Abstract
At present, the onset and progress of diabetes, and the efficacy of potential treatments, can only be assessed through indirect means, i.e., blood glucose, insulin, or C-peptide measurements. The development of non-invasive and reliable methods for 1) quantification of pancreatic beta islet cell mass in vivo, 2) determining endogenous islet function and survival, and 3) visualizing the biodistribution, survival, and function of transplanted exogenous islets are critical to further advance both basic science research and islet cell therapy in diabetes. Islet cell imaging using magnetic resonance (MR), bioluminescence, positron emission tomography (PET), or single photon emission computed tomography (SPECT) may provide us with a direct means to interrogate islet cell distribution, survival, and function. Current state-of-the-art strategies for beta cell imaging are discussed and reviewed here in context of their clinical relevance.
Keywords: Diabetes, islets, transplantation, magnetic resonance imaging, positron emission tomography, bioluminescent imaging
Introduction
Diabetes is a chronic, complicated disease resulting from pancreatic islet cells that stop functioning, leading to impaired insulin levels and sustained hyperglycemia. At present, the correlation between the loss of beta cell mass and the clinical course of diabetes can only be assessed through indirect means, i.e., blood sugar, insulin, and C-peptide measurements. Hence, the development of accurate, reproducible, and non-invasive imaging methods to detect and quantify endogenous beta islet mass in vivo is critically important. Such methods may also offer the potential for early diagnosis of beta cell-related metabolic disorders, and for evaluation of potential islet graft recipients. In the most severe form of diabetes, type I diabetes mellitus (T1DM), the current treatment of regular insulin administration is inadequate to achieve optimal control of blood glucose levels. Precise temporal regulation of insulin can potentially be realized by engraftment of pancreatic beta islet cells, but the insulin-independence rates of islet recipients can be as low as 20% at five years post-engraftment [1]. Reliable imaging methods to guide the transplantation process, to monitor and quantify islet graft survival in vivo, and to correlate the viability and function of transplanted islets with the graft anatomical location and route of delivery, are essential both for improving islet transplantation and for detecting complications post-surgery.
The challenges associated with islet imaging pertain to the size (diameter=150 μm) and density of islets, as well as their anatomical location within the pancreas surrounded by the liver and gastrointestinal system. Endogenous beta cells comprise only 2–3% of the total cells in healthy pancreatic tissue, making their detection even more challenging. In islet transplantation studies, the relative volume of islet graft/host tissue is, at best, less than 0.2%. For imaging of endogenous islets, probes with specificity and affinity for islet cells are administered with subsequent binding in vivo; for imaging of transplanted islet cells, probes are added to cells in vitro before transplantation. In this short review article, we aim to discuss some of the recent advances in islet imaging and their clinical relevance.
Imaging of endogenous pancreatic islet cells
Due to their relative high sensitivity, PET and SPECT imaging have been the primary clinical imaging modalities for visualizing endogenous pancreatic beta cells (see Table 1). At present, the availability of a specific, low molecular weight imaging agent has been an elusive goal but is of critical importance to advance our understanding and treatment of diabetes. Most existing probes also bind to other organs, and some recent efforts have been directed towards redirecting probe biodistribution through chemical derivatization. The ideal probe should only bind to pancreatic beta cells, and not to alpha or delta cells or the exocrine pancreas.
Table 1.
Comparison of non-invasive techniques used for imaging of pancreatic islet cells. N/A is not applicable (i.e., cannot or has not been used for this purpose).
| Endogenous islets | Exogenous (transplanted) islets | |||
|---|---|---|---|---|
| Strengths | Weaknesses | Strengths | Weaknesses | |
| PET | High sensitivity, clinically applicable, signal quantification | Ionizing radiation, no anatomical information, low spatial resolution high non-specific uptake by liver and GI tract | Only live cells generate signal, clinically applicable | Genetic manipulation, ionizing radiation, no anatomical information, low spatial resolution |
| SPECT | High sensitivity, clinically applicable, signal quantification | Ionizing radiation, no anatomical information, high non-specific uptake by liver and GI tract | Only live cells generate signal, clinically applicable | Genetic manipulation, ionizing radiation, no anatomical information, low spatial resolution |
| BLI | N/A | N/A | Only live cells generate signal, widely available, no ionizing radiation | Genetic manipulation, not clinically applicable, low spatial resolution |
| MRI | N/A | N/A | High sensitivity, high spatial resolution, clinically applicable, use of real-time MR- guided injections, no ionizing radiation | No discrimination between live and dead cells, difficult to do in patients with liver iron overload |
Beta cell targets that have been explored for developing specific probes include the dopamine receptor, the vesicular monoamine transporter type 2 (VMAT2), the glucagon-like peptide 1 receptor), sulfonylurea receptors, and certain islet cell surface antigens (i.e. plasma membrane gangliosides). VMAT2 has recently been suggested to be one of the more specific biomarkers for imaging beta cell mass (BCM), using [11C]dihydrotetrabenazine (DTBZ) as the transporter’s ligand. Much reduced pancreatic uptake has been observed in TD1M patients. However, there are conflicting reports in the literature about the specificity of VMAT2 expression. Even with a 9-[18F]fluoropropyl-(+)DTBZ ([18F]AV133), there are a number of reports on non-specific binding to pancreatic exocrine tissue and the liver [2]; uptake in the latter organ obscures visualization of the pancreas in particular. A significant advance in obtaining specificity was recently achieved using a 18F-labeled epoxide DTBZ-derivative ([18F](+)4)[3], where the non-specific clearance and uptake is shifted from the liver to the kidney. Overall, there is a lot of activity in field of probe development, but the ideal probe has yet to be found.
Imaging of (transplanted) exogenous pancreatic islet cells
Magnetic resonance imaging (MRI)
MRI offers the best spatial resolution and soft-tissue contrast for anatomical imaging of transplanted islets. MRI can also be used to guide the islet engraftment process using real-time, MR-guided injections (see Figure 1) [4]. Islets have been labeled with superparamagnetic iron-oxide (SPIO) nanoparticles [5–8] or paramagnetic gadolinium (Gd)-based agents for negative (hypointense)- and positive (hyperintense) [9]-contrast MRI, respectively. Human islets labeled with a clinical Gd-based agent, gadolinium 1,4,7-tris(carboxymethyl)-10-(2'-hydroxypropyl)-1,4,7,10-tetraazacyclododecane or GdHPDO3A, showed no impaired viability and functionality, and were visible for at least 65 days post-engraftment into immunodeficient mice [9]. However, Gd-based agents have a low sensitivity, and a high concentration of labels is required for in vivo detection. Moreover, prolonged body retention of gadolinium may be undesirable, given the recent adverse events with gadolinium injection in patients with nephrogenic systemic fibrosis.
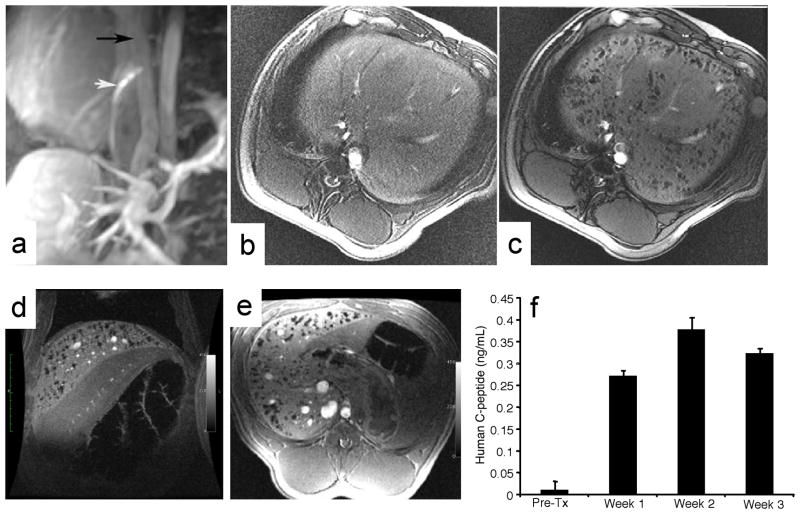
Figure 1.
MR-guided transplantation of magnetocapsules and functionality in vivo in swine. (a) Conventional magnetic resonance angiography/venography of the mesenteric venous system was performed with Gd-DTPA before any punctures. White arrow, active needle; black arrow, portal vein. Needle is seen in the IVC in the proper orientation for portocaval puncture. (b,c) In vivo MRI of magnetocapsules before (b) and 5 min after (c) intraportal infusion of magnetocapsules in a swine. Magnetocapsules can be seen distributed throughout the liver as hypointense signal voids created by the magnetocapsules. (d,e) MRI follow up at 3 weeks shows the persistence of magnetocapsule human islets. (f) Magnetocapsule islets retain functionality in vivo, as assessed by a sustained increase in human C-peptide in plasma. Reproduced, with permission, from Ref. [4].
As for the SPIO labels, the viability and insulin secretion of islets has been reported not to be impaired after labeling. It has been suggested that allograft rejection can be monitored through the use of MRI, as immunorejection leads to islet cell death and subsequent disappearance of hypointensities [7]. In some cases, the signal intensities of SPIO-labeled islets persisted for at least 188 days post-engraftment into mice [5]. Although highly sensitive, a potential pitfall using SPIO-labeling is the hypointensity of the liver commonly encountered in TD1M patients due to hepatic iron overload. There is also no linear correlation between the SPIO concentration and MR signals. A novel MRI approach is the use of perfluorcarbons in islet cell labeling, enabling “hot spot” 19F MRI. When combined with perfluorooctylbromide (PFOB), islets can also be imaged with ultrasound and X-ray/CT imaging [10]. 19F MRI has the advantage that the 19F signal and thus the amount of islets can be directly quantified [11].
Bioluminescence imaging (BLI)
For this imaging modality, islets are engineered to carry a luciferase gene prior to transplantation. When the substrate luciferin is injected into the graft recipients, the enzyme luciferase converts the circulating luciferin to oxyluciferin under the emission of photons thus enabling optical imaging. Transfected islets have been engrafted into mice and imaged by BLI, in some case for more than one year [12–14]. BLI costs less and has a higher throughput than MR imaging. It also provides quantification of viable beta cell mass in vivo with inherently low background. However, BLI is not appropriate for imaging signal coming from deep tissue, and thus unsuitable for human use.
PET and SPECT imaging
Islets have been directly labeled with a PET-trackable glucose analogue, 2-deoxy-2-18F-fluoro-D-glucose ([18F]-FDG) before implantation [15]. Once inside cells, [18F]-FDG is phosphorylated and retained. Dying islet grafts release the radiotracer, but the phosphorylated form of [18F]-FDG cannot be taken up by other cells in vivo. Another candidate for 18F cell labeling is D-mannoheptulose; however, its accumulation in the liver is relatively high and its uptake by human beta cells is potentially low [16]. A major limitation of this approach is the short half-life of the radiotracer (110 minutes for 18F), making it unsuitable for longitudinal imaging.
With a reporter gene method, islets are transfected with a lentivirus expressing herpes simplex virus 1 thymidine kinase (HSV1-tk). Following engraftment, the radiotracer [124I]- or [125I] 5-iodo-1-(2-deoxy-2-fluoro--D-arabino-furanosyl)uracil (FIAU) is administered for PET or SPECT imaging, respectively. The radiotracers are phosphorylated by thymidine kinase inside viable islet cells, and the amount of tracers that is retained by islets in vivo can be measured by PET or SPECT. HSV1-sr39Tk, a mutant form of HSV1-Tk, can be used in combination with the PET radiotracer 9-(4-[18F]-fluoro-3-hydromethylbutyl)guanine ([18F]-FHBG). It was reported that HSV1-sr39Tk more effectively phosphorylates FHBG. Transfected islet grafts in mice could be repeatedly detected by microPET for at least 90 days [17]. The advantages of the reporter gene methods include the exclusive imaging of viable islets and the ability to monitor islet grafts over the long term. Moreover, the magnitude of PET/SPECT signal directly correlates with the engrafted islet mass in mice [18]. However, concerns about viral manipulation of islets and the potential immunogenicity of the reporter may limit the clinical applications of this method.
Multimodal imaging of microencapsulated islets
Microencapsulation of islets presents a multifunctional approach to islet cell therapy. Here, both islets and contrast agents are embedded inside biocompatible microcapsules, with alginate as the common choice of biomaterial. These semi-permeable capsules allow the diffusion of small metabolites, but block the passage of antibodies and immune cells. Capsule rupture and, consequently, the loss of immunoprotection provided by the microcapsules, can be detected, since the loss of signal is caused by the release of contrast agents during rupture [4]. Microencapsulated islets have been labeled with SPIO for 1H MR imaging [4], with barium sulfate or bismuth sulfate for X-ray imaging and fluoroscopy [19], with PFOB for 19F MRI, ultrasound, and CT imaging [20], and with gadolinium-gold or SPIO/gold nanoparticles for 1H MRI, CT, and ultrasound imaging [21, 22] (Figure 2). Microencapsulated islets exhibited an unimpaired function in vitro and restored normoglycemia in diabetic mice after transplantation, without the use of immunosuppressive drugs [4, 20–22].
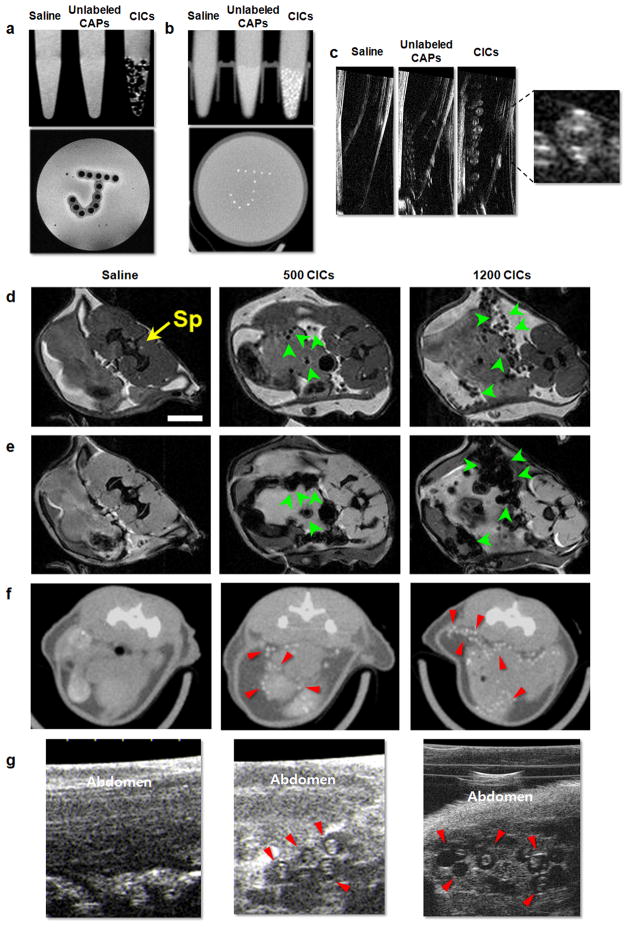
Figure 2.
In vitro and in vivo multimodal imaging using SPIO/gold capsules. (a) Spin echo 9.4 T MRI, (b) micro-CT, and (c) US imaging of phantoms containing saline, unlabeled capsules, and SPIO/gold capsules. (d) Spin echo MRI, (e) gradient echo MRI, (f) micro-CT, and (g) US imaging at 1 day after injection of mouse abdomen injected with saline, 500, or 1200 SPIO/gold capsules. Sp= spinal cord. Note that both in vitro and in vivo, single capsules can be clearly identified (arrows). Reproduced, with permission, from Ref. [22].
Clinical applications
The feasibility and safety of SPIO-labeled islet transplantation into T1DM patients, and the subsequent MR imaging of islet grafts, were recently demonstrated by two research teams in Geneva [23] and Prague [24]. Of the four recipients in the Geneva study, two patients achieved insulin independence for up to 12 months and one was insulin-independent for up to 24 months post-treatment. In the Prague study, significant C-peptide production was detected and lower doses of exogenous insulin were required to maintain normoglycemia in all eight patients. Furthermore, one recipient became insulin-independent for up to six months post-engraftment. In both cases, engrafted islets in the hepatic portal vein could be repeatedly visualized as hypointense spots by a clinical MRI scanner for at least 24 weeks. However, no correlation between the number of implanted islets and the detected hypointense spots was observed. At present, it is not clear how well this technique can provide information about islet survival and function in vivo, but there appears a tendency for hypointense spots to disappear with islet attrition.
In a clinical PET study in Stockholm [15], five patients received hepatic transplantation of unlabeled and labeled islet mixtures. A fraction of the islets (23%) was labeled with [18F]-FDG. For up to four weeks post-treatment, circulating C-peptide was detected and all patients required a reduced exogenous insulin administration. This study revealed that 25% of transplanted islets were lost or damaged during transplantation, as indicated by evaluation of PET signal and circulating C-peptide levels. Due to the short half-life of the 18F radioisotope, this radiotracer is only suitable for assessing the fate of islets up to a few hours after transplantation.
In summary, imaging of transplanted, exogenous pancreatic islet cells can be accomplished using established labeling protocols. Proper imaging of endogenous islets however remains a daunting task, due to the lack of suitable probes specific for the endocrine beta cell.
Acknowledgments
The authors are supported by NIH RO1 EB007825 and the Maryland Nanotechnology Fund.
References
- 1.Low G, Hussein N, Owen RJ, Toso C, Patel VH, Bhargava R, Shapiro AM. Role of imaging in clinical islet transplantation. Radiographics. 2010;30(2):353–366. doi: 10.1148/rg.302095741. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson O, Jahan M, Johnstrom P, Korsgren O, Sundin A, Halldin C, Johansson L. In vivo and in vitro characterization of [18F]-FE-(+)-DTBZ as a tracer for beta-cell mass. Nucl Med Biol. 2010;37(3):357–363. doi: 10.1016/j.nucmedbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Kung MP, Hou C, Lieberman BP, Oya S, Ponde DE, Blankemeyer E, Skovronsky D, Kilbourn MR, Kung HF. In vivo imaging of beta-cell mass in rats using 18F-FP-(+)-DTBZ: a potential PET ligand for studying diabetes mellitus. J Nucl Med. 2008;49(7):1171–1176. doi: 10.2967/jnumed.108.051680. [DOI] [PubMed] [Google Scholar]
- 4.Barnett BP, Arepally A, Karmarkar PV, Qian D, Gilson WD, Walczak P, Howland V, Lawler L, Lauzon C, Stuber M, Kraitchman DL, Bulte JWM. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat Med. 2007;13(8):986–991. doi: 10.1038/nm1581. [DOI] [PubMed] [Google Scholar]
- 5.Evgenov NV, Medarova Z, Dai GP, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med. 2006;12(1):144–148. doi: 10.1038/nm1316. [DOI] [PubMed] [Google Scholar]
- 6.Evgenov NV, Medarova Z, Pratt J, Pantazopoulos P, Leyting S, Bonner-Weir S, Moore A. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes. 2006;55(9):2419–2428. doi: 10.2337/db06-0484. [DOI] [PubMed] [Google Scholar]
- 7.Jirak D, Kriz J, Herynek V, Andersson B, Girman P, Burian M, Saudek F, Hajek M. MRI of transplanted pancreatic islets. Magn Reson Med. 2004;52(6):1228–1233. doi: 10.1002/mrm.20282. [DOI] [PubMed] [Google Scholar]
- 8.Tai JH, Foster P, Rosales A, Feng B, Hasilo C, Martinez V, Ramadan S, Snir J, Melling CW, Dhanvantari S, Rutt B, White DJ. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes. 2006;55(11):2931–2938. doi: 10.2337/db06-0393. [DOI] [PubMed] [Google Scholar]
- 9.Biancone L, Crich SG, Cantaluppi V, Romanazzi GM, Russo S, Scalabrino E, Esposito G, Figliolini F, Beltramo S, Perin PC, Segoloni GP, Aime S, Camussi G. Magnetic resonance imaging of gadolinium-labeled pancreatic islets for experimental transplantation. NMR Biomed. 2007;20(1):40–48. doi: 10.1002/nbm.1088. [DOI] [PubMed] [Google Scholar]
- 10.Barnett BP, Ruiz-Cabello J, Hota P, Ouwerkerk R, Shamblott MJ, Lauzon C, Walczak P, Gilson WD, Chacko VP, Kraitchman DL, Arepally A, Bulte JWM. Use of perfluorocarbon nanoparticles for non-invasive multimodal cell tracking of human pancreatic islets. Contrast Media Mol Imaging. 2011 doi: 10.1002/cmmi.424. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JW. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011;24(2):114–129. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Dang H, Middleton B, Zhang Z, Washburn L, Campbell-Thompson M, Atkinson MA, Gambhir SS, Tian J, Kaufman DL. Bioluminescent monitoring of islet graft survival after transplantation. Mol Ther. 2004;9(3):428–435. doi: 10.1016/j.ymthe.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Virostko J, Radhika A, Poffenberger G, Chen Z, Brissova M, Gilchrist J, Coleman B, Gannon M, Jansen ED, Powers AC. Bioluminescence imaging in mouse models quantifies beta cell mass in the pancreas and after islet transplantation. Mol Imaging Biol. 2010;12(1):42–53. doi: 10.1007/s11307-009-0240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Zhang X, Larson CS, Baker MS, Kaufman DB. In vivo bioluminescence imaging of transplanted islets and early detection of graft rejection. Transplantation. 2006;81(10):1421–1427. doi: 10.1097/01.tp.0000206109.71181.bf. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson O, Eich T, Sundin A, Tibell A, Tufveson G, Andersson H, Felldin M, Foss A, Kyllonen L, Langstrom B, Nilsson B, Korsgren O, Lundgren T. Positron emission tomography in clinical islet transplantation. Am J Transplant. 2009;9(12):2816–2824. doi: 10.1111/j.1600-6143.2009.02844.x. [DOI] [PubMed] [Google Scholar]
- 16.Crenier L, Courtois P, Malaisse WJ. Uptake of tritiated D-mannoheptulose by liver, pancreatic exocrine and endocrine cells. Int J Mol Med. 2001;8(2):155–157. doi: 10.3892/ijmm.8.2.155. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y, Dang H, Middleton B, Campbell-Thompson M, Atkinson MA, Gambhir SS, Tian J, Kaufman DL. Long-term monitoring of transplanted islets using positron emission tomography. Mol Ther. 2006;14(6):851–856. doi: 10.1016/j.ymthe.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Doudet DJ, Studenov AR, Nian C, Ruth TJ, Gambhir SS, McIntosh CH. Quantitative micro positron emission tomography (PET) imaging for the in vivo determination of pancreatic islet graft survival. Nat Med. 2006;12(12):1423–1428. doi: 10.1038/nm1458. [DOI] [PubMed] [Google Scholar]
- 19.Barnett BP, Kraitchman DL, Lauzon C, Magee CA, Walczak P, Gilson WD, Arepally A, Bulte JW. Radiopaque alginate microcapsules for X-ray visualization and immunoprotection of cellular therapeutics. Mol Pharm. 2006;3(5):531–538. doi: 10.1021/mp060056l. [DOI] [PubMed] [Google Scholar]
- 20.Barnett BP, Ruiz-Cabello J, Hota P, Liddell R, Walczak P, Howland V, Chacko VP, Kraitchman DL, Arepally A, Bulte JW. Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology. 2011;258(1):182–191. doi: 10.1148/radiol.10092339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arifin DR, Long CM, Gilad AA, Alric C, Roux S, Tillement O, Link TW, Arepally A, Bulte JWM. Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked using positive contrast MR, CT, and ultrasound imaging. Radiology. 2011 doi: 10.1148/radiol.11101608. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Arifin DR, Muja N, Kim T, Gilad AA, Kim H, Arepally A, Hyeon T, Bulte JW. Multifunctional capsule-in-capsules for immunoprotection and trimodal imaging. Angewandte Chemie. 2011;50(10):2317–2321. doi: 10.1002/anie.201007494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toso C, Vallee JP, Morel P, Ris F, Demuylder-Mischler S, Lepetit-Coiffe M, Marangon N, Saudek F, James Shapiro AM, Bosco D, Berney T. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant. 2008;8(3):701–706. doi: 10.1111/j.1600-6143.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 24.Saudek F, Jirak D, Girman P, Herynek V, Dezortova M, Kriz J, Peregrin J, Berkova Z, Zacharovova K, Hajek M. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation. 2010;90(12):1602–1606. doi: 10.1097/tp.0b013e3181ffba5e. [DOI] [PubMed] [Google Scholar]