Abstract
Stress urinary incontinence (SUI) is a common health problem significantly affecting the quality of life of women worldwide. Animal models that simulate SUI enable the assessment of the mechanism of risk factors for SUI in a controlled fashion, including childbirth injuries, and enable preclinical testing of new treatments and therapies for SUI. Animal models that simulate childbirth are presently being utilized to determine the mechanisms of the maternal injuries of childbirth that lead to SUI with the goal of developing prophylactic treatments. Methods of assessing SUI in animals that mimic diagnostic methods used clinically have been developed to evaluate the animal models. Use of these animal models to test innovative treatment strategies has the potential to improve clinical management of SUI. This chapter provides a review of the available animal models of SUI, as well as a review of the methods of assessing SUI in animal models, and potential treatments that have been tested on these models.
Keywords: Animal models, Bladder pressure, Leak point pressure, Mechanism of injury, Simulated childbirth, Stress urinary incontinence, Urethral pressure, Urinary incontinence, Urodynamics
1 Introduction
Stress urinary incontinence (SUI) is jointly defined by the International Continence Society and International Urogynecological Association as the complaint of involuntary loss of urine on effort or physical exertion (e.g., sporting activities), or on sneezing or coughing (Haylen et al. 2010). It results from a sudden increase of abdominal pressure in the absence of detrusor contraction and is associated with both intrinsic sphincter dysfunction and urethral hypermobility (McGuire 2004). SUI is a common health problem worldwide and significantly impacts both the society and individuals (Birnbaum et al. 2004; Hampel et al. 2004). It is correlated to various risk factors, including general disease, obesity, smoking, being female, advanced age, surgical trauma, and, most significantly, childbirth (DeLancey et al. 2008). However, its pathophysiology is not completely understood.
No animal model can completely simulate the human situation and multifactorial basis for SUI. Nonetheless, animal models can be utilized to further our understanding of the pathophysiology of SUI and enable preclinical testing of potential treatments. They also allow us to assess the mechanism of specific risk factors or contributing elements to SUI in a controlled fashion and to use this knowledge to improve or optimize the management strategy for SUI. Translational investigation of SUI using animal models could potentially lead to an understanding of the importance of each event during the progression of SUI and also to the development of preventive interventions to be delivered before the occurrence of significant events.
2 Methods of Determining SUI in Animal Models
SUI is a behavioral condition that involves unintentional urine leakage; however, animals cannot indicate intent. Therefore, the functional surrogate of urethral resistance to leakage has been used to assess SUI in animal models. In the clinical case, sophisticated urodynamics and other related tests may be performed to narrow diagnosis and treatment (McGuire 2004). The methods used to assess urethral resistance in animal models are based on clinical methods of diagnosing SUI; therefore, the principals of assessing SUI are relatively similar between the two situations.
Other differences between clinical and experimental animal methods arise because untrained animals do not easily follow instructions. Therefore, anesthesia is required for animal testing to immobilize the animal. In addition, invasive and nonsurvival studies examinations can be performed in animals, unlike in humans.
Urethral resistance contributing to urinary continence involves both passive and active mechanisms (Rovner and Wein 2004; Haab et al. 1996), including pressure transmission, urethral smooth muscle contraction, urethral striated muscle contraction, pelvic floor muscle contraction, as well as mucosal coaptation and elasticity of the urethra. Deficiency of these factors is associated with insufficient urethral resistance and may result in symptoms of SUI and decreased leak point pressure (LPP) during a urodynamics study (McGuire 2004). Several methods of determining and characterizing SUI in animal models have been established and developed in the last decade (Hijaz et al. 2008) and are reviewed below.
2.1 Sneeze Testing
The sneeze test was introduced by Lin et al. in 1998 for use in rats to test for decreased urethral resistance to leakage and presumptive SUI (Lin et al. 1998). To perform this test, chili powder or a clipped whisker is placed in the rat’s nose, inducing sneezing (Lin et al. 1998). The sneezing response transiently increases abdominal pressure on the bladder and induces leakage in 30% of animals 4 weeks after simulated childbirth injury. No leakage was induced in uninjured rats (Lin et al. 1998). This method has been used extensively to study SUI in rodent models (Conway et al. 2005; Kaiho et al. 2007; Kamo et al. 2003, 2006) and has also been used in cats and other animals (Julia-Guilloteau et al. 2007; Bernabe et al. 2008; Wallois et al. 1995).
Sneeze testing has been utilized to investigate the nature of the continence reflex response to increased abdominal pressure due to sneezes (Kamo et al. 2003; Julia-Guilloteau et al. 2007). It is presumed that the bladder is squeezed by the abdominal wall during sneezing since bladder pressure was significantly decreased after opening the abdomen during sneeze testing (Kamo et al. 2003). Pressure in the middle urethra increased before bladder pressure increased from sneeze testing, suggesting that continence mechanisms are activated in preparation for sneezing (Kamo et al. 2003). These active closure pressure increases elicited by a reflex response to sneezing are believed to result from contraction of the external urethral sphincter and pelvic floor muscles occurring in the middle urethra in female rats (Kamo et al. 2003).
Urethral smooth muscle contraction also actively contributes to active closure mechanisms during a sneeze reflex since intrathecal phentolamine, prazosin, and intravenous nisoxetine decrease the increased pressure response to sneezing in the urethra of rats (Kaiho et al. 2007). Therefore, the continence response to sneezing includes the noradrenergic system, which can be enhanced with a norepinephrine reuptake inhibitor to prevent SUI via α1-adrenoceptors (Kaiho et al. 2007), as utilized by duloxetine in rats (Miyazato et al. 2008).
2.2 Manual LPP Testing
Manual LPP testing in rats, mimicsking a Crede maneuver, has been developed to test decreased urethral resistance in animal models (Cannon et al. 2002). Because the procedure is relatively easy and highly repeatable, and LPP values can also be achieved from uninjured control animals (Cannon et al. 2002; Damaser et al. 2003), this method of SUI testing enables statistical comparisons of LPP between experimental groups and has therefore become popular (Hijaz et al. 2004; Woo et al. 2009; Wood et al. 2008; Kefer et al. 2008; Lin et al. 2010). Urethane anesthesia is usually used for manual LPP testing since it best maintains urological reflexes (Cannon et al. 2002). It is usually approved only for end-stage use, and animals must be euthanized prior to awakening. Therefore, other anesthetics, such as ketamine and xylazine, have been utilized, when repeat or survival experiments are necessary (Phull et al. 2007). However, ketamine affects activity of the sympathetic nervous system both indirectly, via inhibition of neuronal uptake of catecholamine, and directly by alpha-2 receptor agonism (Aroni et al. 2009), which may affect bladder and urethral function. As a result, use of ketamine and xylazine as an anesthetic has been shown to induce bladder overactivity in rats (Zhang et al. 2010; Cannon and Damaser 2001). However, the effects on urethral resistance have not been determined.
To minimize the effects on urethral resistance by placement of a urethral catheter, a suprapubic bladder catheter is usually placed several days before manual LPP testing (Cannon et al. 2002). However, transurethral catheters have been used for LPP testing in special circumstances, such as during simultaneous measurement of the external urethral sphincter (EUS) electromyogram (EMG) (Jiang et al. 2009a).
To perform manual LPP testing in rats, a passive vesical pressure increase is made by gradually and slowly pressing on the abdomen directly above the bladder until leakage is observed at the urethral meatus. At the moment of urine leakage, the external pressure is rapidly removed and bladder pressure quickly returns to baseline (Cannon et al. 2002). LPP testing is usually performed during filling cystometry with the bladder approximately half full, avoiding times just after voiding or during unstable contractions. If an active bladder pressure contraction is induced by LPP testing, the bladder should be refilled and LPP testing repeated. Bladder pressure increase during LPP testing can be easily differentiated from a bladder contraction or voiding since bladder pressure decreases more slowly after completion of voiding and more fluid is evacuated during voiding. Manual LPP testing has been validated in several SUI animal models (Kefer et al. 2008). Recent efforts to standardize the procedure led to the development of a device consisting of a soft-tipped force applicator with a force sensor, laser crosshairs, and a hand-held remote control system (Shoffstall et al. 2008). Although the device decreased the variability of novices at the LPP test in rats, it had no impact on the variability of testing performed by experienced investigators (Shoffstall et al. 2008).
2.3 Vertical Tilt Table LPP Testing
In this method, a rat is mounted on a vertical tilt table mimicking the erect situation in humans. A saline reservoir is connected to a suprapubic catheter to increase bladder pressure when raised (Lee et al. 2003; Kamo et al. 2004; Kwon et al. 2006). Results using the tilt table technique have been compared to those using manual LPP testing and indicate that they are similar and repeatable (Conway et al. 2005). Prior to the procedure, the spinal cord is usually transected at T8–T9 to eliminate voiding reflexes. However, the test has also been utilized successfully without spinal cord transection (Takahashi et al. 2006).
Although the vertical position of the rats during this test better simulates the human erect body position, the bladder volume is significantly increased as the reservoir height is increased. This change in bladder volume increase may induce additional bladder reflexes and active responses, creating the need for the spinal cord transection. Although the spinal cord transection abolishes unwanted neurogenic activity of the bladder, it also eliminates supraspinal regulation of Onuf’s nucleus and other spinal reflex centers important for continence (Sugaya et al. 2005). Thus, while this method of testing has advantages, such as the erect posture of the animals, it also has disadvantages, both of which must be considered when selecting a testing method for a specific investigation.
2.4 Electrical Stimulation LPP Testing
LPP testing by electrical stimulation of the abdominal muscles, inducing a sudden increase in abdominal pressure, was introduced recently in female rats (Kamo and Hashimoto 2007) to mimic a cough reflex (Widdicombe 1995). As with tilt table testing, to eliminate surpraspinal reflex voiding, the spinal cord is transected at the T8–T9 level. The abdominal skin near the right and left tips of the 11th–13th costae are cut to expose the abdominal oblique muscles, where stimulation needle electrodes are inserted. The only study using this method to date demonstrated that reflex urethral closure mechanisms via bladder–spinal cord–urethral sphincter and pelvic floor muscles greatly contribute to a continence reflex response in increased urethral resistance to prevent leakage (Kamo and Hashimoto 2007).
Electrical stimulation LPP testing is excellent for simulating a sudden increase in abdominal pressure. The LPP value is greater than that from other testing methods because of the contribution of pressure transmission from abdominal muscle contraction. A shortcoming of this method is that in some animals, there is no leakage even at the maximum bearable stimulation level (Kamo and Hashimoto 2007). Moreover, as above, to inhibit bladder contraction, a spinal cord transection has to be performed, eliminating supraspinal regulation of continence reflexes.
2.5 Urethral Closure Pressure Testing
Direct testing of urethral closure pressure has also been performed via utilization of a special catheter, initially introduced transvesically through an incision in the bladder dome and then placed into the bladder neck or the urethra (Bae et al. 2001). This technique was further modified for retrograde urethral perfusion pressure (RUPP) testing in rats via introduction of a dual catheter system via the urethral meatus and placement of a watertight suture (Rodriguez et al. 2005). The pressure sensor is usually placed at the mid-urethra since the highest pressure and greatest continence reflex response in female rats corresponds with the location of urethral striated muscles (Kamo et al. 2003). In contrast, in cat (Julia-Guilloteau et al. 2007; Bernabe et al. 2008) and in dog (Thuroff et al. 1982), the greatest urethral closure pressure active response is located at the distal urethra.
In contrast to vesical pressure measurement, urethral pressure measurement can be inconsistent and difficult to reproduce. The clinical usefulness of urethral pressure profile remains unclear (Lose et al. 2002). To address this, microtransducer-tipped pressure-sensing catheters have been utilized for the measurement of urethral closure pressure (Kamo et al. 2003, 2004; Kamo and Hashimoto 2007) and other urethral pressure measurements (Phull et al. 2007). However, since the micro-tip is actually a point force sensor, different measurement sites, even with the same area, could lead to different results. Therefore, reproducibility of urethral closure pressure and RUPP measurements remains in question.
3 SUI Animal Models
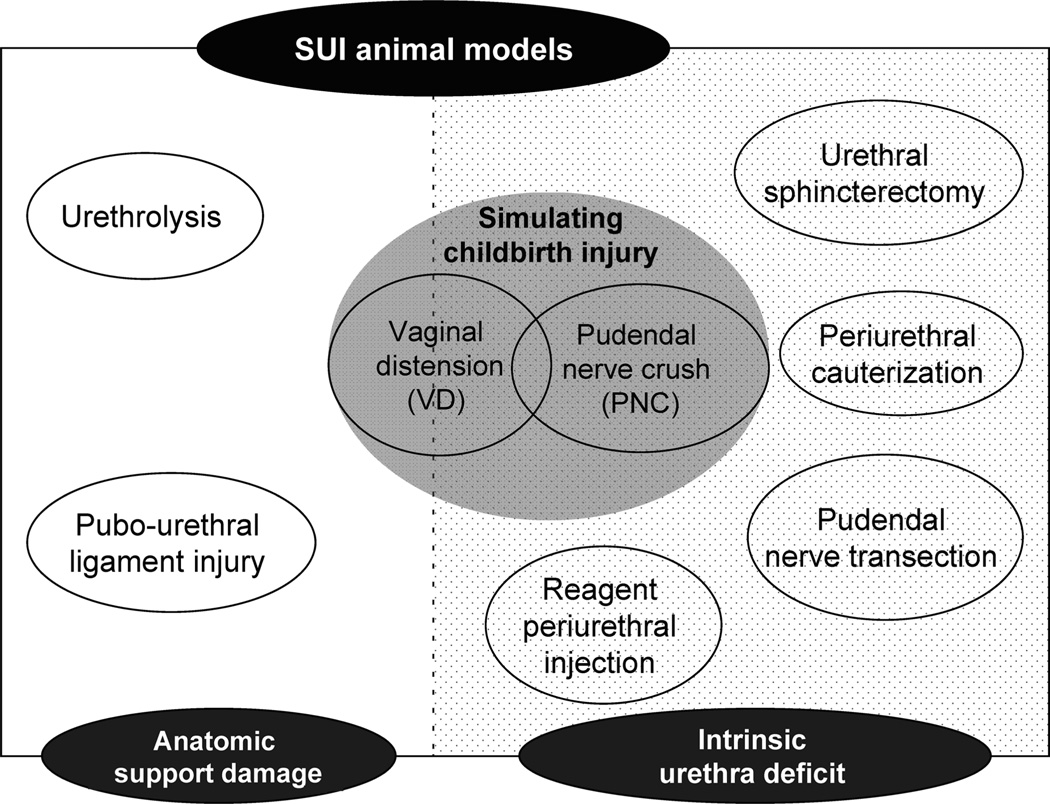
Animal models for SUI in rodents were first introduced by performing vaginal distension (VD) to simulate the maternal injuries of childbirth in female rats (Lin et al. 1998; Sievert et al. 2001). Since then, more and more SUI animal models have been developed using various methods to injure different aspects of the urinary continence mechanism, such as nerve injury (Bernabe et al. 2008; Damaser et al. 2003, 2007; Hijaz et al. 2004; Peng et al. 2006; Ahn et al. 2005), urethral cauterization (Chermansky et al. 2004), urethrolysis (Rodriguez et al. 2005), and pubourethral ligament injury (Kefer et al. 2008, 2009). These animal models enable us to further understand how these factors contribute to development of SUI and to test novel potential therapies (Fig. 1).
Fig. 1.
Animal models for stress urinary incontinence (SUI) can be divided into two broad categories of those that create damage to anatomical support and those that create an intrinsic urethral deficit. Vaginal distension falls into both categories and, along with pudendal nerve crush, provides a simulation of the maternal injuries of childbirth
3.1 Models Simulating Childbirth Injury
3.1.1 Vaginal Distension
Most studies using VD to simulate the maternal injuries of childbirth use similar methods: a Foley catheter is inserted into the rat’s vagina under anesthesia and the balloon is inflated to distend the vagina and injure organs and tissues nearby for an extended period of time (Hijaz et al. 2008). The VD model has became the most used model to study mechanisms of injury and tissue recovery in response to childbirth, since it was introduced over 10 years ago (Lin et al. 1998). Urethral resistance is significantly decreased after VD as confirmed by sneeze testing, LPP testing, and urethral closure pressure testing (Lin et al. 1998; Kamo et al. 2006; Pan et al. 2007). The decrease in urethral resistance recovers in a few weeks, depending primarily on the duration of time the balloon was left distended in the vagina (Pan et al. 2007).
VD has been widely adopted as a model of SUI in rats, however, with some variability in its execution. The selection of catheter size and balloon dilation volume should depend on the age, weight, and breeding status of the rat or on the damage level desired for a specific study. Lin et al. initially used a 12Fr Foley catheter inflated with 2.0 ml in virgin rats and 2.5 ml in retired breeder rats for 4 h (Lin et al. 1998). A larger 22Fr catheter was utilized immediately postpartum in rats with a 5 ml balloon distension for 3 h (Sievert et al. 2004). In addition, the rat was place in a prone position with the symphysis at the edge of the table and the VD catheter attached to a 130 g weight to simulate stretch of tissues during vaginal delivery. Resplande et al. (2002) performed VD in rats immediately postpartum using an 18Fr catheter inflated to 5 ml for 4 h.
In our experience, VD is most repeatable when the vagina is first accommodated and predilated by inserting and removing increasing sizes of urethral dilators (24–32Fr.) (Cannon et al. 2002). We use a modified 10Fr. Foley catheter for VD, inflate it to 3 ml, secure it with a single suture in the skin, and leave it in place for up to 4 h (Damaser et al. 2003; Woo et al. 2009; Wood et al. 2008; Jiang et al. 2009a; Pan et al. 2007, 2009). We use virgin rats in our studies; whereas others use rats immediately postpartum (Sievert et al. 2001, 2004; Lin et al. 2008a). Even though no study has yet performed a head to head comparison of outcomes, the results are similar in the two studies with a predictable decrease in urethral resistance after VD (Hijaz et al. 2008; Damaser et al. 2003, 2005; Woo et al. 2009; Wood et al. 2008; Jiang et al. 2009a; Sievert et al. 2001, 2004; Pan et al. 2007, 2009; Lin et al. 2008a). These variations in VD methodology may correspond to differences in outcomes; however, it is generally accepted that, regardless of the specific methodology, VD damages urethral function in the short-term and reduces urethral resistance to leakage, resulting in SUI that recovers with time.
VD damages the muscular and neurological structures responsible for continence since these tissues are compressed between the dilated balloon and the pelvis, particularly the pubic symphysis (Sievert et al. 2004). Marked cellular swelling and edema in the levator muscles has been shown 1 day after VD (Lin et al. 1998). Smooth muscle between the vagina and the urethra in incontinent rats was also disrupted and thinned (Lin et al. 1998). Cannon et al. (2002) similarly documented extensive disruption of the skeletal muscle layer and marked thinning of the smooth muscle fibers in the urethra following 1 h VD.
VD also results in decreased blood flow to urethra, and hypoxia of the bladder, urethra, and vagina, supportive of hypoxic injury as a possible mechanism of injury leading to SUI (Damaser et al. 2005). Similarly, prolonged VD increases hypoxia-inducible factor 1α expression in the urethra (Wood et al. 2008). VD can also affect expression of other cytokines, including monocyte chemotactic protein-3 (MCP-3) and its receptors (Woo et al. 2007, 2009; Wood et al. 2008). Since MCP-3 attracts mesenchymal stem cells to the local region to facilitate repair (Schenk et al. 2007), this suggests participation of an innate stem cell-mediated repair mechanism after VD. The expression of MCP-3 is also correlated with duration of VD (Wood et al. 2008), confirming a duration-dependent injury and repair mechanism.
Kamo et al. (2006) reported that SUI after VD results from decreased active closure mechanism at the mid-urethra and does not alter abdominal pressure transmission below the bladder neck. Active middle urethra sneeze-induced reflex responses are present but decreased by approximately 50% after VD in rats (Kamo et al. 2006). Furthermore, Jiang et al. (2009a) demonstrated that EUS EMG activity and bladder-to-EUS response activity during LPP testing were significant reduced 4 days after VD. EUS EMG activity recovered by 3 weeks after VD, corresponding with recovery of LPP (Jiang et al. 2009a). These studies confirm that, similar to humans, active urethral closure participates in a sneeze or LPP continence reflex in rats and that the neuromuscular participants in this reflex are damaged by VD, particularly those in the urethra. Therefore, VD simulates mostly urethral mechanisms of SUI rather than hypermobility or pelvic floor mechanisms.
Since birth injury is one of the greatest risk factors in the etiology of SUI in women, VD simultating the maternal injuries of delivery currently provides the best model to study the mechanisms of urethral injury and recovery as well as its pathophysiology. This model further allows us to explore and improve potential treatments to accelerate functional recovery of SUI since insufficient recovery after vaginal delivery injury along with other injuries and factors are strongly correlated with a later development of the SUI (Snooks et al. 1990). Therefore, the best use of the VD model may be in combination with other pathologies, injuries, or genetic manipulations. Work to date in combinatorial models is reviewed later in this chapter.
3.1.2 Pudendal Nerve Injury
The pudendal nerve controls EUS activity, including tonic activity during continence, and activates to strengthen the guarding response to prevent urinary leakage (Park et al. 1997; Evans 1936). It can be trapped and injured during vaginal childbirth when coursing through Alcock’s canal in the ischiorectal fossa, especially between the sacrospinous ligament and the sacrotuberous ligament (Snooks et al. 1986). Increased pudendal nerve terminal motor latency, indicative of nerve injury, correlates with vaginal delivery, advanced age, and SUI (Tetzschner et al. 1997; Olsen et al. 2003; Snooks et al. 1984). Several obstetric factors, such as multiparity, forceps delivery, increased duration of the second stage of labor, third degree perineal tear, and high birth mass, are specifically correlated with pudendal nerve damage (Snooks et al. 1986). While cesarean section can be sparing to the pudendal nerve, cesarean section during labor is not and can put a woman at risk of pudendal nerve damage (Sultan et al. 1994).
In addition to distal pudendal nerve injury at the EUS during vaginal delivery, clinical evidence also demonstrates damage to the pudendal nerve in Alcock’s canal, particularly as the nerve reenters the pelvis at the sacral spine. A novel animal model, pudendal nerve crush (PNC), simulating this damage, has been developed (Kerns et al. 2000). In rats, the rising roots of the pudendal nerve are more proximal than in humans since the pudendal nerve arises from L6–S1 roots in rats and S2–S4 roots in humans (McKenna and Nadelhaft 1986; Pacheco et al. 1989). Nonetheless, innervation of the EUS from the pudendal nerve is similar between rats and humans. In female rats, the motor pudendal nerve bifurcates within Alcock’s canal into separate fascicles that innervate the external anal sphincter and EUS (McKenna and Nadelhaft 1986; Kane et al. 2002).
For PNC, the pudendal nerve is accessed via the dorsal approach and is then crushed bilaterally in the ischiorectal fossa where it reenters the pelvis (Ahn et al. 2005; Kerns et al. 2000). In this model, all three branches of the pudendal nerve are crushed: sensory, EUS motor, and external anal sphincter motor, to simulate the injuries of childbirth. It is possible to injure each independently, however, potentially to test theories regarding reflexes or innervation.
PNC injury appears to result in acute SUI since LPP is significantly reduced until 2 weeks after the injury (Damaser et al. 2007). LPP then trends upward and returns to control levels with increasing time after injury, suggesting that nerve function begins to recover or compensatory changes in the urethra occur (Ahn et al. 2005; Damaser et al. 2007). Despite functional recovery 2 weeks after PNC, the distal nerve and EUS both show evidence of nerve degeneration and early signs of recovery at this time point (Damaser et al. 2007). Only 6 weeks after PNC is full neuroregeneration observed in the pudendal nerve and the EUS (Damaser et al. 2007), suggesting that 2 weeks represents an early time point of initial neuroregeneration and that there is redundancy in the innervation such that incomplete regeneration can lead to functional recovery. Estrogen has been shown to increase the neuroregenerative response of the injured pudendal nerve and promote and facilitate recovery of urethral function (Kane et al. 2004; Ahmed et al. 2006).
EUS EMG and pudendal nerve motor branch potential recordings after PNC support the redundancy theory since they demonstrate significant recovery 3 weeks afterward (Jiang et al. 2009a). However, the increase in amplitude and frequency of EUS EMG activity during LPP testing remains significantly different 3 weeks after PNC compared to normal rats, suggesting that nerve regeneration remains an ongoing process (Jiang et al. 2009a). Three months after PNC, approximately half the crushed pudendal neurons have regenerated to the EUS (Kerns et al. 2000), supporting the idea that incomplete regeneration is sufficient for functional recovery.
PNC injury in female rat, an SUI rodent model, results in a peripheral neurogenic and functional deficit followed by regeneration and recovery in the EUS. It allows evaluation of the pudendal nerve and neuromuscular recovery of the EUS and is useful for investigating mechanisms of neuromuscular recovery and for testing neuroregenerative agents or other potential treatments. It can also be utilized in conjunction with other pathologies, such as diabetes, aging, obesity, or genetic manipulation, to assess mechanism of injury and recovery in populations at increased risk for SUI.
3.1.3 Combination Models
During childbirth trauma, both muscles and nerves can be injured. Thus, the injuries occurring simultaneously during vaginal childbirth may represent a unique compound neuromuscular injury. These two injuries, along with injuries to the pelvic floor, are strongly correlated with later development of SUI (Snooks et al. 1990). Therefore, to further understand the mechanism of SUI after childbirth injury, both VD and PNC have been implemented in combination with each other to investigate the interaction between the different risk factors for SUI.
A dual injury model utilizing both PNC and VD has been developed and may best mimic the injuries incurred due to vaginal delivery (Jiang et al. 2009a). Functional recovery including EUS EMG and pudendal nerve motor branch potentials recorded during both LPP testing and voiding occurs more slowly after both PNC and VD than after either PNC or VD alone (Jiang et al. 2009a, b). Similarly, histological analysis of the EUS and the pudendal nerve confirms this result with slowed anatomical recovery from a dual injury (Pan et al. 2009; Jiang et al. 2009b).
Neurotrophins, particularly brain-derived neurotrophic factor, are upregulated by the target muscle to regenerate peripheral nerves and reinnervate the target muscle after nerve injury; in contrast, they are downregulated after muscle injury (Yan et al. 1994; Friedman et al. 1995; Sakuma et al. 2001). One day after VD, brain-derived neurotrophic factor expression in the EUS was reduced, whereas it was dramatically upregulated in the EUS 1 day after PNC (Pan et al. 2009). After a dual injury of both PNC and VD, brain-derived neurotrophic factor expression was upregulated somewhat but not to the same extent as after PNC, suggesting that insufficient brain-derived neurotrophic factor upregulation may contribute to slowed recovery after dual injury (Pan et al. 2009). Therefore, this model could be utilized to test methods that produce a local increase in neurotrophic factors as potential methods of promoting neuroregeneration and reinnervation after childbirth.
VD has also been combined with other pathophysiologies, such as diabets mellitus or oophorectomy (OVX), to study their interactions. Streptozotocin-induced diabetes causes increased severity and delayed functional recovery after VD (Kim et al. 2007), suggesting that diabetic women likely recover more slowly from maternal birth injuries. When VD follows delivery of rat pups, a later OVX increases the incidence of SUI, although OVX does not increase SUI incidence without VD (Sievert et al. 2001). On the other hand, Kuo (2002)found that OVX affects the intrinsic urethral closure mechanism, but without rat pup delivery, OVX does not affect LPP after VD. Multiple VD procedures have been utilized to simulate multiple deliveries (Kuo 2002; Pauwels et al. 2009). Pauwels et al. (2009) applied repeat VD to postpone recovery of urethral resistance to leakage. LPP is significantly decreased when both OVX and multiple VD were applied (Kuo 2002).
VD has also been performed in mice to induce SUI with the expectation of combining VD with genetic manipulation to study the impact of genetics on SUI (Lin et al. 2008b, 2010). A distension volume of 0.1–0.3 ml results in significantly reduced LPP in C57BL/6 female mice in the short term (Lin et al. 2008b). The density of immunoreactive neurofilaments in the urethra are reduced after VD with a 0.3- or 0.2-ml balloon (Lin et al. 2010). VD in mice could be more useful in future when trangenic models are combined with VD to investigate the genetic dependence of SUI in women after delivery. For example, elastin metabolism can be altered in women with SUI (Chen et al. 2006), and mice with genetically altered elastin metabolism can develop pelvic organ prolapse and decreased LPP after pup delivery (Lee et al. 2008). Combining this genetic model with VD could potentially elucidate the mechanism of elastin recovery after childbirth and identification of women at high risk for SUI.
3.2 Models Simulating Anatomic Support Damage
3.2.1 Urethrolysis
Urethrolysis, both retropubic and vaginal, has been performed for some patients with urethral obstruction or complications of urethral suspension procedures (Foster and McGuire 1993). It has also been utilized to create an animal model of durable urethral dysfunction or decreased urethral resistance, mimicking SUI. Transabdominal urethrolysis in female rats consists of circumferentially detaching the proximal urethra by incising the fascia (Rodriguez et al. 2005). The remaining urethra is then detached from the anterior vagina and pubis, which produces a significant decrease in both LPP and RUPP until 24 weeks after the procedure (Rodriguez et al. 2005). Denervation and an increase in the number of apoptotic cells have also been demonstrated 4–24 weeks after urethrolysis (Rodriguez et al. 2005). Pauwels et al. (2009) further refined this procedure as urethral transposition by surgically freeing the urethra and vagina from their fixation to the abdominal skin, resulting in a durable incontinence and a significant decrease in LPP 8 weeks after injury. Therefore, urethrolysis causes connective tissue damage and a significant diminishment of anatomic support and results in a long lasting or durable decrease in urethral resistance to leakage. It could be best suited for investigation of pathogenesis and treatment of the subset of SUI with obvious bladder neck or urethral hypermobility and could be utilized for preclinical evaluation of slings, injectables, and other potential treatments.
3.2.2 Pubourethral Ligament Injury
The pubourethral ligament (PUL) is believed to strongly attach the ventral surface of the urethra to the pubic bone, and its role in continence is described in the integral theory of continence and SUI (Petros 1998; Petros and Ulmsten 1990). Kefer et al. (2008, 2009) adopted the theory and created a PUL deficiency in female rats as a model of SUI. LPP is significantly decreased both in the short term (10 days) and in the long term (28 days) after suprapubic PUL transection (Kefer et al. 2008, 2009), validating PUL deficiency as an additional model of durable SUI. When transecting this ligament, it is almost impossible to eliminate potential injuries to the other structures, including other pelvic fascia and ligaments, as well as muscles, vessels, and nerves under the pubic symphysis. Both PUL transection and urethrolysis result in urethral mobility, but PUL transection appears to be a milder injury. Therefore, PUL transection has similar uses to urethrolysis as a model of SUI.
3.3 Models Simulating Intrinsic Urethra Deficit
3.3.1 Periurethral Cauterization
Periurethral cauterization was created as a model of SUI in rats by Chermansky et al. (2004). The procedure is performed via trans-abdominal access with a transurethral catheter for support. Electrocauterization of tissues lateral to the midurethra decreased LPP without affecting bladder function, reducing LPP 2 weeks after the procedure and maintaining it as decreased for up to 16 weeks (Chermansky et al. 2004). Histology suggests that damage to striated muscle and nerves contributes to the change in LPP in this model, potentially creating an intrinsic urethral deficit (Chermansky et al. 2004). It is also a durable model of SUI with more significant tissue damage and a long lasting decrease in urethral resistance. It is potentially most useful for investigation of potential interventions, especially those aimed at treating severe and/or complicated SUI.
3.3.2 Urethral Sphincterectomy
A canine SUI model of urethral sphincterectomy has recently been developed by Eberli et al. (2009) in which creation of irreversible damage to the sphincter was the goal. Approximately ¼ of the circumferential sphincter muscle, containing both smooth and striated muscle, was excised through a low midline abdominal incision (Eberli et al. 2009). Both histology and pudendal nerve stimulation indicated an absence of sphincter muscle and its response. Seven months after sphincterectomy, urethral resistance to leakage remained significantly decreased, as evidenced by decreased urethral pressure profile and stress urethral profile as well as decreased LPP. This SUI model represents an extreme and irreversible sphincter deficiency, which could be useful for preclinical evaluation of methods of entirely reproducing sphincter function, such as with an artificial or cell-based sphincter replacement procedure.
3.3.3 Pudendal Nerve Transection
Since PNC recovers relatively rapidly, bilateral pudendal nerve transection has been used to study permanent damage to the pudendal nerve (Conway et al. 2005; Kamo et al. 2003; Hijaz et al. 2004; Jiang et al. 2009a; Peng et al. 2006). The procedure is the same as PNC, except that the pudendal nerve is transected bilaterally instead of being crushed. A segment of nerve (~2 mm long) is removed from the transection site to prevent neuroregeneration. A significant decrease in LPP results, both in the short term and as long as 6 weeks after pudendal nerve transection (PNT) (Conway et al. 2005; Kamo et al. 2003; Jiang et al. 2009a; Peng et al. 2006). The decrease in LPP is only partial if a unilateral PNT is performed (Peng et al. 2006). Peng et al. (2006) demonstrated that voiding efficiency also decreases after PNT, suggesting that EUS bursting activity during voiding facilitates emptying in female rats. These studies all support the idea that the pudendal nerve plays a critical role in the mechanism of urinary continence since as soon as it is injured, the urethral resistance to leakage decreases significantly.
Jiang et al. (2009b) confirmed that 6 weeks after PNT, the EUS showed neurogenic atrophy and contained thin small muscle bundles with no striations. No significant EUS EMG tonic activity and bursting during voiding was recorded 6 weeks after PNT (Jiang et al. 2009a, b). This is in contrast to a previous report by Peng et al., who demonstrated EUS EMG tonic activity and bursting during voiding 6 weeks after PNT despite a decrease in the diameter of striated muscle bundles in the EUS (Peng et al. 2006). This difference could be due to the use of different EMG electrodes in the two studies, suggesting a need to standardize electrodes used for measuring EUS EMG in models of SUI.
PNT has also been investigated in other female animals, such mice, cats, and dogs (Bernabe et al. 2008; Lin et al. 2010; li-El-Dein and Ghoneim 2001). Lin et al. (2010) confirmed permanent effects of PNT in female mice and that both LPP and the density of neurofilaments in the urethra were significantly reduced 4, 10, and 20 days after PNT. In female cats, after unilateral pudendal and pelvic nerve lesions, only distal urethral pressure decreased significantly. In contrast, bilateral pudendal and pelvic nerve lesions may cause urine leakage and significantly decrease the urethral pressure response (Bernabe et al. 2008). Bladder and urethral function after PNT has also been investigated in female dogs (li-El-Dein and Ghoneim 2001). While not specifically designed as an animal model of SUI, the results indicate a decrease in urethral resistance after PNT (li-El-Dein and Ghoneim 2001).
3.3.4 Reagent Periurethral Injection
Botulinum Toxin has been used as a reagent to induce chemical denervation of the urethral sphincter via periurethral injection in rats (Takahashi et al. 2006). A significant decrease in LPP occurred 2 weeks after injection and was supported by evidence of shrinkage of smooth and striated urethral muscle. Six weeks after injection, rats demonstrated functional recovery, making this a model of reversible SUI aimed specifically at injuring the function of smooth and striated muscle. It may be most useful for preclinical testing of treatments to accelerate recovery after chemical or radiation-induced SUI.
4 Treatment Testing on SUI Animal Models
Clinical treatments for SUI include conservative techniques, pharmacologic therapy, and surgical procedures. These therapies aim to strengthen urinary continence via specific or integrated deficiency correction. Although standard interventions such as anti-incontinence surgery have been verifiably successful in restoration of anatomic support and improvement of intrinsic urethral deficiencies (Williams and Klutke 2008), it is still necessary to use animal models of SUI to test new surgical techniques and materials, pharmacologic targets, and therapeutic strategies, such as stem cell therapy. We review here the work to date using these animal models to test potential new treatments.
4.1 Pharmacological Therapy Testing
4.1.1 Serotonin and Norepinephrine Reuptake Inhibitors
Experiments in cats suggested that duloxetine, a serotonin and norepinephrine reuptake inhibitor, could enhance EUS activity through 5HT2 and α1 adrenergic mechanisms (Thor and Katofiasc 1995), indicating promise for the treatment of SUI. After VD in rats, urethral resistance was significantly increased by duloxetine administered intravenously (Miyazato et al. 2008). Intrathecal administration of idazoxan, a selective α2 adrenergic receptor antagonist, indicated that duloxetine can prevent SUI by facilitating noradrenergic and serotonergic systems in the spinal cord, enhancing the sneeze reflex (Miyazato et al. 2008). Other similar SNRIs, including venlafaxine (Bae et al. 2001) and nisoxetine (Kaiho et al. 2007), can also increase urethral pressure in rats and have also been suggested to be useful for treatment of SUI. This class of drug, however, has the potential for central nervous system complications, keeping it from being made available to the general public in the USA (2005).
4.1.2 Fibroblast Growth Factor Testing
Takahashi et al. (2006) evaluated the effects of sustained release of basic fibroblast growth factor (bFGF) in rat urethra denervated by botulinum-A toxin. They incorporated bFGF into 200 µl gelatin hydrogels and injected them into the urethral sphincter, enabling sustained release of bFGF. They determined that bFGF delivered in this fashion produced a significant improvement in urethral sphincter contractility and suggested bFGF as a promising therapy for SUI. Further testing in other animal models and experiments designed to elucidate the mechanism of effect of bFGF ought to be performed to assess the clinical utility of this potential treatment.
4.1.3 Angiotensin II Testing
Using the rat SUI models of PNT and urethrolysis, Phull et al. (2007) demonstrated that angiotensin I and II receptor inhibition significantly decreases urethral resistance to leakage as evidenced by decreased LPP and RUPP. Angiotensin II treatment restored urethral tone in rats with intrinsic sphincter dysfunction, suggesting that angiotensin II may have a functional role in the maintenance of urethral tone and in continence function (Phull et al. 2007). This work could be continued using other models of SUI to determine the mechanism of the role of angiotensin II in continence and SUI in order to assess its clinical utility.
4.2 Sling Surgical Procedures
Since the last century, different urethral suspension techniques and multiple types of slings have been developed (Williams and Klutke 2008). However, there is no consensus on the ideal sling procedure since there is no consensus on the various theories and explanations for the urinary continence mechanism (DeLancey et al. 2008). Today, the tension-free vaginal tape procedure has become one of the most popular sling procedures (Rapp and Kobashi 2008). Nonetheless, animal models of a rat urethral sling procedure have been developed to further evaluate the mechanism of the sling procedure and its complications (Hijaz et al. 2004).
Using LPP testing on a rat PNT model, Hijaz et al. (2004, 2005a) determined that a vaginal sling procedure results in restoration of urethral resistance to leakage in both short- and long-term (5 weeks) testing. They further confirmed that the sling procedure may cause bladder outlet obstruction regardless of whether the mid-urethral segment of the sling was cut after the procedure (Hijaz et al. 2005b). They demonstrated histological changes as a result of both intact and cut slings, including inflammation, localized edema, and differential collagen remodeling, which could account for the preserved anti-incontinence mechanisms of cut or intact polypropylene slings observed clinically (Chen et al. 2009).
To test long-term continence recovery, Cannon et al. (2005) combined tissue engineering and a sling procedure in an SUI rat model in which the proximal sciatic nerve was transected bilaterally. They prepared tissue-engineered slings with muscle-derived cells obtained via the preplate technique and subsequently seeded for 2 weeks on a small intestinal submucosa scaffold. The sling procedure was performed via a transabdominal approach and resulted in significantly increased LPP with no urinary retention, suggesting that the cells may help prevent the outlet obstruction complications of slings (Cannon et al. 2005). Further work on the mechanism of this effect is needed to assess its clinical utility.
4.3 Cell-Based Therapy Testing
Cell-based therapies and tissue engineering hold potential as treatments and preventions for SUI, particularly treatment with autologous multipotent adult stem cells, including mesenchymal stem cells, muscle-derived stem cells (MDSC), and adipose-derived stem cells (ADSC) (Furuta et al. 2007; Nikolavsky and Chancellor 2010). These adult stem cells can be derived from a given subject, expanded in culture, and given back to the subject. Stem cell strategies for SUI have been studied in animal models and are presently undergoing clinical trials (Carr et al. 2008). Although controversy and conflicting results have occurred in the field, preliminary results froma recent clinical trial appear to be promising (Nikolavsky and Chancellor 2010).
4.3.1 Muscle Precursor Cell Therapy
Implantation of muscle precursor cells (MPC) can be traced to three decades ago, when fusion between donor MPCs and host myofibers was first noted in skeletal muscle transfer (Partridge et al. 1978). Cannon et al. applied this theory and tested direct injection of allogenic MPC into rat urethral sphincters denervated by PNT (Cannon et al. 2003). Their results indicated that the cells significantly improved the fast-twitch muscle contraction amplitude of the denervated sphincter to 87% of normal animals. New skeletal muscle fiber formation at the injection site of the urethra was also indicated by immunohistochemistry (Cannon et al. 2003). MPC autografting in a murine model of urethral sphincter injury has also been reported (Yiou et al. 2002). The results demonstrated that this procedure may accelerate sphincter muscle repair by producing a significant increase in the diameter and number of myofibers (Yiou et al. 2002), suggesting that MPC autografting represents a potential new therapeutic approach to urethral sphincter insufficiency.
4.3.2 Stem Cell Homing Signals
Beside direct injection of cells, utilization of cytokines and stem cell homing signals as a treatment for SUI may represent an alternative treatment paradigm. Recently, using a VD rat model of SUI, Woo et al. (2007) demonstrated MCP-3 overexpression in the urethra immediately following VD, as well as a strong correlation between VD duration and the subsequent expression of MCP-3 and one of its associated receptors, CCR1, in the urethra (Wood et al. 2008). This result was consistent regardless of whether an inbred or outbred strain of rat was used (Woo et al. 2009). These studies could form the basis for further evaluation of MCP-3 and stem cell homing for treatment or prevention of SUI (Takacs 2007).
4.3.3 Adipose-Derived Stem Cell Therapy
ADSC can differentiate into adipogenic, myogenic, and osteogenic cells when specific induction factors are present (Zuk et al. 2002). ADSC treatment may also be a promising method for treatment of intrinsic urethral deficiency. Jack et al. (2005) found that human-processed lipoaspirate cells, an accessible source of pluripotent cells, remain viable up to 12 weeks after injection into the urethra with incorporation and differentiation, suggesting that processed lipoaspirate cells may represent a feasible cell source for SUI treatment. They further reported LPP and urethral functional improvement in a rat urethrolysis model of SUI after ADSC injection with a biodegradable microbead carrier (Zeng et al. 2006). Further work is needed to determine the fate of these cells and the mechanism of this effect.
4.3.4 Muscle-Derived Stem Cell Therapy
MDSC display significant regenerative capacity and an improved transplantation capacity compared to MPC implantation (Furuta et al. 2007). In a rat PNT model of SUI, urethral resistance was restored in both the short- and long-term (12 weeks) with periurethral injection of MDSC (Lee et al. 2003, 2004). Although both MDSC and fibroblast injection increased LPP, only the MDSC treatment group had significantly improved urethral muscle contractility (Kwon et al. 2006). MDSC injection also significantly increased LPP 4 and 6 weeks after urethral injury by periurethral cauterization (Chermansky et al. 2004). Therefore, in addition to providing a bulking effect, direct injection of MDSC may physiologically improve urethral sphincter contraction and contribute to continence in animal models of SUI. Clinical trials have commenced, and some results remain controversial (Carr et al. 2008; Aboushwareb and Atala 2008). Therefore, further laboratory investigation is needed to elucidate the mechanism of this effect, and caution is advised in commencing with clinical trials.
5 Summary and Perspective on SUI Animal Models
Investigation into the mechanisms of SUI and development of animal models to test innovative treatments and preventions is relatively new and has blossomed over the course of the last decade. The models and methods of testing provide promise in utilization for preclinical testing of potential treatments and elucidation of the mechanism of SUI development and the mechanism of effect of potential treatments. Much of the testing to date has been observational, documenting the time course of events after an injury, a treatment, or both. Even as the field moves to clinical testing of innovative treatments, more laboratory research needs to be done using interventional studies aimed specifically at determining the mechanism of injury and the mechanism of potential treatments since many questions remain unanswered regarding the mechanism of observed effects
References
- Duloxetine: new drug. For stress urinary incontinence: too much risk, too little benefit. Prescrire Int. 2005;14(80):218–220. [PubMed] [Google Scholar]
- Aboushwareb T, Atala A. Stem cells in urology. Nat Clin Pract Urol. 2008;5(11):621–631. doi: 10.1038/ncpuro1228. [DOI] [PubMed] [Google Scholar]
- Ahmed Y, Lin DL, Ferguson C, Esparza N, Damaser MS. Effect of estrogen on urethral function and nerve regeneration following pudendal nerve crush in the female rat. J Urol. 2006;175(5):1948–1952. doi: 10.1016/S0022-5347(05)00894-3. [DOI] [PubMed] [Google Scholar]
- Ahn H, Lin DL, Esparza N, Damaser MS. Short-term timecourse of bilateral pudendal nerve injury on leak-point pressure in female rats. J Rehabil Res Dev. 2005;42(1):109–114. [PubMed] [Google Scholar]
- Aroni F, Iacovidou N, Dontas I, Pourzitaki C, Xanthos T. Pharmacological aspects and potential new clinical applications of ketamine: reevaluation of an old drug. J Clin Pharmacol. 2009;49(8):957–964. doi: 10.1177/0091270009337941. [DOI] [PubMed] [Google Scholar]
- Bae JH, Moon DG, Lee JG. The effects of a selective noradrenaline reuptake inhibitor on the urethra: an in vitro and in vivo study. BJU Int. 2001;88(7):771–775. doi: 10.1046/j.1464-4096.2001.02389.x. [DOI] [PubMed] [Google Scholar]
- Bernabe J, Julia-Guilloteau V, Denys P, Chartier-Kastler E, Alexandre L, Peeters M, et al. Peripheral neural lesion-induced stress urinary incontinence in anaesthetized female cats. BJU Int. 2008;102(9):1162–1167. doi: 10.1111/j.1464-410X.2008.07795.x. [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, Leong SA, Oster EF, Kinchen K, Sun P. Cost of stress urinary incontinence: a claims data analysis. Pharmacoeconomics. 2004;22(2):95–105. doi: 10.2165/00019053-200422020-00003. [DOI] [PubMed] [Google Scholar]
- Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 2001;69:1193–1202. doi: 10.1016/s0024-3205(01)01182-1. [DOI] [PubMed] [Google Scholar]
- Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Int. 2002;90(4):403–407. doi: 10.1046/j.1464-410x.2002.02918.x. [DOI] [PubMed] [Google Scholar]
- Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003;62(5):958–963. doi: 10.1016/s0090-4295(03)00679-4. [DOI] [PubMed] [Google Scholar]
- Cannon TW, Sweeney DD, Conway DA, Kamo I, Yoshimura N, Sacks M, et al. A tissue-engineered suburethral sling in an animal model of stress urinary incontinence. BJU Int. 2005;96(4):664–669. doi: 10.1111/j.1464-410X.2005.05702.x. [DOI] [PubMed] [Google Scholar]
- Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(6):881–883. doi: 10.1007/s00192-007-0553-z. [DOI] [PubMed] [Google Scholar]
- Chen B, Wen Y, Zhang Z, Guo Y, Warrington JA, Polan ML. Microarray analysis of differentially expressed genes in vaginal tissues from women with stress urinary incontinence compared with asymptomatic women. Hum Reprod. 2006;21(1):22–29. doi: 10.1093/humrep/dei276. [DOI] [PubMed] [Google Scholar]
- Chen CC, Hijaz A, Drazba JA, Damaser MS, Daneshgari F. Collagen remodeling and suburethral inflammation might account for preserved anti-incontinence effects of cut polypropylene sling in rat model. Urology. 2009;73(2):415–420. doi: 10.1016/j.urology.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermansky CJ, Cannon TW, Torimoto K, Fraser MO, Yoshimura N, de Groat WC, et al. A model of intrinsic sphincteric deficiency in the rat: electrocauterization. Neurourol Urodyn. 2004;23(2):166–171. doi: 10.1002/nau.10173. [DOI] [PubMed] [Google Scholar]
- Conway DA, Kamo I, Yoshimura N, Chancellor MB, Cannon TW. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(5):359–363. doi: 10.1007/s00192-004-1263-4. [DOI] [PubMed] [Google Scholar]
- Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003;170(3):1027–1031. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- Damaser MS, Whitbeck C, Chichester P, Levin RM. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol. 2005;98(5):1884–1890. doi: 10.1152/japplphysiol.01071.2004. [DOI] [PubMed] [Google Scholar]
- Damaser MS, Samplaski MK, Parikh M, Lin DL, Rao S, Kerns JM. Time course of neuroanatomical and functional recovery after bilateral pudendal nerve injury in female rats. Am J Physiol Renal Physiol. 2007;293(5):F1614–F1621. doi: 10.1152/ajprenal.00176.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLancey JO, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008;179(6):2286–2290. doi: 10.1016/j.juro.2008.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberli D, Andersson KE, Yoo JJ, Atala A. A canine model of irreversible urethral sphincter insufficiency. BJU Int. 2009;103(2):248–253. doi: 10.1111/j.1464-410X.2008.08001.x. [DOI] [PubMed] [Google Scholar]
- Evans JP. Observations on the nerves of supply to the bladder and urethra of the cat, with a study of their action potentials. J Physiol. 1936;86(4):396–414. doi: 10.1113/jphysiol.1936.sp003375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster HE, McGuire EJ. Management of urethral obstruction with transvaginal urethrolysis. J Urol. 1993;150(5 Pt 1):1448–1451. doi: 10.1016/s0022-5347(17)35805-6. [DOI] [PubMed] [Google Scholar]
- Friedman B, Kleinfeld D, Ip NY, Verge VM, Moulton R, Boland P, et al. BDNF and NT-4/5 exert neurotrophic influences on injured adult spinal motor neurons. J Neurosci. 1995;15(2):1044–1056. doi: 10.1523/JNEUROSCI.15-02-01044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta A, Jankowski RJ, Pruchnic R, Yoshimura N, Chancellor MB. The promise of stem cell therapy to restore urethral sphincter function. Curr Urol Rep. 2007;8(5):373–378. doi: 10.1007/s11934-007-0034-4. [DOI] [PubMed] [Google Scholar]
- Haab F, Zimmern PE, Leach GE. Female stress urinary incontinence due to intrinsic sphincteric deficiency: recognition and management. J Urol. 1996;156(1):3–17. [PubMed] [Google Scholar]
- Hampel C, Artibani W, Espuna PM, Haab F, Jackson S, Romero J, et al. Understanding the burden of stress urinary incontinence in Europe: a qualitative review of the literature. Eur Urol. 2004;46(1):15–27. doi: 10.1016/j.eururo.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An international Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(4):20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- Hijaz A, Daneshgari F, Cannon T, Damaser M. Efficacy of a vaginal sling procedure in a rat model of stress urinary incontinence. J Urol. 2004;172(5 Pt 1):2065–2068. doi: 10.1097/01.ju.0000138476.42556.b8. [DOI] [PubMed] [Google Scholar]
- Hijaz A, Bena J, Daneshgari F. Long-term efficacy of a vaginal sling procedure in a rat model of stress urinary incontinence. J Urol. 2005a;173(5):1817–1819. doi: 10.1097/01.ju.0000154342.75020.05. [DOI] [PubMed] [Google Scholar]
- Hijaz A, Daneshgari F, Huang X, Bena J, Liu G, Saffore L, et al. Role of sling integrity in the restoration of leak point pressure in the rat vaginal sling model. J Urol. 2005b;174(2):771–775. doi: 10.1097/01.ju.0000164721.52278.29. [DOI] [PubMed] [Google Scholar]
- Hijaz A, Daneshgari F, Sievert KD, Damaser MS. Animal models of female stress urinary incontinence. J Urol. 2008;179(6):2103–2110. doi: 10.1016/j.juro.2008.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack GS, Almeida FG, Zhang R, Alfonso ZC, Zuk PA, Rodriguez LV. Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implications for the treatment of stress urinary incontinence and bladder reconstruction. J Urol. 2005;174(5):2041–2045. doi: 10.1097/01.ju.0000176489.96993.84. [DOI] [PubMed] [Google Scholar]
- Jiang HH, Pan HQ, Gustilo-Ashby MA, Gill B, Glaab J, Zaszczurynski P, et al. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol Urodyn. 2009a;28(3):229–235. doi: 10.1002/nau.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HH, Gustilo-Ashby AM, Salcedo LB, Pan HQ, Sypert DF, Butler RS, et al. Electrophysiological function during voiding after simulated childbirth injuries. Exp Neurol. 2009b;215(2):342–348. doi: 10.1016/j.expneurol.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julia-Guilloteau V, Denys P, Bernabe J, Mevel K, Chartier-Kastler E, Alexandre L, et al. Urethral closure mechanisms during sneezing-induced stress in anesthetized female cats. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1357–R1367. doi: 10.1152/ajpregu.00003.2007. [DOI] [PubMed] [Google Scholar]
- Kaiho Y, Kamo I, Chancellor MB, Arai Y, de Groat WC, Yoshimura N. Role of noradrenergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2007;292(2):F639–F646. doi: 10.1152/ajprenal.00226.2006. [DOI] [PubMed] [Google Scholar]
- Kamo I, Hashimoto T. Involvement of reflex urethral closure mechanisms in urethral resistance under momentary stress condition induced by electrical stimulation of rat abdomen. Am J Physiol Renal Physiol. 2007;293(3):F920–F926. doi: 10.1152/ajprenal.00466.2006. [DOI] [PubMed] [Google Scholar]
- Kamo I, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N. Urethral closure mechanisms under sneeze-induced stress condition in rats: a new animal model for evaluation of stress urinary incontinence. Am J Physiol Regul Integr Comp Physiol. 2003;285(2):R356–R365. doi: 10.1152/ajpregu.00010.2003. [DOI] [PubMed] [Google Scholar]
- Kamo I, Cannon TW, Conway DA, Torimoto K, Chancellor MB, de Groat WC, et al. The role of bladder-to-urethral reflexes in urinary continence mechanisms in rats. Am J Physiol Renal Physiol. 2004;287(3):F434–F441. doi: 10.1152/ajprenal.00038.2004. [DOI] [PubMed] [Google Scholar]
- Kamo I, Kaiho Y, Canon TW, Chancellor MB, de Groat WC, Prantil RL, et al. Functional analysis of active urethral closure mechanisms under sneeze induced stress condition in a rat model of birth trauma. J Urol. 2006;176(6 Pt 1):2711–2715. doi: 10.1016/j.juro.2006.07.139. [DOI] [PubMed] [Google Scholar]
- Kane DD, Shott S, Hughes WF, Kerns JM. Motor pudendal nerve characterization in the female rat. Anat Rec. 2002;266(1):21–29. doi: 10.1002/ar.10029. [DOI] [PubMed] [Google Scholar]
- Kane DD, Kerns JM, Lin DL, Damaser MS. Early structural effects of oestrogen on pudendal nerve regeneration in the rat. BJU Int. 2004;93(6):870–878. doi: 10.1111/j.1464-410X.2003.04792.x. [DOI] [PubMed] [Google Scholar]
- Kefer JC, Liu G, Daneshgari F. Pubo-urethral ligament transection causes stress urinary incontinence in the female rat: a novel animal model of stress urinary incontinence. J Urol. 2008;179(2):775–778. doi: 10.1016/j.juro.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefer JC, Liu G, Daneshgari F. Pubo-urethral ligament injury causes long-term stress urinary incontinence in female rats: an animal model of the integral theory. J Urol. 2009;181(1):397–400. doi: 10.1016/j.juro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, et al. Effects of pudendal nerve injury in the female rat. Neurourol Urodyn. 2000;19(1):53–69. doi: 10.1002/(sici)1520-6777(2000)19:1<53::aid-nau7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kim JH, Huang X, Liu G, Moore C, Bena J, Damaser MS, et al. Diabetes slows the recovery from urinary incontinence due to simulated childbirth in female rats. Am J Physiol Regul Integr Comp Physiol. 2007;293(2):R950–R955. doi: 10.1152/ajpregu.00686.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HC. Effects of vaginal trauma and oophorectomy on the continence mechanism in rats. Urol Int. 2002;69(1):36–41. doi: 10.1159/000064358. [DOI] [PubMed] [Google Scholar]
- Kwon D, Kim Y, Pruchnic R, Jankowski R, Usiene I, de MF, et al. Periurethral cellular injection: comparison of muscle-derived progenitor cells and fibroblasts with regard to efficacy and tissue contractility in an animal model of stress urinary incontinence. Urology. 2006;68(2):449–454. doi: 10.1016/j.urology.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(1):31–37. doi: 10.1007/s00192-002-1004-5. [DOI] [PubMed] [Google Scholar]
- Lee JY, Paik SY, Yuk SH, Lee JH, Ghil SH, Lee SS. Long term effects of muscle-derived stem cells on leak point pressure and closing pressure in rats with transected pudendal nerves. Mol Cells. 2004;18(3):309–313. [PubMed] [Google Scholar]
- Lee UJ, Gustilo-Ashby AM, Daneshgari F, Kuang M, Vurbic D, Lin DL, et al. Lower urogenital tract anatomical and functional phenotype in lysyl oxidase like-1 knockout mice resembles female pelvic floor dysfunction in humans. Am J Physiol Renal Physiol. 2008;295(2):F545–F555. doi: 10.1152/ajprenal.00063.2008. [DOI] [PubMed] [Google Scholar]
- li-El-Dein B, Ghoneim MA. Effects of selective autonomic and pudendal denervation on the urethral function and development of retention in female dogs. J Urol. 2001;166(4):1549–1554. [PubMed] [Google Scholar]
- Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52(1):143–151. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- Lin G, Shindel AW, Banie L, Deng D, Wang G, Hayashi N, et al. Molecular mechanisms related to parturition-induced stress urinary incontinence. Eur Urol. 2008a;55:1213–1223. doi: 10.1016/j.eururo.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Liu G, Daneshgari F. A mouse model of simulated birth trauma induced stress urinary incontinence. Neurourol Urodyn. 2008b;27(4):353–358. doi: 10.1002/nau.20509. [DOI] [PubMed] [Google Scholar]
- Lin YH, Liu G, Li M, Xiao N, Daneshgari F. Recovery of continence function following simulated birth trauma involves repair of muscle and nerves in the urethra in the female mouse. Eur Urol. 2010;57(3):512–513. doi: 10.1016/j.eururo.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lose G, Griffiths D, Hosker G, Kulseng-Hanssen S, Perucchini D, Schafer W, et al. Standardization Sub-Committee, International Continence Society. Standardization of urethral pressure measurement: report from the Standardization Sub-Committee of the International Continence Society. Neurourol Urodyn. 2002;21:258–260. doi: 10.1002/nau.10051. [DOI] [PubMed] [Google Scholar]
- McGuire EJ. Pathophysiology of stress urinary incontinence. Rev Urol. 2004;6 suppl 5:S11–S17. [PMC free article] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248(4):532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- Miyazato M, Kaiho Y, Kamo I, Chancellor MB, Sugaya K, de Groat WC, et al. Effect of duloxetine, a norepinephrine and serotonin reuptake inhibitor, on sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2008;295(1):F264–F271. doi: 10.1152/ajprenal.90241.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolavsky D, Chancellor MB. Stem cell therapy for stress urinary incontinence. Neurourol Urodyn. 2010;29 suppl 1:S36–S41. doi: 10.1002/nau.20833. [DOI] [PubMed] [Google Scholar]
- Olsen AL, Ross M, Stansfield RB, Kreiter C. Pelvic floor nerve conduction studies: establishing clinically relevant normative data. Am J Obstet Gynecol. 2003;189(4):1114–1119. doi: 10.1067/s0002-9378(03)00551-9. [DOI] [PubMed] [Google Scholar]
- Pacheco P, Martinez-Gomez M, Whipple B, Beyer C, Komisaruk BR. Somato-motor components of the pelvic and pudendal nerves of the female rat. Brain Res. 1989;490(1):85–94. doi: 10.1016/0006-8993(89)90433-2. [DOI] [PubMed] [Google Scholar]
- Pan HQ, Kerns JM, Lin DL, Liu S, Esparza N, Damaser MS. Increased duration of simulated childbirth injuries results in increased time to recovery. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1738–R1744. doi: 10.1152/ajpregu.00784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HQ, Kerns JM, Lin DL, Sypert D, Steward J, Hoover CR, et al. Dual simulated childbirth injury delays anatomic recovery. Am J Physiol Renal Physiol. 2009;296(2):F277–F283. doi: 10.1152/ajprenal.90602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Bloom DA, McGuire EJ. The guarding reflex revisited. Br J Urol. 1997;80(6):940–945. doi: 10.1046/j.1464-410x.1997.00488.x. [DOI] [PubMed] [Google Scholar]
- Partridge TA, Grounds M, Sloper JC. Evidence of fusion between host and donor myoblasts in skeletal muscle grafts. Nature. 1978;273(5660):306–308. doi: 10.1038/273306a0. [DOI] [PubMed] [Google Scholar]
- Pauwels E, De WS, Wyndaele JJ. Evaluation of different techniques to create chronic urinary incontinence in the rat. BJU Int. 2009;103(6):782–785. doi: 10.1111/j.1464-410X.2008.08158.x. [DOI] [PubMed] [Google Scholar]
- Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn. 2006;25(4):388–396. doi: 10.1002/nau.20229. [DOI] [PubMed] [Google Scholar]
- Petros PE. The pubourethral ligaments–an anatomical and histological study in the live patient. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9(3):154–157. doi: 10.1007/BF02001085. [DOI] [PubMed] [Google Scholar]
- Petros PE, Ulmsten UI. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl. 1990;153:7–31. doi: 10.1111/j.1600-0412.1990.tb08027.x. [DOI] [PubMed] [Google Scholar]
- Phull H, Salkini M, Escobar C, Purves T, Comiter CV. The role of angiotensin II in stress urinary incontinence: A rat model. Neurourol Urodyn. 2007;26(1):81–88. doi: 10.1002/nau.20339. [DOI] [PubMed] [Google Scholar]
- Rapp DE, Kobashi KC. The evolution of midurethral slings. Nat Clin Pract Urol. 2008;5(4):194–201. doi: 10.1038/ncpuro1052. [DOI] [PubMed] [Google Scholar]
- Resplande J, Gholami SS, Graziottin TM, Rogers R, Lin CS, Leng W, et al. Long-term effect of ovariectomy and simulated birth trauma on the lower urinary tract of female rats. J Urol. 2002;168(1):323. [PubMed] [Google Scholar]
- Rodriguez LV, Chen S, Jack GS, de AF, Lee KW, Zhang R. New objective measures to quantify stress urinary incontinence in a novel durable animal model of intrinsic sphincter deficiency. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1332–R1338. doi: 10.1152/ajpregu.00760.2004. [DOI] [PubMed] [Google Scholar]
- Rovner ES, Wein AJ. Treatment options for stress urinary incontinence. Rev Urol. 2004;6 suppl 3:S29–S47. [PMC free article] [PubMed] [Google Scholar]
- Sakuma K, Watanabe K, Sano M, Uramoto I, Nakano H, Li YJ, et al. A possible role for BDNF, NT-4 and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection and axotomy. Brain Res. 2001;907(1)(2):1–19. doi: 10.1016/s0006-8993(01)02288-0. [DOI] [PubMed] [Google Scholar]
- Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25(1):245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- Shoffstall AJ, Zaszczurynski PJ, Butler RS, Damase RMS. Development of a device to standardize leak point pressure experiments in rats. Neurourol Urodyn. 2008;27(6):553. doi: 10.1002/nau.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert KD, Emre BM, Tsai T, Dahms SE, Nunes L, Lue TF. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol. 2001;166(1):311–317. [PubMed] [Google Scholar]
- Sievert KD, Bakircioglu ME, Tsai T, Nunes L, Lue TF. The effect of labor and/or ovariectomy on rodent continence mechanism–the neuronal changes. World J Urol. 2004;22(4):244–250. doi: 10.1007/s00345-004-0444-6. [DOI] [PubMed] [Google Scholar]
- Snooks SJ, Barnes PR, Swash M. Damage to the innervation of the voluntary anal and periurethral sphincter musculature in incontinence: an electrophysiological study. J Neurol Neurosurg Psychiatry. 1984;47(12):1269–1273. doi: 10.1136/jnnp.47.12.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snooks SJ, Swash M, Henry MM, Setchell M. Risk factors in childbirth causing damage to the pelvic floor innervation. Int J Colorectal Dis. 1986;1(1):20–24. doi: 10.1007/BF01648831. [DOI] [PubMed] [Google Scholar]
- Snooks SJ, Swash M, Mathers SE, Henry MM. Effect of vaginal delivery on the pelvic floor: a 5-year follow-up. BRJ Surg. 1990;77(12):1358–1360. doi: 10.1002/bjs.1800771213. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Nishijima S, Miyazato M, Ogawa Y. Central nervous control of micturition and urine storage. J Smooth Muscle Res. 2005;41(3):117–132. doi: 10.1540/jsmr.41.117. [DOI] [PubMed] [Google Scholar]
- Sultan AH, Kamm MA, Hudson CN. Pudendal nerve damage during labour: prospective study before and after childbirth. BR J Obstet Gynaecol. 1994;101(1):22–28. doi: 10.1111/j.1471-0528.1994.tb13005.x. [DOI] [PubMed] [Google Scholar]
- Takacs EB. The potential use of cytokines and stem cell homing signals in the treatment of stress urinary incontinence. J Urol. 2007;177(4):1227–1228. doi: 10.1016/j.juro.2007.01.074. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Chen Q, Ogushi T, Fujimura T, Kumagai J, Matsumoto S, et al. Periurethral injection of sustained release basic fibroblast growth factor improves sphincteric contractility of the rat urethra denervated by botulinum-a toxin. J Urol. 2006;176(2):819–823. doi: 10.1016/j.juro.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Tetzschner T, Sorensen M, Jonsson L, Lose G, Christiansen J. Delivery and pudendal nerve function. Acta Obstet Gynecol Scand. 1997;76(4):324–331. doi: 10.1111/j.1600-0412.1997.tb07986.x. [DOI] [PubMed] [Google Scholar]
- Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther. 1995;274(2):1014–1024. [PubMed] [Google Scholar]
- Thuroff JW, Bazeed MA, Schmidt RA, Tanagho EA. Mechanisms of urinary continence: an animal model to study urethral responses to stress conditions. J Urol. 1982;127(6):1202–1206. doi: 10.1016/s0022-5347(17)54297-4. [DOI] [PubMed] [Google Scholar]
- Wallois F, Gros F, Masmoudi K, Larnicol N. C-Fos-like immunoreactivity in the cat brainstem evoked by sneeze-inducing air puff stimulation of the nasal mucosa. Brain Res. 1995;687(1):2, 143–154. doi: 10.1016/0006-8993(95)00487-b. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG. Neurophysiology of the cough reflex. Eur Respir J. 1995;8(7):1193–1202. doi: 10.1183/09031936.95.08071193. [DOI] [PubMed] [Google Scholar]
- Williams ER, Klutke CG. Stress urinary incontinence: the evolution of the sling. Expert Rev Med Devices. 2008;5(4):507–523. doi: 10.1586/17434440.5.4.507. [DOI] [PubMed] [Google Scholar]
- Woo LL, Hijaz A, Kuang M, Penn MS, Damaser MS, Rackley RR. Over expression of stem cell homing cytokines in urogenital organs following vaginal distention. J Urol. 2007;177(4):1568–1572. doi: 10.1016/j.juro.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Woo LL, Hijaz A, Pan HQ, Kuang M, Rackley RR, Damase MS. Simulated childbirth injuries in an inbred rat strain. Neurourol Urodyn. 2009;28(4):356–361. doi: 10.1002/nau.20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood HM, Kuang M, Woo L, Hijaz A, Butler RS, Penn M, et al. Cytokine expression after vaginal distention of different durations in virgin Sprague-Dawley rats. J Urol. 2008;180(2):753–759. doi: 10.1016/j.juro.2008.03.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Matheson C, Lopez OT, Miller JA. The biological responses of axotomized adult motoneurons to brain-derived neurotrophic factor. J Neurosci. 1994;14(9):5281–5291. doi: 10.1523/JNEUROSCI.14-09-05281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiou R, Dreyfus P, Chopin DK, Abbou CC, Lefaucheur JP. Muscle precursor cell auto-grafting in a murine model of urethral sphincter injury. BJU Int. 2002;89(3):298–302. doi: 10.1046/j.1464-4096.2001.01618.x. [DOI] [PubMed] [Google Scholar]
- Zeng X, Jack GS, Zhang R, Wu B, Rodriguez LV. Treatment of SUI using adipose derived stem cells: Restoration of urethral function. J Urol. 2006;175(4):900. [Google Scholar]
- Zhang Y, Bicek AD, Wang G, Timm GW. Effects of periurethral neuromuscular electrical stimulation on the voiding frequency in rats. Int Urogynecol J Pelvic Floor Dysfunct. 2010;21(10):1279–1284. doi: 10.1007/s00192-010-1189-y. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]