Abstract
Folate (vitamin B9) is an essential nutrient that is required for DNA replication and as a substrate for a range of enzymatic reactions involved in amino acid synthesis and vitamin metabolism. Demands for folate increase during pregnancy because it is also required for growth and development of the fetus. Folate deficiency has been associated with abnormalities in both mothers (anemia, peripheral neuropathy) and fetuses (congenital abnormalities). This article reviews the metabolism of folic acid, the appropriate use of folic acid supplementation in pregnancy, and the potential benefits of folic acid, as well as the possible supplementation of l-methylfolate for the prevention of pregnancy-related complications other than neural tube defects.
Key words: Folic acid, l-methylfolate, Dietary supplements
Folate (vitamin B9) is an essential nutrient that is required for DNA replication and as a substrate for a range of enzymatic reactions involved in amino acid synthesis and vitamin metabolism. Demands for folate increase during pregnancy because it is also required for growth and development of the fetus. Folate deficiency has been associated with abnormalities in both mothers (anemia, peripheral neuropathy) and fetuses (congenital abnormalities). Dietary supplementation with folic acid around the time of conception has long been known to reduce the risk of neural tube defects (NTDs) in the offspring.1–4 This article reviews the metabolism of folic acid, the appropriate use of folic acid supplementation in pregnancy, and the potential benefits of folic acid, as well as the possible supplementation of l-methylfolate for the prevention of pregnancy-related complications other than NTD.
Folate, Folic Acid, and l-Methylfolate
Defining the terminology is important to any discussion of the role of folate in nutrition and reproductive biology. The term folate is typically used as a generic name for the group of chemically related compounds based on the folic acid structure. Folate, or vitamin B9, is thought of as one of the 13 essential vitamins. It cannot be synthesized de novo by the body, and must be obtained either from diet or supplementation. Dietary folate is a naturally occurring nutrient found in foods such as leafy green vegetables, legumes, egg yolk, liver, and citrus fruit. Folic acid is a synthetic dietary supplement that is present in artificially enriched foods and pharmaceutical vitamins. Neither folate nor folic acid is metabolically active. Both must be reduced to participate in cellular metabolism. l-5-Methyltetrahydrofolate (l-methylfolate) is the predominant micronutrient form of folate that circulates in plasma and that is involved in biologic processes.5
Folic Acid Metabolism
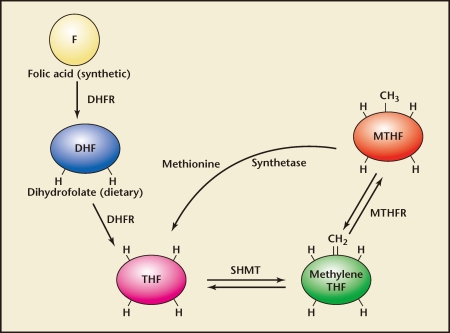
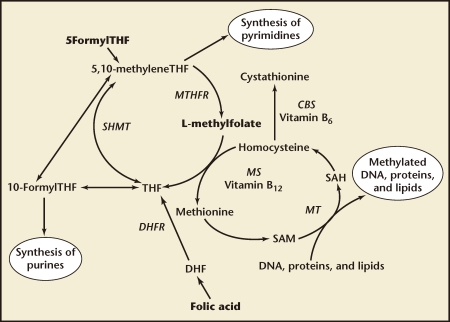
To become metabolically active, folic acid must first be converted to dihydrofolate (DHF) and then tetrahydrofolate (THF) through enzymatic reduction, a process catalyzed by the enzyme DHF reductase (DHFR). Thereafter, THF can be converted to the biologically active l-methylfolate by the enzyme methylenetetrahydrofolate reductase (MTHFR) (Figure 1). This key conversion is necessary to provide l-methylfolate for the one-carbon transfer reactions (methyl donations) needed for purine/pyrimidine synthesis during DNA and RNA assembly, for DNA methylation, and to regulate homocysteine metabolism (Figure 2).6,7 MTHFR is the critical enzyme for almost all biologic processes that involve the metabolism of folate and methionine.
Figure 1.
Formation of l-methylfolate from folic acid. CH2, methylene; CH3, methyl group; DHF, dihydrofolate; DHFR, dihydrofolate reductase, F, folic acid; H, hydrogen; SHMT, serine hydroxy-methyl transferase; THF, tetrahydrofolate. Reproduced with permission from Stahl SM, Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, 3rd ed. New York: Cambridge University Press; 2008.
Figure 2.
Folate metabolic pathway. CBS, cystathionine b synthase; DHF, dihydrofolate; DHFR, dihydrofolate reductase; MS, methionine synthase; MT, methyltransferase; MTHFR, methylenetetrahydrofolate reductase; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SHMT, serine hydroxyl-methyltransferase; THF, tetrahydrofolate. Adapted with permission from Bodnar LM et al.6
Genetic Polymorphisms and Folic Acid Metabolism
Genetic variations (polymorphisms) are common within the human genome and, in some cases, can result in the production of proteins with altered biologic activity. Several such polymorphisms have been identified in the genes encoding proteins involved in folate metabolism. As noted, metabolic processes requiring methyl group donations are regulated by the enzyme MTHFR. In the United States, up to approximately 60% of the population are intermediate metabolizers of folate or heterozygous for genetic polymorphism of the MTHFR enzyme,8 whereas up to 25% of certain populations are homozygous for these genetic variations.5 In varying degrees, these polymorphisms impair the conversion of folate to its active form, l-methylfolate. For example, individuals who are poor metabolizers of folate are homozygous for the common variant MTHFR 677C->T genotype and show approximately 30% of the enzyme activity found in those with the wild-type (CC) variant, whereas heterozygotes for the same genetic polymorphism have around 65% of wild-type enzyme activity.9 With another variant, MTHFR 1298A->C, homozygous individuals can display catalytic activity of the enzyme that is reduced to 68% of the wild-type activity.10
Based on the high prevalence of MTHFR genetic polymorphisms in the general population and concerns about reduced enzymatic activity and, therefore, less biologically available l-methylfolate, newer research in this area has focused on supplementation with l-methylfolate rather than folic acid as a means of preventing folate-related pathology.
Folic Acid and the Prevention of NTDs
Dietary supplementation with folic acid around the time of conception has long been known to reduce the risk of NTD in the offspring.1–4 Although such an intervention is effective, it targets only women planning a pregnancy or recently pregnant. Additional measures to increase the intake of folic acid in the general population include multivitamin supplementation and fortification of grain-based products such as flour, cereal, and pasta. Fortification of grain products with folic acid has been mandatory in the United States since January 199811 and in Canada since December 1998.12 Within months, these legislative mandates were associated with a significant increase in the concentrations of erythrocyte folate among women of childbearing age13,14 and a decrease in the prevalence of infants born with NTDs.15–17 To assure that women have adequate folate stores during pregnancy, the US National Institutes of Health (NIH) and Institute of Medicine (IOM) have recommended that 600 µg of folic acid be taken daily by pregnant women, and that this supplementation be continued throughout pregnancy and reduced to 500 µg during lactation.18 Current US recommendations include (a) for women at high risk of having a child with a NTD (such as those with a personal or family history of NTD, a prior child with a NTD, or those on anticonvulsant medications), dietary supplementation with 5 mg folic acid daily prior to conception; and (b) for all other reproductive-aged women, .4 to 1 mg folic acid daily for at least 2 to 3 months prior to conception, throughout pregnancy, and during the postpartum period. Current recommendations in Canada vary depending on the patient’s demographic characteristics, lifestyle, and a priori risk of having a fetus with a structural abnormality (summarized in Table 1).19 Furthermore, in a recent double-blind, randomized, placebo-controlled trial of 144 women of childbearing age, Lamers and colleagues demonstrated that supplementation with l-methylfolate was more effective than folic acid at increasing red blood cell folate concentrations.20
Table 1.
Canadian Recommendations for Folic Acid and Multivitamin Supplementation in Pregnancy
| Option | Population | Folic Acid Dose | Duration of Supplementation |
| A | Patients with no personal health risks, planned pregnancy | Good diet of folate-rich foods; daily supplementation with folic acid 0.4–1.0 mg | At least 2 to 3 months before conception and throughout pregnancy and the postpartum period (4–6 weeks and as long as breastfeeding continues) |
| B | Patients with health risks, family history of neural tube defect, high-risk ethnic group | (1) Folate-rich foods, daily supplementation with 5 mg folic acid | (1) Beginning at least 3 months before conception and continuing until 10 to 12 weeks postconception |
| (2) Daily supplementation with folic acid 0.4–1.0 mg | (2) From 12 weeks postconception and continuing throughout pregnancy and the postpartum period (4–6 weeks or as long as breastfeeding continues) | ||
| C | Patients with history of poor compliance with medications, additional lifestyle issues, variable diet, no consistent birth control, and possible teratogenic substance use | Folate-rich foods, daily supplementation with 5 mg folic acid | Counsel about folic acid supplementation to prevent birth defects and additional health benefits |
Adapted with permission from Wilson RD et al.19
Folic Acid and the Prevention of Anemia
Blood volume expansion resulting from an increase in both plasma and erythrocytes is a normal physiologic change of pregnancy. Although more plasma than erythrocytes is usually added to the maternal circulation, the increase in erythrocyte volume is considerable, averaging about 450 mL.21 Because of the increase in plasma volume, hemoglobin concentration and hematocrit normally decrease slightly during pregnancy. However, although hemoglobin concentrations at term average 12.5 g/dL, approximately 5% of women are anemic, with hemoglobin concentrations below 11.0 g/dL (see Table 2).22
Table 2.
Hemoglobin Concentrations in 85 Healthy Women With Proven Iron tores
| Hemoglobin (g/dL) | Nonpregnant | Midpregnancy | Late Pregnancy |
| Mean | 13.7 | 11.5 | 12.3 |
| Less than 12.0 | 1% | 72% | 36% |
| Less than 11.0 | None | 29% | 6% |
| Less than 10.0 | None | 4% | 1% |
| Lowest | 11.7 | 9.7 | 9.8 |
Adapted with permission from Cunningham F et al.22
Erythropoiesis is the process by which red blood cells are produced in the hematopoietic tissue of the bone marrow. Among the many requirement for active erythropoiesis are the need for adequate supplies of three key nutrients: folate, cobalamin (vitamin B12), and iron. Although a complete detailing of the role of these nutrients in erythropoiesis is beyond the scope of this review, it is important to understand that the reaction in normal erythropoieses involving both folate and vitamin B12 is the transfer of a methyl group from l-methylfolate to homocysteine via methylcobalamin for the regeneration of methionine.23 Thus, in settings of low folate and/or vitamin B12, anemia will likely ensue. As an example, in a recent retrospective analysis of anemia in pregnancy by Bentley and colleagues, pregnant women prescribed prenatal medical food with 1.13 mg of l-methylfolate in addition to 0.4 mg of folic acid and 500 to 1000 µg vitamin B12 (high folate, high vitamin B12) were compared with pregnant women prescribed standard prenatal vitamins containing only 0.8 to 1.0 mg of folic acid and 0 to 12 µg of vitamin B12 (low folate, low vitamin B12).24 The women in the high folate, high-dose vitamin B12 group demonstrated significantly higher hemoglobin levels at delivery (11.8 g/dL vs 10.7 g/dL; P = .001) than the control standard prenatal vitamin group. How these findings will pan out in prospective, randomized trials and what impact direct l-methylfolate supplementation will have on other folate-related processes remain to be determined.
Folic Acid and the Prevention of Preterm Birth
Preterm birth (PTB), defined as delivery prior to 37 weeks of gestation, complicates 12.5% (1 in 8) of all deliveries in the United States. It is a major cause of neonatal mortality and morbidity. Infants born preterm are at risk of short-term respiratory, gastrointestinal, immunologic, and central nervous system complications, as well as long-term motor, cognitive, and neurobehavioral sequelae. As a result, the societal costs of preterm deliveries in the United States alone exceed $26 billion a year.25 Preterm labor represents a syndrome rather than a diagnosis because the etiologies are varied. Approximately 20% of preterm deliveries are iatrogenic and are performed for maternal or fetal indications, including intrauterine growth restriction, preeclampsia, placenta previa, and nonreassuring fetal testing. Although the causes of the remaining 80% of PTB are not well understood, four basic pathophysiologic mechanisms are described: (a) premature activation of the fetal hypothalamic-pituitaryadrenal (HPA) axis; (b) intrauterine infection/inflammation; (c) decidual hemorrhage (placental abruption); and (d) pathological distension of the uterus.26
The treatment of preterm labor has focused primarily on inhibiting uterine contractions, which has been shown neither to reduce the incidence of PTB nor improve neonatal outcome.27 In the face of such therapeutic nihilism, attention has turned instead to prevention. One of the agents under investigation to prevent PTB in both low- and high-risk populations is folic acid.
Epidemiologic Evidence
Indirect evidence suggests that folate may indeed be important in the timing of labor. In observational studies, a shorter duration of pregnancy has been associated with low serum folate levels6,28 and with the absence of folic acid supplementation during pregnancy.29
Initial intervention studies focused on multiple micronutrient supplementation and showed a significant reduction in pregnancy complications with such supplementation, including low birth weight, small for gestational age (SGA), and maternal anemia30–33; such studies were not sufficiently powered to show a difference in PTB, preterm premature rupture of membranes (PPROM), or placental abruption. Interestingly, these differences lost significance when multiple micronutrient supplementation was compared with iron/folic acid supplementation alone,29 suggesting that it may be these elements that are most important. Subsequent studies suggest that folic acid supplementation alone may protect against PTB, without increasing the risk of miscarriage, structural anomalies, multiple pregnancy, or stillbirth.29,34–36 The largest such study was a secondary analysis of the prospective observational FASTER trial carried out in the United States from 1999–2002.36 This secondary analysis included 34,480 women with singleton pregnancies dated by first trimester ultrasound who delivered between 20-0/7 and 42-0/7 weeks of gestation. Spontaneous PTB was defined as delivery between 20-0/7 and 36-6/7 weeks in the absence of any medical or obstetric complications or indications; pregnancies that ended in elective termination or stillbirth and those with fetal chromosomal or structural abnormalities were excluded. All subjects were asked about their diet and specifically about dose and duration of folic acid supplementation at their initial enrollment in the first trimester. In this cohort, compared with no supplementation, preconceptional folate supplementation for ≥ 1 year was associated with a significant reduction in spontaneous PTB (hazard ratio [HR] 0.22; 95% confidence interval [CI], 0.08–0.61; P = .004 for delivery at 20–28 weeks; HR 0.45; 95% CI, 0.24–0.83; P = .010 for delivery at 28–32 weeks). The authors concluded that preconceptional folate supplementation decreased the risk of spontaneous PTB, and that this association was strong, specific, dose-dependent, consistent with other studies, biologically plausible, and essentially unchanged after adjustment for potential confounders.36 Recent data suggest that the duration of folic acid supplementation may be as important as the dose. In the large prospective cohort study reviewed above,36 the risk of spontaneous PTB was inversely related to the duration of folic acid supplementation, and was lowest in women who reported using folic acid supplementation for more than 1 year prior to conception.
Biologic Plausibility
If folic acid supplementation is indeed associated with a reduction in PTB, what is the mechanism? This question is complicated by the fact that, despite years of research, little is known about the molecular mechanisms responsible for the onset of labor in humans, both at term and preterm, let alone about how to rescue pregnancies complicated by preterm labor. What is becoming clear is that many cases of PTB are associated with an abnormal inflammatory response, which may be triggered by intrauterine infection or hemorrhage.22 Folate is known to be important for normal immune function. Folate-deficient individuals, for example, demonstrate dysfunction of both cell-mediated and humoral immunity.37 Moreover, the phagocytic and bactericidal capacities of polymorphonuclear leukocytes are decreased in folate-deficient humans, thereby increasing their susceptibility to infections such as asymptomatic bacteriuria.38 In such individuals, dietary supplementation with folic acid has been shown to improve immune function and decrease circulating biomarkers of inflammation, including α1-acid glycoprotein and C-reactive protein.39
More recently, several genetic variations have been described in key genes involved in folate metabolism that appear to confer an increased risk for spontaneous PTB. One such variant involves a 19-base pair (bp) deletion in the DHFR gene.40 DHFR is a critical enzyme in the folate metabolic cascade because, as discussed above, ingested folate must first be fully reduced before further metabolic processing can occur. The DHFR 19-bp deletion allele thus interferes with folate metabolism and the transport of reduced folate across the placenta.
Another example is a gene sequence variation in the SHMT1 gene, known as the SHMT1(1420)T variant. This variant results in less serine hydroxymethyltransferase 1 transcriptional activity and is associated with an increased risk of spontaneous PTB; this effect was most pronounced in patients who had low folic acid intake.41 Findings such as these raise the possibility that even women with “adequate” folate intake may be at risk of PTB if they carry a particular genetic variant. Whether such women would benefit from higher folic supplementation or supplementation with l-methylfolate directly is not known at this time.
Additional Benefits of Folic Acid Supplementation
In addition to the prevention of NTD, periconceptional supplementation with folic acid also appears to have other beneficial effects, including the prevention of congenital heart disease and oral clefts42–45 and possibly preterm birth (discussed above). The mechanism by which folic acid prevents structural anomalies in the fetus is not known, but may involve the regulation of homocysteine metabolism.46
Several investigators have suggested that folic acid supplementation may have additional benefits on pregnancy outcome. This line of investigation began because of epidemiologic studies showing that pregnancies exposed to folic acid antagonists have significantly higher rates of placenta-related pregnancy complications.47–50 Folic acid antagonists encompass a broad spectrum of drugs used for a variety of clinical indications, including the treatment of seizure disorders, mood disorders, and urinary tract infections. Folic acid antagonists can be divided into two groups: (a) DHFR inhibitors (eg, sulfamethoxazole-trimethoprim), which block the conversion of folate to its more active metabolites (Figure 1), and (b) other folic acid antagonists, a group consisting primarily of anticonvulsant medications (phenobarbital, phenytoin, primidone, and carbamazepine) but also including Spasmophen (an antispasmodic drug that contains low doses of phenobarbital) and cholestyramine. In one study, pregnancies exposed to either DHFR inhibitors (n = 12,546) or other folic acid antagonists (n = 1565) were noted to be at increased risk of developing preeclampsia (adjusted odds ratio [OR] 1.52; 95% CI, 1.39–1.66), severe preeclampsia (OR 1.77; 95% CI, 1.38–2.28), placental abruption (OR 1.32; 95% CI, 1.12–1.57), fetal growth restriction = 10th percentile (OR 1.07; 95% CI, 1.01–1.13), fetal growth restriction < 3rd percentile (OR 1.22; 95% CI, 1.11–1.34), and fetal death (OR 1.35; 95% CI, 1.07–1.70).49 These adverse events have one thing in common: they all appear to result from abnormalities in implantation and placentation that occur early in gestation. Because folic acid has been shown to regulate trophoblast invasion,51 it is biologically plausible that folate deficiency may interfere with the early stages of placental development leading to complications later in gestation.
Risks of High-Dose Folate Supplementation
Although folic acid supplementation to supraphysiologic levels has demonstrated many of the benefits to pregnant women and fetuses noted above, the potential risk of high-dose folate supplementation must also be considered. First, folate supplementation can mask vitamin B12 deficiency (pernicious anemia) and care must be taken with susceptible individuals to avoid missing this diagnosis. Also, concerns have been raised about the potentially untoward effects of unmetabolized synthetic folic acid with regard to cancer, depression, and cognitive impairment. 52 With all these concerns, early data suggest supplementation with l-methylfolate rather than folic acid may mitigate these risks.53
Conclusions
Periconceptional folic acid supplementation protects against fetal structural anomalies, including NTD and congenital heart defects. Recent data suggest that it may also protect against preterm birth. The importance of genetic polymorphisms in the genes regulating folate metabolism (particularly the MTHFR gene) and how it affects the bioavailability of l-methylfolate and thereby strategies for folate supplementation, is not very well understood. Although additional studies are needed to better define the precise timing, dosing, and formulation, existing data suggest that dietary folic acid supplementation is a good idea for all reproductiveaged women. Women with known MTHFR mutations may benefit from direct supplementation with l-methylfolate but, at present, there are insufficient data to conclusively make that determination.
Main Points.
Demands for folate increase during pregnancy because it is also required for growth and development of the fetus. Folate deficiency has been associated with abnormalities in both mothers (anemia, peripheral neuropathy) and fetuses (congenital abnormalities).
The term folate is typically used as a generic name for the group of chemically related compounds based on the folic acid structure. Folate, or vitamin B9, is thought of as one of the 13 essential vitamins. It cannot be synthesized de novo by the body, and must be obtained either from diet or supplementation. Folic acid is a synthetic dietary supplement that is present in artificially enriched foods and pharmaceutical vitamins. Neither folate nor folic acid is metabolically active. Both must be reduced to participate in cellular metabolism. l-5-Methyl-tetrahydrofolate (l-methylfolate) is the predominant micronutrient form of folate that circulates in plasma and that is involved in biologic processes.
Based on the high prevalence of methyltetrahydrofolate reductase (MTHFR) genetic polymorphisms in the general population and concerns about reduced enzymatic activity and, therefore, less biologically available l-methylfolate, newer research in this area has focused on supplementation with l-methylfolate rather than folic acid as a means of preventing folate-related pathology.
In a recent double-blind, randomized, placebo-controlled trial of 144 women of childbearing age, Lamers and colleagues demonstrated that supplementation with l-methylfolate was more effective than folic acid at increasing red blood cell folate concentrations.
Periconceptional folic acid supplementation protects against fetal structural anomalies, including neural tube and congenital heart defects. Recent data suggest that it may also protect against preterm birth. Although additional studies are needed to better define the precise timing, dosing, and formulation, existing data suggest that dietary folic acid supplementation is a good idea for all reproductive-aged women.
References
- 1.Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group, authors. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 2.Rieder MJ. Prevention of neural tube defects with periconceptional folic acid. Clin Perinatol. 1994;21:483–503. [PubMed] [Google Scholar]
- 3.Pitkin RM. Folate and neural tube defects. Am J Clin Nutr. 2007;85:285S–288S. doi: 10.1093/ajcn/85.1.285S. [DOI] [PubMed] [Google Scholar]
- 4.De Wals P, Tairou F, Van Allen MI, et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med. 2007;357:135–142. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- 5.Pietrzik K, Bailey L, Shane B. Folic acid and l-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2010;48:535–548. doi: 10.2165/11532990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Bodnar LM, Himes KP, Venkataramanan R, et al. Maternal serum folate species in early pregnancy and risk of preterm birth. Am J Clin Nutr. 2010;92:864–871. doi: 10.3945/ajcn.2010.29675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh JR, Herbig AK, Stover PJ. New perspectives on folate catabolism. Annu Rev Nutr. 2001;21:255–282. doi: 10.1146/annurev.nutr.21.1.255. [DOI] [PubMed] [Google Scholar]
- 8.Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008;13:216–226. [PubMed] [Google Scholar]
- 9.Ulrich CM, Kampman E, Bigler J, et al. Lack of association between the C677T MTHFR polymorphism and colorectal hyperplastic polyps. Cancer Epidemiol Biomarkers Prev. 2000;9:427–433. [PubMed] [Google Scholar]
- 10.Weisberg IS, Jacques PF, Selhub J, et al. The 1298 A ->C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis. 2001;156:409–415. doi: 10.1016/s0021-9150(00)00671-7. [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration, authors. Food Standards. Amendment of standards of identity for enriched grain products to require addition of folic acid. Fed Regist. 1996;61:8781–8796. [Google Scholar]
- 12.Bureau of Food Regulatory. International and Interagency Affairs, Health C, authors. Regulatory impact analysis statement. Canada Gazette Part II. 1998;132:3029–3033. [Google Scholar]
- 13.Jacques PF, Selhub J, Bostom AG, et al. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–1454. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich M, Brown CJ, Block G. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J Am Coll Nutr. 2005;24:266–274. doi: 10.1080/07315724.2005.10719474. [DOI] [PubMed] [Google Scholar]
- 15.Honein MA, Paulozzi LJ, Mathews TJ, et al. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981–2986. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- 16.Williams LJ, Mai CT, Edmonds LD, et al. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology. 2002;66:33–39. doi: 10.1002/tera.10060. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC), authors Spina bifida and anencephaly before and after folic acid mandate-United States, 1995–1996 and 1999–2000. MMWR Morb Mortal Wkly Rep. 2004;53:362–365. [PubMed] [Google Scholar]
- 18.Institute of Medicine, Food and Nutrition Board, authors. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 19.Wilson RD, Johnson JA, Wyatt P, et al. Genetics Committee of the Society of Obstetricians and Gynaecologists of Canada and The Motherrisk Program. Pre-conceptional vitamin/folic acid supplementation 2007: the use of folic acid in combination with a multivitamin supplement for the prevention of neural tube defects and other congenital anomalies. J Obstet Gynaecol Can. 2007;29:1003–1026. doi: 10.1016/S1701-2163(16)32685-8. [DOI] [PubMed] [Google Scholar]
- 20.Lamers Y, Prinz-Langenohl R, Brämswig S, Pietrzik K. Red blood cell folate concentrations increase more after supplementation with [6S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am J Clin Nutr. 2006;84:156–161. doi: 10.1093/ajcn/84.1.156. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard JA, Adams RH. Erythrocyte production and destruction during pregnancy. Am J Obstet Gynecol. 1960;79:750–757. doi: 10.1016/0002-9378(60)90633-5. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham F, Leveno K, Bloom S, et al. Williams Obstetrics. 23rd ed. New York: McGraw-Hill; 2009. Hematological disorders. [Google Scholar]
- 23.Koury MJ, Ponka P. New insights into erythropoiesis: the role of folate, vitamin B12 and iron. Annu Rev Nutr. 2004;24:105–131. doi: 10.1146/annurev.nutr.24.012003.132306. [DOI] [PubMed] [Google Scholar]
- 24.Bentley S, Hermes A, Phillips D, et al. Comparative effectiveness of a prenatal medical food to prenatal vitamins on hemoglobin levels and adverse outcomes: a retrospective analysis. Clin Therapeut. 2011;33:204–210. doi: 10.1016/j.clinthera.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Institute of Medicine. Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- 26.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. 2001;15:78–89. doi: 10.1046/j.1365-3016.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 27.Fisk NM, Atun R. Market failure and the poverty of new drugs in maternal health. PLoS Med. 2008;5:e22. doi: 10.1371/journal.pmed.0050022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malinow MR, Rajkovic A, Duell PB, et al. The relationship between maternal and neonatal umbilical cord plasma homocyst(e)ine suggests a potential role for maternal homocysteine in fetal metabolism. Am J Obstet Gynecol. 1998;178:228–233. doi: 10.1016/s0002-9378(98)80005-7. [DOI] [PubMed] [Google Scholar]
- 29.Tchernia G, Blot I, Rey A, et al. Maternal folate status, birthweight and gestational age. Dev Pharmacol Ther. 1982;4(suppl):58–65. [PubMed] [Google Scholar]
- 30.Keen CL, Clegg MS, Hanna LA, et al. The plausibility of micronutrient deficiencies being a significant contributing factor to the occurrence of pregnancy complications. J Nutr. 2003;133(5 suppl 2):1597S–1605S. doi: 10.1093/jn/133.5.1597S. [DOI] [PubMed] [Google Scholar]
- 31.Neggers Y, Goldenberg RL. Some thoughts on body mass index, micronutrient intakes and pregnancy outcome. J Nutr. 2003;133(5 suppl 2):1737S–1740S. doi: 10.1093/jn/133.5.1737S. [DOI] [PubMed] [Google Scholar]
- 32.Allen LH. Multiple micronutrients in pregnancy and lactation: an overview. Am J Clin Nutr. 2005;81:1206S–1212S. doi: 10.1093/ajcn/81.5.1206. [DOI] [PubMed] [Google Scholar]
- 33.Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2006;4):CD004905. doi: 10.1002/14651858.CD004905.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Czeizel AE, Puhó EH, Langmar Z, et al. Possible association of folic acid supplementation during pregnancy with reduction of preterm birth: a population-based study. Eur J Obstet Gynecol Reprod Biol. 2010;148:135–140. doi: 10.1016/j.ejogrb.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Muggli EE, Halliday JL. Folic acid and risk of twinning: a systematic review of the recent literature, July 1994 to July 2006. Med J Aust. 2007;186:243–248. doi: 10.5694/j.1326-5377.2007.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 36.Bukowski R, Malone FD, Porter FT, et al. Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med. 2009;6:e1000061. doi: 10.1371/journal.pmed.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Courtemanche C, Elson-Schwab I, Mashiyama ST, et al. Folate deficiency inhibits the proliferation of primary human CD8+ T lymphocytes in vitro. J Immunol. 2004;173:3186–3192. doi: 10.4049/jimmunol.173.5.3186. [DOI] [PubMed] [Google Scholar]
- 38.Christian P, Jiang T, Khatry SK, et al. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. Am J Clin Nutr. 2006;83:788–794. doi: 10.1093/ajcn/83.4.788. [DOI] [PubMed] [Google Scholar]
- 39.Dhur A, Galan P, Hercberg S. Folate status and the immune system. Prog Food Nutr Sci. 1991;15:43–60. [PubMed] [Google Scholar]
- 40.Johnson WG, Scholl TO, Spychala JR, et al. Common dihydrofolate reductase 19-base pair deletion allele: a novel risk factor for preterm delivery. Am J Clin Nutr. 2005;81:664–668. doi: 10.1093/ajcn/81.3.664. [DOI] [PubMed] [Google Scholar]
- 41.Engel SM, Olshan AF, Siega-Riz AM, et al. Polymorphisms in folate metabolizing genes and risk for spontaneous preterm and small-for-gestational age birth. Am J Obstet Gynecol. 2006;195:1231.e1–e11. doi: 10.1016/j.ajog.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Botto LD, Mulinare J, Erickson JD. Do multivitamin or folic acid supplements reduce the risk for congenital heart defects? Evidence and gaps. Am J Med Genet A. 2003;121A:95–101. doi: 10.1002/ajmg.a.20132. [DOI] [PubMed] [Google Scholar]
- 43.Bailey LB, Berry RJ. Folic acid supplementation and the occurrence of congenital heart defects, orofacial clefts, multiple births, and miscarriage. Am J Clin Nutr. 2005;81:1213S–1217S. doi: 10.1093/ajcn/81.5.1213. [DOI] [PubMed] [Google Scholar]
- 44.Huhta JC, Linask K, Bailey L. Recent advances in the prevention of congenital heart disease. Curr Opin Pediatr. 2006;18:484–489. doi: 10.1097/01.mop.0000245347.45336.d7. [DOI] [PubMed] [Google Scholar]
- 45.Ionescu-Ittu R, Marelli AJ, Mackie AS, Pilote L. Prevalence of severe congenital heart disease after folic acid fortification of grain products: time trend analysis in Quebec, Canada. BMJ. 2009;338;b1673 doi: 10.1136/bmj.b1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huhta JC, Hernandez-Robles JA. Homocysteine, folate, and congenital heart defects. Fetal Pediatr Pathol. 2005;24:71–79. doi: 10.1080/15227950591008240. [DOI] [PubMed] [Google Scholar]
- 47.Wen SW, Chen XK, Rodger M, et al. Folic acid supplementation in early second trimester and the risk of preeclampsia. Am J Obstet Gynecol. 2008;198:45.e1–e7. doi: 10.1016/j.ajog.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 48.Ray JG, Laskin C. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: a systematic review. Placenta. 1999;20:519–529. doi: 10.1053/plac.1999.0417. [DOI] [PubMed] [Google Scholar]
- 49.Wen SW, Zhou J, Yang Q, et al. Maternal exposure to folic acid antagonists and placenta mediated adverse pregnancy outcomes. CMAJ. 2008;179:1263–1268. doi: 10.1503/cmaj.080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, Xie RH, Krewski D, et al. Exposure to trimethoprim/sulfamethoxazole but not other FDA category C and D anti-infectives is associated with increased risks of preterm birth and low birth weight. Int J Infect Dis. 2011;15:e336–e341. doi: 10.1016/j.ijid.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Williams PJ, Bulmer JN, Innes BA, Broughton Pipkin F. Possible roles for folic acid in the regulation of trophoblast invasion and placental development in normal early human pregnancy. Biol Reprod. 2011;84:1148–1153. doi: 10.1095/biolreprod.110.088351. [DOI] [PubMed] [Google Scholar]
- 52.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr. 2010;91:1733–1744. doi: 10.3945/ajcn.2009.28671. [DOI] [PubMed] [Google Scholar]
- 53.Frankenburg FR. Folate supplementation: is it safe and effective? J Clin Psychiatry. 2009;70:767. [PubMed] [Google Scholar]