss, with anti-EGFR panitumumab was shown to both increase anti-tumor efficacy and reduce metastatic bone breakdown.164 Thus, combination of immunomodulatory mAbs with others that affect growth, apoptosis or angiogenesis represents a future direction for cancer therapy investigation.
Anti-CD20 mAb Combinations
Rituximab (Rituxan®, Genentech/Biogen Idec), a chimeric anti-CD20 mAb, has become the standard therapy for many CD20-positive B-cell lymphomas and was the first mAb approved for any oncology indication.165–167 Although rituximab significantly improves long-term survival in combination with conventional chemotherapy, it is not curative in the majority of B-cell non-Hodgkin's lymphoma (NHL) patients and rituximab resistance has been observed.165,166 Hence, additional treatment strategies, including novel mAb combinations with anti-CD20 therapies, are being evaluated to improve survival and cure rates with varying degrees of success. Mechanistic studies indicate that rituximab exerts its anti-tumor activity by inducing apoptosis, ADCC and CDC. Phase 1/2 studies in hematological malignancies with rituximab in combination with either epratuzumab, galiximab or alemtuzumab, which target the B-cell antigens CD22, CD80 and CD52, respectively, demonstrated that these combinations were well-tolerated and resulted in clinical responses equal to or greater than single-agent therapy alone (Table 2).168–171 Preclinical evidence for expanded hematological indications for the combination of anti-CD20 and anti-CD52 mAbs have been reported and may influence further development of this combination,172,173 although issues with adventitious infections may complicate its use as consolidation therapy.174,175 A Phase 2b study testing the combination of rituximab and lumiliximab, which targets the B-cell marker CD23, did not achieve clinical superiority even though there were favorable signals in earlier trials.176 Milatuzumab, which recognizes the B-cell integral membrane protein CD74, has shown interesting activity in mantle cell lymphoma cell lines in both in vitro and in vivo models when combined with rituximab, where; rituximab alone has shown marginal efficacy.177,178 Milatuzumab is now in a Phase 1/2 study in combination with anti-CD20 veltuzumab in NHL. Recently, treatment of human NHL-engrafted mice with anti-CD47 mAbs reduced lymphoma burden and improved survival, while combination treatment with rituximab led to elimination of lymphoma and cure. This outcome was thought to be the result of combining IgG-Fc receptor (FcγR)-dependent and FcγR-independent stimulation of phagocytosis.179
Other combination strategies under evaluation involve combinations of mAbs directed at B cells with mAbs that provide added benefit through alternative mechanisms. Addition of anti-VEGF bevacizumab to rituximab therapy in diffuse large B-cell lymphoma was found to be safely tolerated in a Phase 1 study,180 and the combination is now being pursued in multiple hematological indications with the most advanced in Phase 3 (Table 2). The combination of rituximab with anti-CD2, T-cell-/NK-cell-depleting siplizumab is undergoing evaluation in clinical studies of patients with T-cell/NK-cell NHL, which will be a test case for the possible emergence of adventitious infections.181 Multiple clinical evaluations are underway to investigate the combination of immunomodulatory mAbs such as anti-PD1 CT-011or anti-CD40 SGN-40 with rituximab (Table 2).
Futures
Although the reports to date suggest that substantial potential exists for mAb combinations as future cancer therapies, there are numerous logistical hurdles that must be overcome. The mAb therapies currently marketed are costly in part because of the large investment necessary for their development. Will it be feasible to charge premium (or even sub-premium) prices for two separate therapeutic mAbs? Co-development of multiple mAbs within a single drug product is one mechanism for overcoming this hurdle; however, the development costs may be higher for such a product because of the added complexity of the development path. Considering the hurdles faced when combining two mAbs, the possibility of developing a treatment regimen that includes more than two seems even more remote.
Despite the challenges, innovative ways of combining biologic therapies are emerging. The ability to produce recombinant “polyclonal-like” antibodies is one mechanism for producing complex mixtures of antibodies to treat complex diseases,182 although this approach is still in its infancy. Research designed to deliver combinatorial targeted therapies appear to be moving rapidly toward multi-specific antibody-like therapeutics;183 for example, bispecific antibodies have been reported for some time.184–187 The “hybrid hybridoma” technology resulted in the first approval of a bispecific product in oncology, catumaxomab, which is a treatment for malignant ascites. Although drugability issues inherent in using antibody or protein fragments as building blocks for antibody-like proteins have been widely recognized,188,189 new methods for overcoming the challenges of modifying the archetypal antibody structure for the recognition of multiple antigens, such as combining them with antibody fragments190 or designed protein scaffolds191 to build protein molecules that recognize multiple epitopes or antigens, are emerging139,192–200 and at least some of these are likely to find success in the clinic before the end of the decade.
Conclusions
Advances in personalized medicine have allowed the differentiation of individual diseases states within a broadly defined indication. Within oncology, this advancement has enabled researchers to not only identify the affected tissue, stage of cancer and metastatic status, but to also define the specific cellular and molecular patterns that make each tumor unique. Continuing translational research along with rational and computational methods for integrating complex sets of tumor markers will result in opportunities for combining multiple targeted therapies to more effectively treat tumors that use multiple molecular mechanisms to grow, survive and spread.201 Indeed, new publications on preclinical-stage mAb-based therapeutics now often include an assessment of their activity in combination with other targeted therapies. With strong clinical advances in mAb-based treatments in oncology, the next step will be to identify the most effective mAb combinations that help individual cancer patients achieve more durable and lasting responses.
Abbreviations
- ADCC
antibody dependent cell-mediated cytotoxicity
- BsAb
bispecific antibody
- CRC
colorectal cancer
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- CDC
complement dependent cytotoxicity
- EGFR
epidermal growth factor receptor
- GITR
glucocorticoid-induced TNFR family-related protein
- HER2
human epidermal growth factor receptor 2
- HGF/SF
hepatocyte growth factor/scatter factor
- IGF-1R
type I insulin-like growth factor receptor
- JAK
janus kinase
- mAb
monoclonal antibody
- MAPK
mitogen-activated protein kinase
- MOA
mechanism of action
- NSCLC
non-small cell lung cancer
- PD-1
programmed death-1
- PI3K
phosphoinositide-3-kinase
- RTK
receptor tyrosine kinase
- STAT
signal transducers and activators of transcription
- TKI
small molecule tyrosine kinase inhibitor
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis inducing ligand
- VEGF
vascular endothelial growth factor
References
- 1.Reichert J. Probabilities of success for antibody therapeutics. mAbs. 2009;1:387–389. doi: 10.4161/mabs.1.4.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu A, Huang P. Receptor tyrosine kinase coactivation networks in cancer. Cancer Res. 2010;70:3857–3860. doi: 10.1158/0008-5472.CAN-10-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes N, Lane H. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler D, Huang S, Kruser T, Nechrebecki M, Armstrong E, Benavente S, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritter C, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman J, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 6.Lee-Hoeflich S, Crocker L, Yao E, Pham T, Munroe X, Hoeflich K, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 7.Sergina N, Rausch M, Wang D, Blair J, Hann B, Shokat K, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye D, Mendelsohn J, Fan Z. Augmentation of a humanized anti-HER2 mAb 4D5 induced growth inhibition by a human-mouse chimeric anti-EGF receptor mAb C225. Oncogene. 1999;18:731–738. doi: 10.1038/sj.onc.1202319. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi Y, Kono K, Mimura K, Mitsui F, Sugai H, Akaike H, et al. Targeting EGFR and HER-2 with cetuximab- and trastuzumab-mediated immunotherapy in oesophageal squamous cell carcinoma. Br J Cancer. 2007;97:494–501. doi: 10.1038/sj.bjc.6603885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Half E, Sun Y, Sinicrope F. Anti-EGFR and ErbB-2 antibodies attenuate cyclooxygenase-2 expression and cooperatively inhibit survival of human colon cancer cells. Cancer Lett. 2007;251:237–246. doi: 10.1016/j.canlet.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Meira D, de Almeida V, Mororo J, Nobrega I, Bardella L, Silva R, et al. Combination of cetuximab with chemoradiation, trastuzumab or MAPK inhibitors: mechanisms of sensitisation of cervical cancer cells. Br J Cancer. 2009;101:782–791. doi: 10.1038/sj.bjc.6605216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narayan M, Wilken J, Harris L, Baron A, Kimbler K, Maihle N. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res. 2009;69:2191–2194. doi: 10.1158/0008-5472.CAN-08-1056. [DOI] [PubMed] [Google Scholar]
- 13.Larbouret C, Robert B, Navarro-Teulon I, Thezenas S, Ladjemi M, Morisseau S, et al. In vivo therapeutic synergism of anti-epidermal growth factor receptor and anti-HER2 monoclonal antibodies against pancreatic carcinomas. Clin Cancer Res. 2007;13:3356–3362. doi: 10.1158/1078-0432.CCR-06-2302. [DOI] [PubMed] [Google Scholar]
- 14.Schoeberl B, Faber A, Li D, Liang M, Crosby K, Onsum M, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res. 2010;70:2485–2494. doi: 10.1158/0008-5472.CAN-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 16.Clemmons D. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 17.Cohen B, Baker D, Soderstrom C, Tkalcevic G, Rossi A, Miller P, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063–2073. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 18.Rowinsky E, Youssoufian H, Tonra J, Solomon P, Burtrum D, Ludwig D. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549–5555. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 19.Hewish M, Chau I, Cunningham D. Insulin-like growth factor 1 receptor targeted therapeutics: novel compounds and novel treatment strategies for cancer medicine. Recent Pat Anticancer Drug Discov. 2009;4:54–72. doi: 10.2174/157489209787002515. [DOI] [PubMed] [Google Scholar]
- 20.Adams T, McKern N, Ward C. Signalling by the type 1 insulin-like growth factor receptor: interplay with the epidermal growth factor receptor. Growth Factors. 2004;22:89–95. doi: 10.1080/08977190410001700998. [DOI] [PubMed] [Google Scholar]
- 21.Riedemann J, Takiguchi M, Sohail M, Macaulay V. The EGF receptor interacts with the type 1 IGF receptor and regulates its stability. Biochem Biophys Res Commun. 2007;355:707–714. doi: 10.1016/j.bbrc.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Hendrickson A, Haluska P. Resistance pathways relevant to insulin-like growth factor-1 receptor-targeted therapy. Curr Opin Investig Drugs. 2009;10:1032–1040. [PubMed] [Google Scholar]
- 23.Weroha S, Haluska P. IGF-1 receptor inhibitors in clinical trials—early lessons. J Mammary Gland Biol Neoplasia. 2008;13:471–483. doi: 10.1007/s10911-008-9104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goetsch L, Gonzalez A, Leger O, Beck A, Pauwels P, Haeuw J, et al. A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer. 2005;113:316–328. doi: 10.1002/ijc.20543. [DOI] [PubMed] [Google Scholar]
- 25.Galer C, Corey C, Wang Z, Younes M, Gomez-Rivera F, Jasser S, et al. Dual inhibition of epidermal growth factor receptor and insulin-like growth factor receptor I: Reduction of angiogenesis and tumor growth in cutaneous squamous cell carcinoma. Head Neck. 2011;33:189–198. doi: 10.1002/hed.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong J, Sereno A, Aivazian D, Langley E, Miller B, Snyder W, et al. A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity. mAbs. 2011;3:273–288. doi: 10.4161/mabs.3.3.15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reidy D, Vakiani E, Fakih M, Saif M, Hecht J, Goodman-Davis N, et al. Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:4240–4246. doi: 10.1200/JCO.2010.30.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung C, Pohlmann P, Rothenberg M, Burkey B, Parker J, Palka K, et al. Insulin-like growth factor-1 receptor inhibitor, AMG-479, in cetuximab-refractory head and neck squamous cell carcinoma. Head Neck. 2010 doi: 10.1002/hed.21478. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eder J, Vande Woude G, Boerner S, LoRusso P. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- 30.O'Toole J, Rabenau K, Burns K, Lu D, Mangalampalli V, Balderes P, et al. Therapeutic implications of a human neutralizing antibody to the macrophagestimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 2006;66:9162–9170. doi: 10.1158/0008-5472.CAN-06-0283. [DOI] [PubMed] [Google Scholar]
- 31.Engelman J, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto W, Okamoto I, Tanaka K, Hatashita E, Yamada Y, Kuwata K, et al. TAK-701, a humanized monoclonal antibody to hepatocyte growth factor, reverses gefitinib resistance induced by tumor-derived HGF in non-small cell lung cancer with an EGFR mutation. Mol Cancer Ther. 2010;9:2785–2792. doi: 10.1158/1535-7163.MCT-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stabile L, Rothstein M, Keohavong P, Lenzner D, Land S, Gaither-Davis A, et al. Targeting of both the c-Met and EGFR pathways results in additive inhibition of lung tumorigenesis in transgenic mice. Cancers. 2010;2:2153–2170. doi: 10.3390/cancers2042153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lal B, Goodwin C, Sang Y, Foss C, Cornet K, Muzamil S, et al. EGFRvIII and c-Met pathway inhibitors synergize against PTEN-null/EGFRvIII+ glioblastoma xenografts. Mol Cancer Ther. 2009;8:1751–1760. doi: 10.1158/1535-7163.MCT-09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachleitner-Hofmann T, Sun M, Chen C, Tang L, Song L, Zeng Z, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther. 2008;7:3499–3508. doi: 10.1158/1535-7163.MCT-08-0374. [DOI] [PubMed] [Google Scholar]
- 36.Ferrara N, Gerber H, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 37.Kim K, Li B, Winer J, Armanini M, Gillett N, Phillips H, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 38.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 39.Alitalo K, Tammela T, Petrova T. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 40.Ellis L. The role of neuropilins in cancer. Mol Cancer Ther. 2006;5:1099–1107. doi: 10.1158/1535-7163.MCT-05-0538. [DOI] [PubMed] [Google Scholar]
- 41.Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 42.Persaud K, Tille J, Liu M, Zhu Z, Jimenez X, Pereira D, et al. Involvement of the VEGF receptor 3 in tubular morphogenesis demonstrated with a human anti-human VEGFR-3 monoclonal antibody that antagonizes receptor activation by VEGF-C. J Cell Sci. 2004;117:2745–2756. doi: 10.1242/jcs.01138. [DOI] [PubMed] [Google Scholar]
- 43.Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 44.Van de Veire S, Stalmans I, Heindryckx F, Oura H, Tijeras-Raballand A, Schmidt T, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 2010;141:178–190. doi: 10.1016/j.cell.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 45.Bais C, Wu X, Yao J, Yang S, Crawford Y, McCutcheon K, et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141:166–177. doi: 10.1016/j.cell.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 46.Pan Q, Chanthery Y, Liang W, Stawicki S, Mak J, Rathore N, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Augustin H, Koh G, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 48.Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575–585. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 49.Brown J, Cao Z, Pinzon-Ortiz M, Kendrew J, Reimer C, Wen S, et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Mol Cancer Ther. 2010;9:145–156. doi: 10.1158/1535-7163.MCT-09-0554. [DOI] [PubMed] [Google Scholar]
- 50.Coxon A, Bready J, Min H, Kaufman S, Leal J, Yu D, et al. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody. Mol Cancer Ther. 2010;9:2641–2651. doi: 10.1158/1535-7163.MCT-10-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Avraamides C, Garmy-Susini B, Varner J. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desgrosellier J, Cheresh D. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramakrishnan V, Bhaskar V, Law D, Wong M, DuBridge R, Breinberg D, et al. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol. 2006;5:273–286. [PubMed] [Google Scholar]
- 54.Kim T, Landen C, Lin Y, Mangala L, Lu C, Nick A, et al. Combined anti-angiogenic therapy against VEGF and integrin alphaVbeta3 in an orthotopic model of ovarian cancer. Cancer Biol Ther. 2009;8:2263–2272. doi: 10.4161/cbt.8.23.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, et al. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol. 2001;158:1111–1120. doi: 10.1016/S0002-9440(10)64058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Su Y, Volpert O, Vande Woude G. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci USA. 2003;100:12718–12723. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen P, Sweeney C, Park D, Beaupre D, Deng H, Leitch I, et al. A phase Ib study of AMG 102 in combination with bevacizumab or motesanib in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2677–2687. doi: 10.1158/1078-0432.CCR-09-2862. [DOI] [PubMed] [Google Scholar]
- 58.Shen J, Vil MD, Zhang H, Tonra J, Rong L, Damoci C, et al. An antibody directed against PDGF receptorbeta enhances the antitumor and the anti-angiogenic activities of an anti-VEGF receptor 2 antibody. Biochem Biophys Res Commun. 2007;357:1142–1147. doi: 10.1016/j.bbrc.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 59.Shen J, Vil M, Prewett M, Damoci C, Zhang H, Li H, et al. Development of a fully human anti-PDGFRbeta antibody that suppresses growth of human tumor xenografts and enhances antitumor activity of an anti-VEGFR2 antibody. Neoplasia. 2009;11:594–604. doi: 10.1593/neo.09278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rothlin C, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pasquale E. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linger R, Keating A, Earp H, Graham D. TAM receptor tyrosine kinases: biologic functions, signaling and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Ye X, Tan C, Hongo J, Zha J, Liu J, et al. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28:3442–3455. doi: 10.1038/onc.2009.212. [DOI] [PubMed] [Google Scholar]
- 64.Krasnoperov V, Kumar S, Ley E, Li X, Scehnet J, Liu R, et al. Novel EphB4 monoclonal antibodies modulate angiogenesis and inhibit tumor growth. Am J Pathol. 2010;176:2029–2038. doi: 10.2353/ajpath.2010.090755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu-Lowe D, Chen E, Zhang L, Watson K, Mancuso P, Lappin P, et al. Targeting activin receptor-like kinase 1 inhibits angiogenesis and tumorigenesis through a mechanism of action complementary to anti-VEGF therapies. Cancer Res. 2011;71:1362–1373. doi: 10.1158/0008-5472.CAN-10-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jakobsson L, Bentley K, Gerhardt H. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochem Soc Trans. 2009;37:1233–1236. doi: 10.1042/BST0371233. [DOI] [PubMed] [Google Scholar]
- 67.Thurston G, Noguera-Troise I, Yancopoulos G. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7:327–331. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 68.Li J, Harris A. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Front Biosci. 2009;14:3094–3110. doi: 10.2741/3438. [DOI] [PubMed] [Google Scholar]
- 69.Thurston G, Kitajewski J. VEGF and Delta-Notch: interacting signalling pathways in tumour angiogenesis. Br J Cancer. 2008;99:1204–1209. doi: 10.1038/sj.bjc.6604484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoey T, Yen W, Axelrod F, Basi J, Donigian L, Dylla S, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 71.Fischer M, Yen W, Kapoun A, Wang M, O'Young G, Lewicki J, et al. Anti-DLL4 inhibits growth and reduces tumor-initiating cell frequency in colorectal tumors with oncogenic KRAS mutations. Cancer Res. 2011;71:1520–1525. doi: 10.1158/0008-5472.CAN-10-2817. [DOI] [PubMed] [Google Scholar]
- 72.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang W, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 73.Yan M, Callahan C, Beyer J, Allamneni K, Zhang G, Ridgway J, et al. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:6–7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- 74.Pennell N, Lynch T., Jr Combined inhibition of the VEGFR and EGFR signaling pathways in the treatment of NSCLC. Oncologist. 2009;14:399–411. doi: 10.1634/theoncologist.2008-0276. [DOI] [PubMed] [Google Scholar]
- 75.Langer C, Soria J. The role of anti-epidermal growth factor receptor and anti-vascular endothelial growth factor therapies in the treatment of non-small-cell lung cancer. Clin Lung Cancer. 2010;11:82–90. doi: 10.3816/CLC.2010.n.011. [DOI] [PubMed] [Google Scholar]
- 76.Cohen D, Hochster H. Update on clinical data with regimens inhibiting angiogenesis and epidermal growth factor receptor for patients with newly diagnosed metastatic colorectal cancer. Clin Colorectal Cancer. 2007;7:21–27. doi: 10.3816/ccc.2008.s.004. [DOI] [PubMed] [Google Scholar]
- 77.Geva R, Prenen H, Topal B, Aerts R, Vannoote J, Van Cutsem E. Biologic modulation of chemotherapy in patients with hepatic colorectal metastases: the role of anti-VEGF and anti-EGFR antibodies. J Surg Oncol. 2010;102:937–945. doi: 10.1002/jso.21760. [DOI] [PubMed] [Google Scholar]
- 78.Diao Y, Tian X, Huang Y, Chen L, Lin X, Zhuang Z. Enhanced cancer therapy with the combination of EGFR and VEGFR-2 targeting in an orthotopic glioblastoma model. J Chemother. 2010;22:407–412. doi: 10.1179/joc.2010.22.6.407. [DOI] [PubMed] [Google Scholar]
- 79.Shaheen R, Ahmad S, Liu W, Reinmuth N, Jung Y, Tseng W, et al. Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. Br J Cancer. 2001;85:584–589. doi: 10.1054/bjoc.2001.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jung Y, Mansfield P, Akagi M, Takeda A, Liu W, Bucana C, et al. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002;38:1133–1140. doi: 10.1016/s0959-8049(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 81.Lamszus K, Brockmann M, Eckerich C, Bohlen P, May C, Mangold U, et al. Inhibition of glioblastoma angiogenesis and invasion by combined treatments directed against vascular endothelial growth factor receptor-2, epidermal growth factor receptor and vascular endothelial-cadherin. Clin Cancer Res. 2005;11:4934–4940. doi: 10.1158/1078-0432.CCR-04-2270. [DOI] [PubMed] [Google Scholar]
- 82.Sano D, Choi S, Milas Z, Zhou G, Galer C, Su Y, et al. The effect of combination anti-endothelial growth factor receptor and anti-vascular endothelial growth factor receptor 2 targeted therapy on lymph node metastasis: a study in an orthotopic nude mouse model of squamous cell carcinoma of the oral tongue. Arch Otolaryngol Head Neck Surg. 2009;135:411–420. doi: 10.1001/archoto.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tonra J, Deevi D, Corcoran E, Li H, Wang S, Carrick F, et al. Synergistic antitumor effects of combined epidermal growth factor receptor and vascular endothelial growth factor receptor-2 targeted therapy. Clin Cancer Res. 2006;12:2197–2207. doi: 10.1158/1078-0432.CCR-05-1682. [DOI] [PubMed] [Google Scholar]
- 84.Wong N, Fernando N, Nixon A, Cushman S, Aklilu M, Bendell J, et al. A phase II study of capecitabine, oxaliplatin, bevacizumab and cetuximab in the treatment of metastatic colorectal cancer. Anticancer Res. 2011;31:255–261. [PMC free article] [PubMed] [Google Scholar]
- 85.Ocean A, Polite B, Christos P, Horvath L, Hamilton A, Matulich D, et al. Cetuximab is associated with excessive toxicity when combined with bevacizumab Plus mFOLFOX6 in metastatic colorectal carcinoma. Clin Colorectal Cancer. 2010;9:290–296. doi: 10.3816/CCC.2010.n.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spigel D, Greco F, Waterhouse D, Shipley D, Lane C, Vazquez E, et al. Phase II trial of FOLFOX6, bevacizumab and cetuximab in the first-line treatment of metastatic colorectal cancer. Clin Adv Hematol Oncol. 2010;8:480–485. [PubMed] [Google Scholar]
- 87.Hecht J, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 88.Tol J, Koopman M, Cats A, Rodenburg C, Creemers G, Schrama J, et al. Chemotherapy, bevacizumab and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 89.Emlet D, Brown K, Kociban D, Pollice A, Smith C, Ong B, et al. Response to trastuzumab, erlotinib and bevacizumab, alone and in combination, is correlated with the level of human epidermal growth factor receptor-2 expression in human breast cancer cell lines. Mol Cancer Ther. 2007;6:2664–2674. doi: 10.1158/1535-7163.MCT-07-0079. [DOI] [PubMed] [Google Scholar]
- 90.du Manoir J, Francia G, Man S, Mossoba M, Medin J, Viloria-Petit A, et al. Strategies for delaying or treating in vivo acquired resistance to trastuzumab in human breast cancer xenografts. Clin Cancer Res. 2006;12:904–916. doi: 10.1158/1078-0432.CCR-05-1109. [DOI] [PubMed] [Google Scholar]
- 91.Li H, Adachi Y, Yamamoto H, Min Y, Ohashi H, Ii M, et al. Insulin-like growth factor-I receptor blockade reduces tumor angiogenesis and enhances the effects of bevacizumab for a human gastric cancer cell line, MKN45. Cancer. 2011;117:3135–3147. doi: 10.1002/cncr.25893. [DOI] [PubMed] [Google Scholar]
- 92.Sakaguchi M, Kajio T, Kawahara K, Kato K. Antibodies against basic fibroblast growth factor inhibit the autocrine growth of pulmonary artery endothelial cells. FEBS Lett. 1988;233:163–166. doi: 10.1016/0014-5793(88)81376-0. [DOI] [PubMed] [Google Scholar]
- 93.Kurokawa M, Doctrow S, Klagsbrun M. Neutralizing antibodies inhibit the binding of basic fibroblast growth factor to its receptor but not to heparin. J Biol Chem. 1989;264:7686–7691. [PubMed] [Google Scholar]
- 94.Schmitz K, Ferguson K. Interaction of antibodies with ErbB receptor extracellular regions. Exp Cell Res. 2009;315:659–670. doi: 10.1016/j.yexcr.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gutowski M, Briggs S, Johnson D. Epidermal growth factor receptor-reactive monoclonal antibodies: xenograft antitumor activity alone and as drug immunoconjugates. Cancer Res. 1991;51:5471–5475. [PubMed] [Google Scholar]
- 96.Schiller J. Developments in epidermal growth factor receptor-targeting therapy for solid tumors: focus on matuzumab (EMD 72000) Cancer Invest. 2008;26:81–95. doi: 10.1080/07357900701511847. [DOI] [PubMed] [Google Scholar]
- 97.Schmiedel J, Blaukat A, Li S, Knochel T, Ferguson K. Matuzumab binding to EGFR prevents the conformational rearrangement required for dimerization. Cancer Cell. 2008;13:365–373. doi: 10.1016/j.ccr.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mishima K, Johns T, Luwor R, Scott A, Stockert E, Jungbluth A, et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res. 2001;61:5349–5354. [PubMed] [Google Scholar]
- 99.Johns T, Adams T, Cochran J, Hall N, Hoyne P, Olsen M, et al. Identification of the epitope for the epidermal growth factor receptor-specific monoclonal antibody 806 reveals that it preferentially recognizes an untethered form of the receptor. J Biol Chem. 2004;279:30375–30384. doi: 10.1074/jbc.M401218200. [DOI] [PubMed] [Google Scholar]
- 100.Perera R, Narita Y, Furnari F, Gan H, Murone C, Ahlkvist M, et al. Treatment of human tumor xenografts with monoclonal antibody 806 in combination with a prototypical epidermal growth factor receptorspecific antibody generates enhanced antitumor activity. Clin Cancer Res. 2005;11:6390–6399. doi: 10.1158/1078-0432.CCR-04-2653. [DOI] [PubMed] [Google Scholar]
- 101.Pedersen M, Jacobsen H, Koefoed K, Hey A, Pyke C, Haurum J, et al. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res. 2010;70:588–597. doi: 10.1158/0008-5472.CAN-09-1417. [DOI] [PubMed] [Google Scholar]
- 102.Spangler J, Neil J, Abramovitch S, Yarden Y, White F, Lauffenburger D, et al. Combination antibody treatment downregulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc Natl Acad Sci USA. 2010;107:13252–13257. doi: 10.1073/pnas.0913476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meira D, Nobrega I, de Almeida V, Mororo J, Cardoso A, Silva R, et al. Different antiproliferative effects of matuzumab and cetuximab in A431 cells are associated with persistent activity of the MAPK pathway. Eur J Cancer. 2009;45:1265–1273. doi: 10.1016/j.ejca.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 104.Dechant M, Weisner W, Berger S, Peipp M, Beyer T, Schneider-Merck T, et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res. 2008;68:4998–5003. doi: 10.1158/0008-5472.CAN-07-6226. [DOI] [PubMed] [Google Scholar]
- 105.Kamat V, Donaldson J, Kari C, Quadros M, Lelkes P, Chaiken I, et al. Enhanced EGFR inhibition and distinct epitope recognition by EGFR antagonistic mAbs C225 and 425. Cancer Biol Ther. 2008;7:726–733. doi: 10.4161/cbt.7.5.6097. [DOI] [PubMed] [Google Scholar]
- 106.Cho H, Mason K, Ramyar K, Stanley A, Gabelli S, Denney D, Jr, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 107.Molina M, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- 108.Ghosh R, Narasanna A, Wang S, Liu S, Chakrabarty A, Balko J, et al. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res. 2011;71:1871–1882. doi: 10.1158/0008-5472.CAN-10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Franklin M, Carey K, Vajdos F, Leahy D, de Vos A, Sliwkowski M. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 110.Baselga J, Swain S. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 111.Drebin J, Link V, Greene M. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic anti-tumor effects in vivo. Oncogene. 1988;2:273–277. [PubMed] [Google Scholar]
- 112.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 113.Nahta R, Hung M, Esteva F. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 114.Yao E, Zhou W, Lee-Hoeflich S, Truong T, Haverty P, Eastham-Anderson J, et al. Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab and pertuzumab. Clin Cancer Res. 2009;15:4147–4156. doi: 10.1158/1078-0432.CCR-08-2814. [DOI] [PubMed] [Google Scholar]
- 115.Baselga J, Gelmon K, Verma S, Wardley A, Conte P, Miles D, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Portera C, Walshe J, Rosing D, Denduluri N, Berman A, Vatas U, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res. 2008;14:2710–2716. doi: 10.1158/1078-0432.CCR-07-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jacobs S, Cook S, Svoboda M, Van Wyk J. Interaction of the monoclonal antibodies alpha IR-1 and alpha IR-3 with insulin and somatomedin-C receptors. Endocrinology. 1986;118:223–226. doi: 10.1210/endo-118-1-223. [DOI] [PubMed] [Google Scholar]
- 118.Arteaga C, Kitten L, Coronado E, Jacobs S, Kull F, Jr, Allred D, et al. Blockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. J Clin Invest. 1989;84:1418–1423. doi: 10.1172/JCI114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soos M, Field C, Lammers R, Ullrich A, Zhang B, Roth R, et al. A panel of monoclonal antibodies for the type I insulin-like growth factor receptor. Epitope mapping, effects on ligand binding and biological activity. J Biol Chem. 1992;267:12955–12963. [PubMed] [Google Scholar]
- 120.Doern A, Cao X, Sereno A, Reyes C, Altshuler A, Huang F, et al. Characterization of inhibitory anti-insulin-like growth factor receptor antibodies with different epitope specificity and ligand-blocking properties: implications for mechanism of action in vivo. J Biol Chem. 2009;284:10254–10267. doi: 10.1074/jbc.M809709200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dong J, Demarest S, Sereno A, Tamraz S, Langley E, Doern A, et al. Combination of two insulin-like growth factor-I receptor inhibitory antibodies targeting distinct epitopes leads to an enhanced antitumor response. Mol Cancer Ther. 2010;9:2593–2604. doi: 10.1158/1535-7163.MCT-09-1018. [DOI] [PubMed] [Google Scholar]
- 122.Dong J, Sereno A, Snyder W, Miller B, Tamraz S, Doern A, et al. Stable IgG-like bispecific antibodies directed toward the Type I insulin-like growth factor receptor demonstrate enhanced ligand blockade and anti-tumor activity. J Biol Chem. 2011;286:4703–4717. doi: 10.1074/jbc.M110.184317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cao B, Su Y, Oskarsson M, Zhao P, Kort E, Fisher R, et al. Neutralizing monoclonal antibodies to hepatocyte growth factor/scatter factor (HGF/SF) display antitumor activity in animal models. Proc Natl Acad Sci USA. 2001;98:7443–7448. doi: 10.1073/pnas.131200498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gao C, Xie Q, Zhang Y, Su Y, Zhao P, Cao B, et al. Therapeutic potential of hepatocyte growth factor/scatter factor neutralizing antibodies: inhibition of tumor growth in both autocrine and paracrine hepatocyte growth factor/scatter factor: c-Met-driven models of leiomyosarcoma. Mol Cancer Ther. 2009;8:2803–2810. doi: 10.1158/1535-7163.MCT-09-0125. [DOI] [PubMed] [Google Scholar]
- 125.Jun H, Sun J, Rex K, Radinsky R, Kendall R, Coxon A, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res. 2007;13:6735–6742. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- 126.Kim K, Wang L, Su Y, Gillespie G, Salhotra A, Lal B, et al. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res. 2006;12:1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 127.van der Horst E, Chinn L, Wang M, Velilla T, Tran H, Madrona Y, et al. Discovery of fully human anti-MET monoclonal antibodies with antitumor activity against colon cancer tumor models in vivo. Neoplasia. 2009;11:355–364. doi: 10.1593/neo.81536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tvorogov D, Anisimov A, Zheng W, Leppanen V, Tammela T, Laurinavicius S, et al. Effective suppression of vascular network formation by combination of antibodies blocking VEGFR ligand binding and receptor dimerization. Cancer Cell. 2010;18:630–640. doi: 10.1016/j.ccr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 129.Ashkenazi A, Dixit V. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 130.Mahalingam D, Szegezdi E, Keane M, de Jong S, Samali A. TRAIL receptor signalling and modulation: Are we on the right TRAIL? Cancer Treat Rev. 2009;35:280–288. doi: 10.1016/j.ctrv.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 131.Chen L, Park S, Tumanov A, Hau A, Sawada K, Feig C, et al. CD95 promotes tumour growth. Nature. 2010;465:492–496. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang J, Adams A, Ridky T, Tao S, Khavari P. Tumor necrosis factor receptor 1/c-Jun-NH2-kinase signaling promotes human neoplasia. Cancer Res. 2007;67:3827–3834. doi: 10.1158/0008-5472.CAN-06-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang A, Wilson N, Ashkenazi A. Proapoptotic DR4 and DR5 signaling in cancer cells: toward clinical translation. Curr Opin Cell Biol. 2010;22:837–844. doi: 10.1016/j.ceb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 134.Stagg J, Sharkey J, Pommey S, Young R, Takeda K, Yagita H, et al. Antibodies targeted to TRAIL receptor-2 and ErbB-2 synergize in vivo and induce an antitumor immune response. Proc Natl Acad Sci USA. 2008;105:16254–16259. doi: 10.1073/pnas.0806849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maddipatla S, Hernandez-Ilizaliturri F, Knight J, Czuczman M. Augmented antitumor activity against B-cell lymphoma by a combination of monoclonal antibodies targeting TRAIL-R1 and CD20. Clin Cancer Res. 2007;13:4556–4564. doi: 10.1158/1078-0432.CCR-07-0680. [DOI] [PubMed] [Google Scholar]
- 136.Vega M, Huerta-Yepez S, Jazirehi A, Garban H, Bonavida B. Rituximab (chimeric anti-CD20) sensitizes B-NHL cell lines to Fas-induced apoptosis. Oncogene. 2005;24:8114–8127. doi: 10.1038/sj.onc.1208954. [DOI] [PubMed] [Google Scholar]
- 137.Daniel D, Yang B, Lawrence D, Totpal K, Balter I, Lee W, et al. Cooperation of the proapoptotic receptor agonist rhApo2L/TRAIL with the CD20 antibody rituximab against non-Hodgkin lymphoma xenografts. Blood. 2007;110:4037–4046. doi: 10.1182/blood-2007-02-076075. [DOI] [PubMed] [Google Scholar]
- 138.Marini P, Denzinger S, Schiller D, Kauder S, Welz S, Humphreys R, et al. Combined treatment of colorectal tumours with agonistic TRAIL receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy: enhanced effects in vitro and dose-dependent growth delay in vivo. Oncogene. 2006;25:5145–5154. doi: 10.1038/sj.onc.1209516. [DOI] [PubMed] [Google Scholar]
- 139.Michaelson J, Demarest S, Miller B, Amatucci A, Snyder W, Wu X, et al. Anti-tumor activity of stability-engineered IgG-like bispecific antibodies targeting TRAIL-R2 and LTbetaR. mAbs. 2009;1:128–141. doi: 10.4161/mabs.1.2.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler R, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 141.Peggs K, Quezada S, Allison J. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 142.Paterson A, Sharpe A. Taming tissue-specific T cells: CTLA-4 reins in self-reactive T cells. Nat Immunol. 2010;11:109–111. doi: 10.1038/ni0210-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Inman B, Frigola X, Dong H, Kwon E. Costimulation, coinhibition and cancer. Curr Cancer Drug Targets. 2007;7:15–30. doi: 10.2174/156800907780006878. [DOI] [PubMed] [Google Scholar]
- 144.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 145.Butte M, Keir M, Phamduy T, Sharpe A, Freeman G. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Parry R, Chemnitz J, Frauwirth K, Lanfranco A, Braunstein I, Kobayashi S, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fife B, Pauken K, Eagar T, Obu T, Wu J, Tang Q, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rudd C. The reverse stop-signal model for CTLA4 function. Nat Rev Immunol. 2008;8:153–160. doi: 10.1038/nri2253. [DOI] [PubMed] [Google Scholar]
- 149.Curran M, Montalvo W, Yagita H, Allison J. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mangsbo S, Sandin L, Anger K, Korman A, Loskog A, Totterman T. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother. 2010;33:225–235. doi: 10.1097/CJI.0b013e3181c01fcb. [DOI] [PubMed] [Google Scholar]
- 151.Yu P, Steel J, Zhang M, Morris J, Waldmann T. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16:6019–6028. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brahmer J, Drake C, Wollner I, Powderly J, Picus J, Sharfman W, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shevach E, Stephens G. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 154.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ascierto P, Simeone E, Sznol M, Fu Y, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37:508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 156.Kocak E, Lute K, Chang X, May K, Jr, Exten K, Zhang H, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66:7276–7284. doi: 10.1158/0008-5472.CAN-05-2128. [DOI] [PubMed] [Google Scholar]
- 157.Molckovsky A, Siu L. First-in-class, first-in-human phase I results of targeted agents: highlights of the 2008 American society of clinical oncology meeting. J Hematol Oncol. 2008;1:20. doi: 10.1186/1756-8722-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Takeda K, Kojima Y, Uno T, Hayakawa Y, Teng M, Yoshizawa H, et al. Combination therapy of established tumors by antibodies targeting immune activating and suppressing molecules. J Immunol. 2010;184:5493–5501. doi: 10.4049/jimmunol.0903033. [DOI] [PubMed] [Google Scholar]
- 159.Saha A, Chatterjee S. Combination of CTL-associated antigen-4 blockade and depletion of CD25 regulatory T cells enhance tumour immunity of dendritic cell-based vaccine in a mouse model of colon cancer. Scand J Immunol. 2010;71:70–82. doi: 10.1111/j.1365-3083.2009.02355.x. [DOI] [PubMed] [Google Scholar]
- 160.Ito D, Ogasawara K, Iwabuchi K, Inuyama Y, Onoe K. Induction of CTL responses by simultaneous administration of liposomal peptide vaccine with anti-CD40 and anti-CTLA-4 mAb. J Immunol. 2000;164:1230–1235. doi: 10.4049/jimmunol.164.3.1230. [DOI] [PubMed] [Google Scholar]
- 161.Melero I, Martinez-Forero I, Dubrot J, Suarez N, Palazon A, Chen L. Palettes of vaccines and immunostimulatory monoclonal antibodies for combination. Clin Cancer Res. 2009;15:1507–1509. doi: 10.1158/1078-0432.CCR-08-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ngiow S, von Scheidt B, Akiba H, Yagita H, Teng M, Smyth M. Anti-TIM3 antibody promotes T cell IFN-{gamma}-mediated anti-tumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 163.Teng M, von Scheidt B, Duret H, Towne J, Smyth M. Anti-IL-23 monoclonal antibody synergizes in combination with targeted therapies or IL-2 to suppress tumor growth and metastases. Cancer Res. 2011;71:2077–2086. doi: 10.1158/0008-5472.CAN-10-3994. [DOI] [PubMed] [Google Scholar]
- 164.Canon J, Bryant R, Roudier M, Osgood T, Jones J, Miller R, et al. Inhibition of RANKL increases the anti-tumor effect of the EGFR inhibitor panitumumab in a murine model of bone metastasis. Bone. 2010;46:1613–1619. doi: 10.1016/j.bone.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 165.Cheson B, Leonard J. Monoclonal antibody therapy for B-cell non-Hodgkin's lymphoma. N Engl J Med. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 166.Fanale M, Younes A. Monoclonal antibodies in the treatment of non-Hodgkin's lymphoma. Drugs. 2007;67:333–350. doi: 10.2165/00003495-200767030-00002. [DOI] [PubMed] [Google Scholar]
- 167.Sikder M, Friedberg J. Beyond rituximab: The future of monoclonal antibodies in B-cell non-Hodgkin lymphoma. Curr Hematol Malig Rep. 2008;3:187–193. doi: 10.1007/s11899-008-0027-5. [DOI] [PubMed] [Google Scholar]
- 168.Leonard J, Friedberg J, Younes A, Fisher D, Gordon L, Moore J, et al. A phase I/II study of galiximab (an anti-CD80 monoclonal antibody) in combination with rituximab for relapsed or refractory, follicular lymphoma. Ann Oncol. 2007;18:1216–1223. doi: 10.1093/annonc/mdm114. [DOI] [PubMed] [Google Scholar]
- 169.Leonard J, Coleman M, Ketas J, Ashe M, Fiore J, Furman R, et al. Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:5044–5051. doi: 10.1200/JCO.2005.13.821. [DOI] [PubMed] [Google Scholar]
- 170.Leonard J, Schuster S, Emmanouilides C, Couture F, Teoh N, Wegener W, et al. Durable complete responses from therapy with combined epratuzumab and rituximab: final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer. 2008;113:2714–2723. doi: 10.1002/cncr.23890. [DOI] [PubMed] [Google Scholar]
- 171.Faderl S, Ferrajoli A, Wierda W, O'Brien S, Lerner S, Keating M. Alemtuzumab by continuous intravenous infusion followed by subcutaneous injection plus rituximab in the treatment of patients with chronic lymphocytic leukemia recurrence. Cancer. 2010;116:2360–2365. doi: 10.1002/cncr.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cruz R, Hernandez-Ilizaliturri F, Olejniczak S, Deeb G, Knight J, Wallace P, et al. CD52 overexpression affects rituximab-associated complement-mediated cytotoxicity but not antibody-dependent cellular cytotoxicity: preclinical evidence that targeting CD52 with alemtuzumab may reverse acquired resistance to rituximab in non-Hodgkin lymphoma. Leuk Lymphoma. 2007;48:2424–2436. doi: 10.1080/10428190701647879. [DOI] [PubMed] [Google Scholar]
- 173.Nijmeijer B, van Schie M, Halkes C, Griffioen M, Willemze R, Falkenburg J. A mechanistic rationale for combining alemtuzumab and rituximab in the treatment of ALL. Blood. 2010;116:5930–5940. doi: 10.1182/blood-2010-01-262006. [DOI] [PubMed] [Google Scholar]
- 174.Lin T, Donohue K, Byrd J, Lucas M, Hoke E, Bengtson E, et al. Consolidation therapy with subcutaneous alemtuzumab after fludarabine and rituximab induction therapy for previously untreated chronic lymphocytic leukemia: final analysis of CALGB 10,101. J Clin Oncol. 2010;28:4500–4506. doi: 10.1200/JCO.2010.29.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Elter T, Eichhorst B, Wendtner C. Use of alemtuzumab and rituximab consolidation in CLL: Pros and cons. Curr Hematol Malig Rep. 2009;4:43–46. doi: 10.1007/s11899-009-0006-5. [DOI] [PubMed] [Google Scholar]
- 176.Byrd J, Kipps T, Flinn I, Castro J, Lin T, Wierda W, et al. Phase 1/2 study of lumiliximab combined with fludarabine, cyclophosphamide and rituximab in patients with relapsed or refractory chronic lymphocytic leukemia. Blood. 2010;115:489–495. doi: 10.1182/blood-2009-08-237727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Alinari L, Yu B, Christian B, Yan F, Shin J, Lapalombella R, et al. Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma. Blood. 2011;117:4530–4541. doi: 10.1182/blood-2010-08-303354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stein R, Qu Z, Cardillo T, Chen S, Rosario A, Horak I, et al. Antiproliferative activity of a humanized anti-CD74 monoclonal antibody, hLL1, on B-cell malignancies. Blood. 2004;104:3705–3711. doi: 10.1182/blood-2004-03-0890. [DOI] [PubMed] [Google Scholar]
- 179.Chao M, Alizadeh A, Tang C, Myklebust J, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ganjoo K, An C, Robertson M, Gordon L, Sen J, Weisenbach J, et al. Rituximab, bevacizumab and CHOP (RA-CHOP) in untreated diffuse large B-cell lymphoma: safety, biomarker and pharmacokinetic analysis. Leuk Lymphoma. 2006;47:998–1005. doi: 10.1080/10428190600563821. [DOI] [PubMed] [Google Scholar]
- 181.O'Mahony D, Morris J, Stetler-Stevenson M, Matthews H, Brown M, Fleisher T, et al. EBV-related lymphoproliferative disease complicating therapy with the anti-CD2 monoclonal antibody, siplizumab, in patients with T-cell malignancies. Clin Cancer Res. 2009;15:2514–2522. doi: 10.1158/1078-0432.CCR-08-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tolstrup A, Frandsen T, Bregenholt S. Development of recombinant human polyclonal antibodies for the treatment of complex human diseases. Expert Opin Biol Ther. 2006;6:905–912. doi: 10.1517/14712598.6.9.905. [DOI] [PubMed] [Google Scholar]
- 183.Chan A, Carter P. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 184.Merchant A, Zhu Z, Yuan J, Goddard A, Adams C, Presta L, et al. An efficient route to human bispecific IgG. Nat Biotechnol. 1998;16:677–681. doi: 10.1038/nbt0798-677. [DOI] [PubMed] [Google Scholar]
- 185.Brennan M, Davison P, Paulus H. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science. 1985;229:81–83. doi: 10.1126/science.3925553. [DOI] [PubMed] [Google Scholar]
- 186.Clark M, Gilliland L, Waldmann H. The potential of hybrid antibodies secreted by hybrid-hybridomas in tumour therapy. Int J Cancer Suppl. 1988;2:15–17. doi: 10.1002/ijc.2910410706. [DOI] [PubMed] [Google Scholar]
- 187.Glennie M, McBride H, Worth A, Stevenson G. Preparation and performance of bispecific F(ab'gamma)2 antibody containing thioether-linked Fab' gamma fragments. J Immunol. 1987;139:2367–2375. [PubMed] [Google Scholar]
- 188.Demarest S, Glaser S. Antibody therapeutics, antibody engineering and the merits of protein stability. Curr Opin Drug Discov Devel. 2008;11:675–687. [PubMed] [Google Scholar]
- 189.Worn A, Pluckthun A. Stability engineering of antibody single-chain Fv fragments. J Mol Biol. 2001;305:989–1010. doi: 10.1006/jmbi.2000.4265. [DOI] [PubMed] [Google Scholar]
- 190.Fischer N, Leger O. Bispecific antibodies: molecules that enable novel therapeutic strategies. Pathobiology. 2007;74:3–14. doi: 10.1159/000101046. [DOI] [PubMed] [Google Scholar]
- 191.Binz H, Amstutz P, Pluckthun A. Engineering novel binding proteins from nonimmunoglobulin domains. Nat Biotechnol. 2005;23:1257–1268. doi: 10.1038/nbt1127. [DOI] [PubMed] [Google Scholar]
- 192.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 193.Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol. 2007;25:1290–1297. doi: 10.1038/nbt1345. [DOI] [PubMed] [Google Scholar]
- 194.Emanuel S, Engle L, Chao G, Zhu R, Cao C, Lin Z, et al. A fibronectin scaffold approach to bispecific inhibitors of epidermal growth factor receptor and insulin-like growth factor-I receptor. mAbs. 2011;3:38–48. doi: 10.4161/mabs.3.1.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mabry R, Lewis K, Moore M, McKernan P, Bukowski T, Bontadelli K, et al. Engineering of stable bispecific antibodies targeting IL-17A and IL-23. Protein Eng Des Sel. 2010;23:115–127. doi: 10.1093/protein/gzp073. [DOI] [PubMed] [Google Scholar]
- 196.Dimasi N, Gao C, Fleming R, Woods R, Yao X, Shirinian L, et al. The design and characterization of oligospecific antibodies for simultaneous targeting of multiple disease mediators. J Mol Biol. 2009;393:672–692. doi: 10.1016/j.jmb.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 197.Coloma M, Morrison S. Design and production of novel tetravalent bispecific antibodies. Nat Biotechnol. 1997;15:159–163. doi: 10.1038/nbt0297-159. [DOI] [PubMed] [Google Scholar]
- 198.Johnson S, Burke S, Huang L, Gorlatov S, Li H, Wang W, et al. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol. 2010;399:436–449. doi: 10.1016/j.jmb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 199.Gunasekaran K, Pentony M, Shen M, Garrett L, Forte C, Woodward A, et al. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J Biol Chem. 2010;285:19637–19646. doi: 10.1074/jbc.M110.117382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bostrom J, Yu S, Kan D, Appleton B, Lee C, Billeci K, et al. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science. 2009;323:1610–1614. doi: 10.1126/science.1165480. [DOI] [PubMed] [Google Scholar]
- 201.Mathew J, Taylor B, Bader G, Pyarajan S, Antoniotti M, Chinnaiyan A, et al. From bytes to bedside: data integration and computational biology for translational cancer research. PLoS Comput Biol. 2007;3:12. doi: 10.1371/journal.pcbi.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]