Abstract
Cetuximab, a chimeric mouse-human IgG1 monoclonal antibody against the epidermal growth factor receptor, has proven effective in the treatment of metastatic colorectal cancer and squamous cell carcinoma of the head and neck. However, a high incidence of immediate hypersensitivity reactions (HSR) to cetuximab after the first infusion has been observed. We have developed a test for identification of patients likely to show treatment-related HSR to cetuximab. An enzyme-linked immunosorbent assay (ELISA) for detecting anti-cetuximab IgEs was developed and tested on serum samples collected from cancer patients before start of cetuximab treatment, and from healthy blood donors. Similar levels of anti-cetuximab IgE were detected in pre-treatment patient sera (24/92, 26.1%) and sera from healthy blood donors (33/117, 28.2%). HSR were observed in 14 out of the 92 patients (15.2%), and 8 of these (57.1%) were grade 3–4. Anti-cetuximab IgEs were detected in 7/8 of the patients (87.5%) with severe HSRs as compared with 14/78 patients (17.9%) with no HSR (p = 0.0002). Predictive value of the anti-cetuximab IgE test for HSR events of grades 3–4 was calculated using Receiver Operating Characteristics analysis. With a cut-off value of 29 arbitrary units for the anti-cetuximab IgE, the ELISA test showed a sensitivity of 87.5%, specificity of 82.1%, positive predictive value of 33.3% and negative predictive value of 98.5%. Anti-cetuximab IgE ELISA detection could be a valuable tool to help the physician anticipate an anaphylaxis episode following cetuximab infusion and opt for a suitable alternative treatment.
Key words: anti-cetuximab antibodies, ELISA, hypersensitivity, therapeutic monoclonal antibody, ROC
Introduction
Various monoclonal antibodies (mAb) including rituximab, alemtuzumab, trastuzumab, bevacizumab, cetuximab and panitumumab are now in use for the treatment of malignancies. Although generally well tolerated and less toxic than conventional anticancer agents, mAbs can cause infusion-related reactions. These reactions include cytokine release syndrome and hypersensitivity reactions (HSR). The former, consisting of flulike symptoms of varying intensities that are likely due to the interaction of the mAb with the target itself, as has been observed with OKT3, rituximab and more recently with anti-CD28.1–3 HSR is related to IgE-dependent mechanisms and is usually observed after repeated injections of the mAb.4–7 In contrast to the development of sensitization during treatment, severe HSRs have been reported after the first infusion of some mAb, such as abciximab, OKT3, omalizumab8–10 and cetuximab.11–13
Cetuximab (Erbitux®, Merck KGaA), a chimeric mousehuman IgG1 mAb against the epidermal growth factor receptor (EGFR) is approved for treatment of metastatic colorectal cancers and metastatic or locoregionally advanced head and neck cancers. It improves the efficacy of chemo- and radiotherapy.14–16 However, severe HSR to cetuximab were reported, initially with a low incidence (1–3%) and more recently at levels reaching 22% depending on the geographic location.11 Another study confirmed this frequency and demonstrated that HSR may arise via IgEs that are directed against the galactose-α-1,3-galactose (Galα1,3Gal) glycosylated portion of the Fab region of this chimeric mAb. In that study, 17 out of the 25 patients who displayed HSR had anti-cetuximab IgE antibodies as compared with 1 out of the 51 subjects without any HSR.13 In our experience at François Baclesse Centre (FBC, Caen, France), 9.9% patients presented HSRs after the first infusion of cetuximab and 5.2% were grade 3–4 episodes.
The aim of this study was to develop a predictive test for the anaphylactic reaction at cetuximab treatment initiation. An enzyme-linked immunosorbent assay (ELISA) for quantitation of anti-cetuximab IgEs in serum samples was designed and performed retrospectively on serum samples that had been collected from a cohort of cancer patients prior to start of cetuximab treatment. Correlation between the ELISA results and the incidence of HSR that had been recorded during treatment was assessed. Data were analyzed by the receiver operating characteristics (ROC) method to obtain the values for sensitivity, specificity and reliability of the ELISA for predicting HSR reactions.
Results
Patient characteristics and HSRs.
Between October 2005 to March 2009, 213 patients had been treated with cetuximab at François Baclesse Centre, Caen, France. Of these, 21 exhibited hypersensitivity reactions (HSR) after the first infusion (9.9%), including 11 severe episodes (5.2%) of grade 3–4. Pre-treatment serum samples were available from 92 patients who were included in this study (Table 1). Among the above 92 patients, 14 (15.2%) had HSR after the first injection of cetuximab. Of these, six patients had low-to-moderate reactions (grade 1–2), six had severe reactions (grade 3) and two died following the HSR event.
Table 1.
Patient characteristics (n = 92)
| Characteristics | With HSR reaction (n = 14) | Without HSR reaction (n = 78) |
| Age (years) | ||
| Median (Range) | 58 (40–78) | 62 (36–83) |
| Gender (M/F) | 11/3 | 52/26 |
| Primary tumor site | ||
| Head and neck | 9 | 23 |
| Colorectal | 4 | 53 |
| Other | 1 | 2 |
| Metastasis | ||
| Absence | 8 | 21 |
Histamine and tryptase measurements were performed on serum samples from eight patients who had HSR (Table 2). Histamine and tryptase concentrations increased significantly in all 8 and 7/8 patients, respectively. The observed kinetics of variation of both these markers of hypersensitivity were compatible with mast cell degranulation. In one patient who experienced a grade 1 HSR limited to urticaria, there was no evidence of mast cell degranulation, as indicated by low levels of histamine and no tryptase release. In this patient the anti-cetuximab IgE were at an undetectable level.
Table 2.
Quantitation of biological markers of HSR and the levels of anti-cetuximab IgEs in patients with HSR
| HSR Grade | Histamine (nM) | Tryptase (µg/L) | Anti-cetuximab IgE (EAU) | ||||||
| 45 min | 1.5 h | 3 h | 16 h | 45 min | 1.5 h | 3 h | 16 h | ||
| 4 | 6580 | ND | ND | ND | 277 | ND | ND | ND | 3300 |
| 4 | ND | ND | ND | ND | ND | ND | ND | ND | 40 |
| 3 | 43.9 | 39.6 | 19.6 | 1.9 | 35.3 | 31.7 | 20.9 | 3.3 | 105 |
| 3 | 33.7 | 4.8 | ND | 2.1 | 15.6 | 18 | ND | 4.2 | 70 |
| 3 | ND | ND | ND | ND | ND | ND | ND | ND | 60 |
| 3 | 62.5 | 13.4 | 5.5 | 1.7 | 30.2 | 32.8 | 27.9 | 3.2 | 42 |
| 3 | 590 | 62.3 | 20.3 | 1.5 | 78 | 62.8 | 53.7 | 4.9 | 31 |
| 3 | 321 | 45.5 | 5.1 | 1.7 | 11.5 | 10.9 | 10.1 | 1.2 | 15 |
| 2 | 52.9 | 47.6 | 6.2 | 2.7 | 17.8 | 17.2 | 10.2 | 2.6 | 45 |
| 2 | ND | ND | ND | ND | ND | ND | ND | ND | 16 |
| 2 | ND | ND | ND | ND | ND | ND | ND | ND | 5 |
| 1 | 8.1 | ND | ND | 3 | 3.6 | ND | ND | 3.2 | 0 |
| 1 | ND | ND | ND | ND | ND | ND | ND | ND | 147 |
| 1 | ND | ND | ND | ND | ND | ND | ND | ND | 80 |
| median | 57.7 | 42.6 | 6.2 | 1.9 | 24 | 24.8 | 20.9 | 3.2 | 43.5 |
Biological markers (histamine and tryptase) of IgE-dependent reactions and anti-cetuximab IgE levels in patients with HSR. Blood samples were collected within 24 h after the HSR episode, at the times indicated, as recommended. Serum anti-cetuximab IgE were detected in samples that had been collected prior to the start of treatment. ND, not determined.
Specificity of ELISA for detecting anti-cetuximab antibodies.
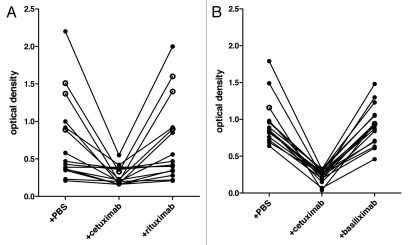
In this assay, we used a positive sample obtained from a healthy donor as a standard. When titrated, the highest positive dilution for this sample was 1/320. The detection limit based on this standard was 3.5 EAU. In order to confirm the specificity of the test, we used a competition assay by incubating positive serum samples with an excess of cetuximab or alternatively with rituximab which is an isotype matched control or with basiliximab which has the same allotype (G1m3).17 As seen in Figure 1, in all positive samples the reaction was inhibited in the presence of excess cetuximab, but not in the presence of PBS or rituximab (Fig. 1A) nor basiliximab (Fig. 1B), confirming the specificity of the assay for anti-cetuximab antibodies, which are probably targeting the known Gal oligosaccharides but not isotypic or allotypic determinants.
Figure 1.
Specificity of anti-cetuximab IgE assay. Diluted blood samples were incubated in coated wells with either phosphate buffered saline (PBS) or an excess of cetuximab or rituximab (A) or basiliximab (B) as indicated. Results from 15 subjects are shown for rituximab (7 control, ●; 5 patients without HS, ●; 3 with HS, ○), and from 17 subjects for basiliximab (16 controls, ●; 1 with HS, ○). Presence of anti-cetuximab IgEs was detected with biotinylated rat monoclonal anti-human-IgE and streptavidin-alkaline phosphatase followed by PNPP. Optical densities corresponding to different patient samples are plotted.
Prevalence of anti-cetuximab antibodies in healthy donors and cancer patients.
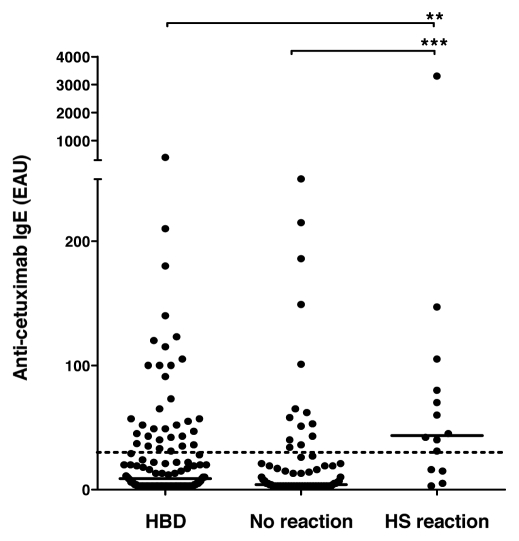
The median value of anti-cetuximab antibodies in the patients was 5 EAU (range, 0–3,300 EAU), which was not significantly different from that of healthy blood donors, 9 EAU (range, 0–400 EAU). In the 14 patients with HSR reaction, anti-cetuximab IgE levels reached a median level of 43.5 EAU (range, 0–3,300 EAU) as compared to 4 EAU (range, 0–250 EAU) in those without reaction, which was highly significant (p = 0.0002; Fig. 2). Out of the two patients who died due to grade 4 HSR, one showed the highest level of anti-cetuximab IgE (3,300 EAU) recorded in this study, while the other patient showed a much lower level (40 EAU). There were no statistically significant differences in the levels of anti-cetuximab IgEs between patients with grade 1–2 HSR and those with grade 3–4 HSR.
Figure 2.
Prevalence of anti-cetuximab IgE. IgE levels were measured in serum samples from a control population of healthy blood donors (HBD) and in samples collected from patients prior to receiving cetuximab treatment. Patients who showed hypersensitivity reaction (HS reaction) and those who did not (no reaction) were included in the study. Median value per group is given as a horizontal bar. Dotted line corresponds to the threshold calculated by the ROC analysis of 29 EAU. ***significant difference between HS group and patients without reaction (p < 0.001); **significant difference between HS group and control population (p < 0.01).
Data from healthy donors indicate an incidence rate of anticetuximab IgE in 33 out of the 117 (28.2%) serum samples, with a comparable prevalence between the two blood banks from Caen (29.3%) and Rouen (27.1%). These values were not significantly different from the frequency of positive samples observed in the cohort of treated patients (24 out of 92, 26.1%).
Predictive value of the ELISA testing for cetuximab-induced HSR.
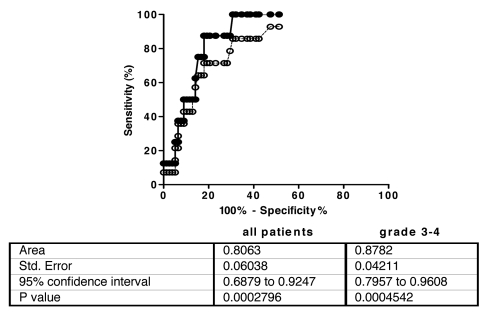
An ROC analysis was performed with data from all patients and the area under the curve was calculated to be 0.806 (95% confidence interval: 0.688 to 0.925; Fig. 3). A threshold of 29 EAU was selected, giving a sensitivity of 71.4% and a specificity of 82.1%. Accordingly, anti-cetuximab IgE were considered positive in 14 out of 78 (17.9%) patients without HSR as compared with 10/14 patients (71.4%) with HSR reaction. When applied to grade 3–4 patients the ROC analysis gives an area under the curve of 0.878 (95% CI: 0.796–0.961; Fig. 3). With the same threshold of 29 EAU the sensitivity of the test was raised to 87.5%, with the same specificity, for this group of patients. The calculated positive predictive value and negative predictive value of the test were 33.3% and 98.5%, respectively (Table 3). The odds ratio for HSR reaction between patients with or without anti-cetuximab IgE was 32 (CI: 3.6–281).
Figure 3.
ROC curve and analysis report. Analysis was performed with all patients (----○----) and patients with grade 3–4 HSR episodes (—●—).
Table 3.
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) in patients with grade 3–4 hypersensitivity reactions
| Anti-cetuximab IgE (>29 EAU) | IgE+ | IgE− | Total | |
| Patients with HSR grade 3–4 (n = 8) | 7 | 1 | 8 | |
| Patients without HSR (n = 78) | 14 | 64 | 78 | |
| Total | 21 | 65 | 86 | |
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| IgE Anti-cetuximab ELISA assay | 87.5 | 82.1 | 33.3 | 98.5 |
Incidence of IgE in patients with grade 3–4 HS reaction.
Discussion
Cetuximab is the first anti-EGFR mAb approved for cancer therapy. Since its introduction in the clinic, occurrence of HSR during treatment has been observed. Anti-cetuximab IgEs involved in the HSR have recently been reported to be directed against the Galα1-3Gal oligosaccharide.13 This motif represents about onethird of the 21 oligosaccharide motifs characterized on both CH2 and Fab fragments of this mAb produced in the murine SP2/0 cell line.18 In order to detect these pre-existing IgE we developed an ELISA to screen patients' sera prior to cetuximab treatment.
This test was based on using cetuximab itself as a coating reagent to allow detection of the specific IgE. This method provided sufficient surface-bound Galα1,3Gal determinants, which are the only known determinants involved in immunization against cetuximab.13 Moreover, since the whole mAb molecule was used, the assay was not restricted to detection of the oligosaccharide determinants but could detect all anti-cetuximab reactivity. Although we did not investigate the oligosaccharide specificity of the bound IgE, we confirmed the specificity of the IgEs to cetuximab by verifying the lack of inhibition of reaction in the presence of an excess of another chimeric mAb of the same isotype (IgG1) or allotype (G1m3).
According to the results of our ELISA, the prevalence of anti-cetuximab IgE observed among the patients was comparable with that in the healthy blood donor cohort, confirming previous reports.11,13 It was higher than the one observed in other populations,12,13,19 and supports the idea of unexplained regional variations.13
Anti-cetuximab IgE were detected in 10/14 patients who had HSR (71.4%) as compared to 14/78 (17.9%) in patients who did not have HSR (p = 0.0002). Incidence rate of anti-cetuximab IgEs reached 87.5% in patients who exhibited grade 3–4 HSR (7/8). The odds ratio of 32 (CI: 3.6–281.4) between IgE+ and IgE− patients was highly significant, strongly suggesting that pre-treatment determination of pre-existing anti-cetuximab IgE might help to manage the risk of HSR. Although there is no clear correlation between the levels of IgE and the severity of the HSR episode, it should be noted that the exceedingly high level of 3,300 EAU observed was associated with severe HSR that resulted in death. On the other hand, in the group of patients who experienced HSR, three patients were negative for IgE. These three episodes being mild (grade 2), blood samples for histamine and tryptase assays had not been collected, but would have been useful to confirm the allergic origin of the clinical episode.
The ELISA described here can predict a higher risk of reaction, but not a reaction. In order to confirm sensitization, other conventional tests used in diagnostic of allergy, including tests for basophil activation or cutaneous reaction might be helpful. Furthermore, a desensitization strategy has been used successfully in a patient, which allowed treatment continuation.20
Alternatively, other treatment options such as inhibitors of tyrosine kinase or another EGFR targeting mAb such as panitumumab21 may be used in case of a predicted infusion reaction against cetuximab in patients with metastatic colorectal cancer.
Although the Galα1,3Gal is a well-known, potent inducer of natural antibodies (IgM or IgG) the origin of IgE sensitisation is still elusive. Among the factors suggested to be involved, the B or AB blood group have been correlated with lower levels of anti-gal antibodies and might be less prone to IgE immunisation.22 However, in this study we did not observe any correlation between the blood group and the level of anticetuximab IgE in patients or control groups (data not shown).
A limiting factor of our retrospective study is the number of serum samples available from patients with a HSR during treatment. Nevertheless, the ROC analysis on this study population indicated a good potential of the ELISA developed here for predicting high grade HSR during cetuximab treatment. A well-designed prospective study is warranted to evaluate the applicability of ELISA testing for anticetuximab IgEs as a predictor of treatment-related HSR.
In summary, pre-existing anti-cetuximab IgEs are known to be associated with severe anaphylactic reactions after the first infusion of cetuximab. A sensitive anti-cetuximab IgE ELISA was developed to identify patients at risk of HSR. This test could be a useful tool to optimize prophylactic measures and management of symptoms or to help the physician select an alternative treatment when available.
Materials and Methods
Control subjects and patients.
Blood samples were obtained prior to cetuximab treatment from 92 patients who were being treated for metastatic colorectal cancer or squamous-cell carcinoma of the head and neck, between October 2005 and March 2009 at François Baclesse Centre, Caen, France. Samples from 117 healthy blood donors were obtained from local blood banks (Etablissement Français du Sang), of which 58 were from Caen and 59 from Rouen.
Grading system of hypersensitivity reactions.
Definition of reaction episodes and grading of hypersensitivity reactions were based on the classification of Ring and Messmer.23 Grade 1 corresponded to generalised skin symptoms (flush or rash, urticaria, angioedema), grade 2 to mild to moderate pulmonary, cardiovascular, and/or gastrointestinal symptoms, grade 3 to severe hypotension or anaphylactic shock and grade 4 to cardiac or respiratory arrest.
Anti-cetuximab IgE detection.
Anti-cetuximab IgEs were measured using an enzyme-linked immunosorbent assay (ELISA). Polystyrene microtiter plates (Maxisorp Nunc, Roskilde, Denmark) were coated with 100 µL of a 0.5 µg/L cetuximab solution (Erbitux®, Merck Serrano) in phosphate buffered saline (PBS), overnight at 4°C. After three washes with PBS containing Tween-20 (0.1%), plates were saturated with a solution of human albumin (0.1%) for 2 h at 37°C. Duplicate serum samples (diluted 1/25) were added and incubated overnight at 4°C. Bound anti-cetuximab IgE antibodies were detected using a biotinylated rat monoclonal anti-human-IgE (LO-HE-17, P.A.R.I.S, Compiègne, France), allowed to react for 1.5 h at 37°C. Streptavidin-alkaline phosphatase Beckman Coulter, Fullerton, USA, 1/2,000 dilution, was added, followed by 1 mg/mL paranitrophenyl phosphate solution (PNPP, Interchim, Montluçon, France). Positive samples were titrated after serial dilutions from 1/50 to 1/200 or more as appropriate. Optical density (OD) was measured at 450 nm (Elx808, KC4 software, Bio-Tek) and the mean of duplicates was calculated. Results were expressed in arbitrary units of IgE (EAU) using a positive serum sample from a healthy donor as a standard.
To assess the specificity of the detection, a competition ELISA was performed on sera diluted 1/25 using an excess of cetuximab or an IgG1 isotype control (rituximab, Mabthera®) or a G1m3 allotype control (basiliximab, Simulect®), at 1.1 mg/mL final concentration.
Histamine and tryptase measurements.
Histamine and tryptase measurements were carried out on blood samples collected from patients who experienced severe hypersensitivity reactions. EDTA-blood samples were obtained at 45 min, 1.5, 3 and 16 h after the HSR. Plasma histamine was measured after alkylation by radio-immunoassay according to the manufacturer's instructions (RIA Histamine, Immunotech, Beckman Coulter). Total tryptase was measured using tryptase fluoroimmunossay UniCAP (Phadia, Uppsala, Sweden) on the same samples. The accepted limits for the pathological values of histamine and tryptase are 6 nM and 12 µgL−1, respectively.24 A doubling of the basal value of tryptase indicates mast cell degranulation.25
Statistical analysis.
Statistical analysis was performed using the non-parametric Kruskal-Wallis test and ad hoc post-tests or Mann-Whitney test. A value of p < 0.05 was considered statistically significant. Area under the ROC curve and 95% confidence interval (CI) were calculated. The threshold for IgE positivity was calculated using the maximized Youden index. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated according to the results of the ROC analysis. All statistical analyses were performed using Prism v5.01, GraphPad Software Inc.
Acknowledgments
We thank Dr. Michel Dupuis (Etablissement Français du Sang, Caen, France) for providing samples from healthy blood donors and Olivier Le Tac (François Baclesse Centre, Caen, France) for his help in collecting sera from cetuximab-treated patients. We also thank Dr. Anuradha Alahari for copy-editing the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Chatenoud L, Ferran C, Reuter A, Legendre C, Gevaert Y, Kreis H, et al. Systemic reaction to the anti-T-cell monoclonal antibody OKT3 in relation to serum levels of tumor necrosis factor and interferon-gamma [corrected] N Engl J Med. 1989;320:1420–1421. doi: 10.1056/NEJM198905253202117. [DOI] [PubMed] [Google Scholar]
- 2.Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8) Blood. 1999;94:2217–2224. [PubMed] [Google Scholar]
- 3.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 4.Abramowicz D, Crusiaux A, Niaudet P, Kreis H, Chatenoud L, Goldman M. The IgE humoral response in OKT3-treated patients. Incidence and fine specificity. Transplantation. 1996;61:577–581. doi: 10.1097/00007890-199602270-00011. [DOI] [PubMed] [Google Scholar]
- 5.Leonard PA, Woodside KJ, Gugliuzza KK, Sur S, Daller JA. Safe administration of a humanized murine antibody after anaphylaxis to a chimeric murine antibody. Transplantation. 2002;74:1697–1700. doi: 10.1097/00007890-200212270-00009. [DOI] [PubMed] [Google Scholar]
- 6.Baudouin V, Crusiaux A, Haddad E, Schandene L, Goldman M, Loirat C, et al. Anaphylactic shock caused by immunoglobulin E sensitization after retreatment with the chimeric anti-interleukin-2 receptor monoclonal antibody basiliximab. Transplantation. 2003;76:459–463. doi: 10.1097/01.TP.0000073809.65502.8F. [DOI] [PubMed] [Google Scholar]
- 7.Deniz YM, Gupta N. Safety and tolerability of omalizumab (Xolair), a recombinant humanized monoclonal anti-IgE antibody. Clin Rev Allergy Immunol. 2005;29:31–48. doi: 10.1385/criai:29:1:031. [DOI] [PubMed] [Google Scholar]
- 8.Georgitis JW, Browning MC, Steiner D, Lorentz WB. Anaphylaxis and desensitization to the murine monoclonal antibody used for renal graft rejection. Ann Allergy. 1991;66:343–347. [PubMed] [Google Scholar]
- 9.Hawkins C, Gatenby P, McGill D. Severe hypotension complicating primary angioplasty: allergy to abciximab. Allergy. 2003;58:688–689. doi: 10.1034/j.1398-9995.2003.00190.x. [DOI] [PubMed] [Google Scholar]
- 10.Limb SL, Starke PR, Lee CE, Chowdhury BA. Delayed onset and protracted progression of anaphylaxis after omalizumab administration in patients with asthma. J Allergy Clin Immunol. 2007;120:1378–1381. doi: 10.1016/j.jaci.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 11.O'Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25:3644–3648. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 12.Saltz LB, Lenz HJ, Kindler HL, Hochster HS, Wadler S, Hoff PM, et al. Randomized phase II trial of cetuximab, bevacizumab and irinotecan compared with cetuximab and bevacizumab alone in irinotecanrefractory colorectal cancer: the BOND-2 study. J Clin Oncol. 2007;25:4557–4561. doi: 10.1200/JCO.2007.12.0949. [DOI] [PubMed] [Google Scholar]
- 13.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 15.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 16.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 17.Magdelaine-Beuzelin C, Vermeire S, Goodall M, Baert F, Noman M, Assche GV, et al. IgG1 heavy chaincoding gene polymorphism (G1m allotypes) and development of antibodies-to-infliximab. Pharmacogenet Genomics. 2009;19:383–387. doi: 10.1097/FPC.0b013e32832a06bf. [DOI] [PubMed] [Google Scholar]
- 18.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364:8–18. doi: 10.1016/j.ab.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Lilenbaum R, Wang X, Gu L, Kirshner J, Lerro K, Vokes E. Randomized phase II trial of docetaxel plus cetuximab or docetaxel plus bortezomib in patients with advanced non-small-cell lung cancer and a performance status of 2: CALGB 30402. J Clin Oncol. 2009;27:4487–4491. doi: 10.1200/JCO.2009.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerath MR, Kwan M, Kannarkat M, Mirakhur B, Carey L, Valgus J, et al. A desensitization protocol for the mAb cetuximab. J Allergy Clin Immunol. 2009;123:260–262. doi: 10.1016/j.jaci.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 21.Helbling D, Borner M. Successful challenge with the fully human EGFR antibody panitumumab following an infusion reaction with the chimeric EGFR antibody cetuximab. Ann Oncol. 2007;18:963–964. doi: 10.1093/annonc/mdm130. [DOI] [PubMed] [Google Scholar]
- 22.Posthumus J, James H, Wang X, Commins S, Platts-Mills TAE. Correlation of blood type with the presence of IgE antibodies to galactose-alpha-1,3-galactose (Alpha-gal): Is there a protective effect of blood group substance B? J Allergy Clin Immunol. 2010;125:203. [Google Scholar]
- 23.Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1:466–469. doi: 10.1016/s0140-6736(77)91953-5. [DOI] [PubMed] [Google Scholar]
- 24.Laroche D, Dubois F, Gerard JL, Lefrancois C, Andre B, Vergnaud MC, et al. Radioimmunoassay for plasma histamine: a study of false positive and false negative values. Br J Anaesth. 1995;74:430–437. doi: 10.1093/bja/74.4.430. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz LB, Bradford TR, Rouse C, Irani AM, Rasp G, Van der Zwan JK, et al. Development of a new, more sensitive immunoassay for human tryptase: use in systemic anaphylaxis. J Clin Immunol. 1994;14:190–204. doi: 10.1007/BF01533368. [DOI] [PubMed] [Google Scholar]