Abstract
Background
While localized delivery of biocomposite materials, such as calcium hydroxyapatite (CHAM), have been demonstrated to potentially attenuate adverse LV remodeling post-myocardial infarction (MI), the underlying biological mechanisms for this effect remain unclear. This study tested the hypothesis that targeted CHAM injections would alter proteolytic pathways (matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs)), and be associated with parameters of post-MI LV remodeling.
Methods and Results
MI was induced in adult sheep followed by 20 targeted injections of a total volume of 1.3 mL (n=6) or 2.6 mL of CHAM (n=5), or saline (n=13), and LV end-diastolic volume (EDV) and MMP/TIMP profiles in the MI region were measured at 8 weeks post-MI. LV EDV decreased with 2.6 mL CHAM vs MI Only (105.4±7.5 vs 80.6±4.2 respectively, p<0.05) but not with 1.3 mL CHAM (94.5±5.0, p=0.32). However, MI thickness increased by 2-fold in both CHAM groups compared to MI Only (p<0.05). MMP-13 increased 40-fold in the MI Only group (p<0.05) but fell by over 6-fold in both CHAM groups (p<0.05). MMP-7 increased approximately 1.5-fold in the MI Only group (p<0.05) but decreased to referent control values in both CHAM groups in the MI region (p<0.05). Collagen content was reduced by approximately 30% in the CHAM groups compared to MI Only (p<0.05).
Conclusions
Differential effects on LV remodeling and MMP/TIMP profiles occurred with CHAM. Thus, targeted injections of a biocomposite material can favorably affect the post-MI remodeling process and therefore holds promise as a treatment strategy in and of itself, or as a matrix with potentially synergistic effects with localized pharmacologic or cellular therapies.
Keywords: Infarct Expansion, Matrix Metalloproteinase, Tissue Inhibitor of Matrix Metalloproteinases
Introduction
Following a myocardial infarction (MI), cellular and interstitial events result in changes in left ventricular (LV) geometry and function which have been collectively described as LV remodeling. The progressive extension of these changes from the MI, to the adjacent borderzone region, and to the surrounding normal myocardium, are driven by the process of infarct expansion which results from progressive changes in infarct material properties over time.1, 2 Past studies have demonstrated that the induction of a family of proteolytic enzymes known as the matrix metalloproteinases (MMPs), along with their tissue inhibitors (TIMPs), can contribute to the rate and course of the post-MI LV remodeling process.3-6 While a large body of evidence exists that biologic signaling molecules can modify MMPs and TIMPs, mechanical stimuli are also likely to play a significant role in the process.7-9 This may be of particular relevance in the post-MI context, as stress-strain patterns and asynchronous contractions are a hallmark of the post-MI remodeling process.10, 11 Specifically, heterogeneous mechanical strain patterns post-MI have been associated with changes in the regional MMP and TIMP portfolio.12, 13 Therefore, it can be hypothesized that modifying regional mechanical stimuli post-MI would in turn alter MMP and TIMP profiles and thereby alter the course of LV remodeling. Strategies to modify stress/strain patterns post-MI utilizing mechanical restraint have been performed at both the global and regional level.14-20 Specifically, application of a mesh patch across the MI region alone in a sheep model,16 as well as targeted injections of a biocomposite material into the MI region in a pig model,20 have both reported attenuation of LV remodeling. However, no studies have examined the relationship between altering regional, mechanical stress/strain patterns and the influence on MMP/TIMP profiles and parameters of LV remodeling. Accordingly, the overall goal of this project was to use a large animal model of post-MI remodeling, perform targeted injections of calcium hydroxyapatite microspheres (CHAM) into the MI region, and examine indices of LV matrix remodeling which included MMP and TIMP profiles, matrix composition and signaling pathways, and the relation to determinants of LV remodeling.
Methods
Animal Model
An MI was surgically induced in 24 adult male sheep (35-40 kg) using a well described method.14-16, 18, 19 Briefly, the animals were anesthetized and underwent left thoracotomy. An anteroapical infarction was produced by ligating the left anterior descending artery and its diagonal branches, resulting in an infarction of approximately 20% of the LV mass. This technique has been shown to reproducibly create MI of consistent magnitude.18, 19 Subsequently, the sheep were randomized to receive either saline or a specific volume of calcium hydroxyapatite microspheres (CHAM). For the purposes of referent control values for the subsequent myocardial biochemical analysis, LV myocardial samples were collected from referent normal female sheep (n=6). All animals were treated and cared for in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (National Research Council, Washington, DC, 1996).
Calcium Hydroxyapatite Microsphere (CHAM) Injection
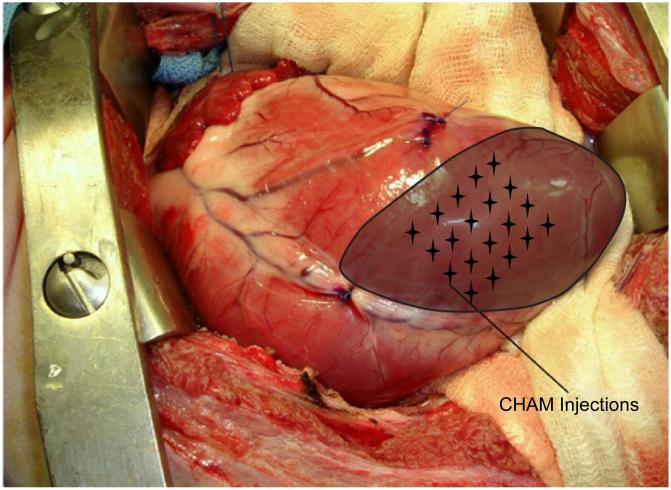
A viscous, biocompatible soft tissue filler of CHAM suspended in an aqueous gel carrier of water, glycerin, and carboxymethylcellulose (Radiesse, Bioform Medical Inc, San Mateo, CA) was prepared in either 1.3 mL or 2.6 mL volumes. Within 60 minutes of MI creation sheep were randomly assigned to receive either 1.3 mL (n=6) or 2.6 mL (n=5) of CHAM or physiologic saline solution (n=13) through direct intramyocardial injection, as shown in the schematic in Figure 1. The injections were performed at 20 uniformly spaced points within the ischemic territory to a depth of approximately 2 mm.
Figure 1.
Intra-operative photograph of an anteroapical MI as seen through a left thoracotomy. The infarct region is shaded and targeted injection sites of CHAM are noted by the stellate grid. Twenty injections were performed to a depth of 2 mm, placing a total of either 1.3 or 2.6 mL CHAM into the MI region.
Left Ventricular Function and Myocardial Sampling
At baseline (prior to MI induction) and at 8 weeks post-MI, quantitative two dimensional echocardiograms were obtained (Philips 7500 ultrasound system using a 5-MHz probe) using a sub-diaphragmatic technique. From these recordings, LV end-diastolic volume (EDV), ejection fraction, and MI length were determined.17-19 At 8 weeks post-MI the sheep were euthanized and the heart was rapidly harvested. The infarct thickness was measured with a digital micrometer. Transmural sections from the MI, remote and borderzone regions were flash frozen. The selection of this 8 week post-MI time point was based upon several considerations. First, the intent of this study was to examine the chronic phase of post-MI remodeling in which the acute wound healing phase and robust inflammatory response had subsided, but the progressive infarct expansion process was fully manifest.1-5 Second, it has been demonstrated previously in other interventional studies that a significant treatment effect upon the remodeling process should be detected by 8 weeks post-MI.5, 14, 16, 18 Third, the purpose of this study was to examine the chronic effects of CHAM placement independent of the acute inflammatory effects of the initial placement and dissolution of the biomaterial matrix into the interstitial space.
LV Myocardial Collagen Content
Total myocardial collagen content was determined by the biochemical measurement of hydroxyproline content as described previously.12 Briefly, LV myocardial samples were weighed, hydrolyzed and subjected to hydroxyproline quantification by spectrophotometry. The spectrophotometric absorption values were then converted to collagen concentration and normalized to wet weight of LV myocardium (ug/mg wwt).
LV Myocardial MMP/TIMP Analysis
LV myocardial samples were homogenized (3- to 30-second bursts) in 5 mL of an ice-cold extraction buffer (1:3 wt/vol) containing cacodylic acid (10 mmol/L), NaCl (0.15 mol/L), ZnCl (20 mmol/L), NaN3 (1.5 mmol/L), and 0.01% Triton X-100 (pH 5.0). The homogenate was then centrifuged (4°C, 10 minutes, 800g) and the supernatant decanted and saved on ice. Final protein concentration of the myocardial extracts was determined with a standardized colorimetric assay (BCA Protein Assay, Pierce). The extracted samples were then aliquoted and stored at −80°C. For this study, the relative abundances of MMPs corresponding to each class were examined by quantitative zymograpy or immunoblotting (10 μg myocardial protein), which has been described in detail previously.5, 12, 21 Abundance of the gelatinase MMP-2 was determined by zymography, as described previously.5, 6, 12 For immunoblotting, following electrophoresis the separated proteins were transferred to a nitrocellulose membrane. After a blocking and washing step, the membranes were incubated in antisera (0.4 μg/mL) corresponding to the regional myocardial abundance of the interstitial collagenases MMP-1 (IM35, Calbiochem), MMP-8 (Ab3316, Chemicon) and MMP-13 (AB8114, Chemicon International), the gelatinase MMP-9 (AB804, Chemicon International), the matrilysin MMP-7 (Ab38996, Abcam), or the membrane-type MMP MT1-MMP (AB815, Chemicon). The TIMPs were measured in identical fashion using specific antisera for TIMP-1 (AB8116, Chemicon), TIMP-2 (Ab38973, Abcam), and TIMP-4 (AB816, Chemicon). After incubation with a secondary antibody, immunoreactive signals were detected by chemiluminescence (Western Lightning Chemiluminescence Reagent Plus, Perkin Elmer). Recombinant standards (Chemicon or Oncogene) were included in all immunoblots as positive controls. The zymograms and immunoblots were analyzed by densitometry (Gel Pro Analyzer, Media Cybernetics). Finally, measurements were performed in order to measure total net myocardial MMP proteolytic activity as described previously.22 The LV myocardial extracts were incubated (37°C, 2 hours) in the presence and absence of the MMP substrate (Anaspec #27074) that is cleaved by all active MMPs with equivalent kinetics.22 This MMP fluorogenic peptide will only yield a detectable UV emission when proteolytically processed at a specific amino acid sequence, and using a specific excitation/emission (328/400, FluoStar Galaxy, BMG Labtech Inc, Cary, NC). Using a recombinant MMP construct of known catalytic activity (MMP-2/9 BIOMOL, SE-237, SE-244), the fluorogenic recordings were converted to units of MMP activity and expressed in terms of protein content (ng/mg/hr).13, 22
LV Myocardial mRNA Profiling
A critical step in the regulation of MMPs and TIMPs is at the transcriptional level,3 accordingly relative mRNA levels for key MMPs (MMP-1,-13,-2, MT1-MMP), TIMPs (TIMP-1,-2,-4) were examined by PCR. In addition, indices of the profibrotic cascade such as transforming growth factor beta 1 (TGF-beta1), the common intracellular TGF signaling component Smad-2,23, 24 as well as the procollagen type I, and procollagen type III mRNA levels were quantified. Custom sheep specific primers were synthesized which corresponded to these mRNA sequences and are shown in Supplemental Table 1. LV myocardial homogenates were subjected to RNA extraction (RNeasy Fibrous Tissue Mini Kit, Qiagen, Valencia, CA) and the quantity and quality of the RNA determined (Experion Automated Electrophoresis System; Bio-Rad Laboratories, Hercules, CA). RNA (1 ug) was reverse transcribed to generate cDNA (iScript cDNA Synthesis Kit; Bio-Rad, Hercules, CA). The cDNA was amplified with gene specific primer/probe sets (TaqMan Universal PCR Master Mix: Cat# 4364321, Applied Biosystems, Foster City, CA) using single-color Real-Time PCR (rtPCR; MyiQ Bio-Rad, Hercules, CA). Negative controls were run to verify the absence of genomic DNA contamination (reverse transcription control), and the absence of overall DNA contamination in the PCR system and working environment (template control). The rtPCR fluorescence signal was converted to cycle times (Ct), and normalized to the 18s signal. Finally, the specific mRNA levels were expressed as a function of referent control values.
LV Myocardial Fibroblasts and Effects of Calcium Hydroxyapatite
In order to assess the potential effects of CHAM independent on myocardial fibroblast response, independent of in-vivo loading conditions as well as assess potential effects of the vehicle alone (2.3% carboxymethylcellulose, 14.5% Glycerin, and 82.5% deionized water), primary cultures of fibroblasts were prepared from referent normal sheep LV myocardium using specific outgrowth techniques described previously.25 Briefly, the LV myocardial samples were minced under a sterile laminar flow hood, transferred to cell culture flasks (75 cm2; Falcon), and allowed to adhere. Sterile growth media was added to the flasks and the cells incubated under standard cell culture conditions (37°C; 21% O2, 5% CO2). Culture media consisted of Fibroblast Growth Medium (FGM) from Promocell (#C23010; Heidelberg, Germany), 20% fetal bovine serum (FBS) and Promocell Supplement Mixture (#C39315) containing Amphotericin B, basic Fibroblast Growth Factor, Gentamicin, and Insulin. After a two-week incubation period, myocardial fibroblasts migrated from the initial myocardial plugs and grew to confluency. At this point, the cells were scraped and transferred to tissue culture flasks (150cm2, Falcon) containing the supplemented FGM and grown to 80% confluency. These initial confluent cultures were then split and plated out into 35mm tissue culture dishes (#430165, Corning) that were pre-coated with either CHAM (0.5mL), vehicle (0.5 mL) or no pretreatment (control). The fibroblasts were then incubated for 24 hours in identical conditions, the fibroblasts harvested, RNA extracted, and subjected to mRNA measurements. All experiments were performed in triplicate.
Data Analysis
The densitometry results obtained from the MMP and TIMP zymography and immunoblotting were converted to a normalized integrated optical density (IODs). IOD values were assessed for normality and homoscedasticity using the Kolmogorov-Smirnov test as well as inspection of the residuals (Q-Q and Residual/Means plots). Where violations occurred, the data were natural log transformed and retested. For each of these variables, transformation using the natural log function altered the distribution pattern to approximate normal distribution. Data were analyzed using repeated measures analysis of variance (ANOVA) with main effects of MI, sub-plots of treatment group, and a within subjects region effect. Unstructured and compound symmetry covariance structures were considered in addition to the robust variance estimator. The unstructured covariance structure was found to fit the data best as measured by Aikake’s information criterion.26 A priori pair-wise comparisons were made following ANOVA model fitting (MI values versus referent control). To adjust for multiple testing, α was conservatively controlled to an estimated within class false discovery rate of 5% using the method described by Benjamini et al. for dependent tests.27 Model estimation was constructed in SAS Proc Mixed and multiple testing adjustments were conducted in SAS (Proc Multtest). Values were also transformed to a percent change from referent normal control values. The individual mRNA levels were normalized to referent control values and then comparisons performed using a t-test with a null hypothesis set to 100. Relative mRNA values across treatment groups and regions were compared by an adjusted t-test. One sample t test analysis was performed against the referent control value set to 100. LV echocardiographic measurements were compared between groups using onewayANOVA. All multiple comparisons were performed among treatment groups (module prcomp, STATA)). For ease of interpretation, values are reported as the mean ± standard error of the mean (SEM) of the untransformed data in all tables and figures. Statistical analyses were performed on the transformed data using either SAS version 9.1 (SAS Institute Inc. Cary, NC, USA) or STATA statistical software (v.8.0, StataCorp, College Station, TX). The authors had full access to the data and take full responsibility for its integrity.
Results
LV and Collagen Remodeling Post-MI; Effects of CHAM
Indices of LV geometry, function and collagen content are summarized in Table 1. At 8 weeks post-MI, LV dilation and reduced pump function occurred in all MI groups. In the high volume CHAM group, however, the degree of LV dilation was reduced and ejection fraction improved from the other MI groups. While LV EDV was reduced in the low volume CHAM treatment group compared to the MI Only group, it did not reach statistical significance (p=0.32). Posterior wall thickness at the site of the MI was significantly decreased in the MI Only group and was increased in both CHAM groups. Despite changes in LV geometry and function following CHAM injection, the absolute MI size was equivalent in all 3 MI groups. LV myocardial collagen content was significantly increased within the MI region of all groups. However, LV myocardial collagen content within the MI region was reduced in CHAM groups, with a significant reduction in the 1.3 mL CHAM group.
Table 1.
LV geometry, function, infarct thickness and myocardial collagen content at eight weeks post-MI; Effects of CHAM
| LV EDV (mL) |
LV EF (%) | MI Thickness (mm) |
MI Size (% LV) |
Collagen Content (μg/mg wet weight) | |||
|---|---|---|---|---|---|---|---|
| MI | BZ | Remote | |||||
| Control | 52.9±3.0 | 52.1±1.3 | 6.08±0.34# | N/A | 8.5±1.1 | ||
| MI Only | 105.4±7.5* | 20.9±2.3* | 2.69±0.23* | 24.7±1.2 | 113.1±13.8* | 11.5±2.5 | 9.2±1.5 |
| 1.3 mL CHAM | 94.5±5.0* | 26.8±1.5* | 5.48±0.34‡ | 27.1±0.8 | 73.1±8.9*‡ | 18.1±4.6 | 7.1±0.7 |
| 2.6 mL CHAM | 80.6±4.2*‡ | 37.2±3.7*‡§ | 5.44±0.15‡ | 24.7±1.0 | 84.9±9.9* | 14.7±4.8 | 6.7±0.8 |
Values are means±SEM;
LVEDV = Left ventricle end diastolic volume, LV EF = Left ventricle ejection fraction.
posterior wall thickness; BZ: Border zone
Sample sizes: Referent Control n=6, MI Only n=13, 1.3 mL CHAM n=6, 2.6 mL CHAM n=5
p<0.05 vs. Referent Control
p<0.05 vs. MI Only
p<0.05 vs. 1.3 mL CHAM
MMP/TIMP Abundance and MMP Proteolytic Activity Post-MI
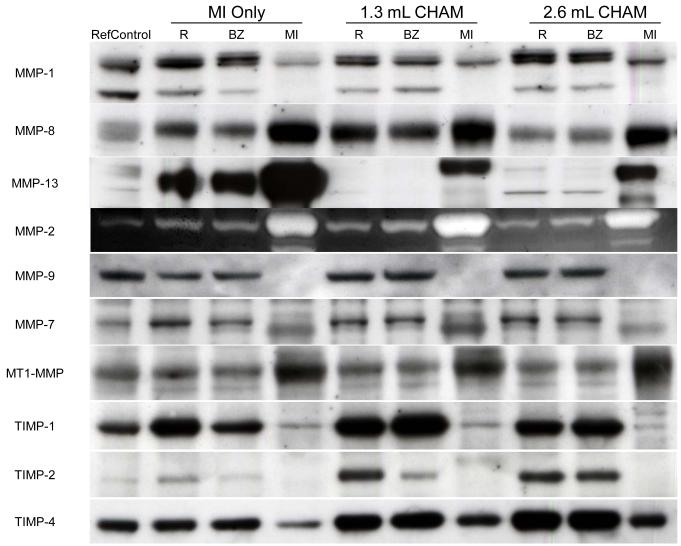
Representative immunoblot/zymogram results by region and treatment for the MMPs and TIMPs of interest are shown in Figure 2. The absolute IOD values obtained from this analysis are summarized in Tables 2 and 3. The ANOVA revealed region dependent changes (remote, borderzone, and MI regions) for all the MMPs, with the exception of MMP-8, and for all the TIMPs examined (p<0.01). For example, a significant reduction in MMP-1 occurred in the MI region while MMP-13 was significantly increased in the MI region. MMP proteolytic activity was increased within the MI and Borderzone region in all post-MI groups. Thus, consistent with past reports, heterogeneity exists between the different regions.5,12 In order to examine the relative changes which occurred between the MI Only and CHAM treatment groups, percent change from referent control values were calculated and the results from this analysis are summarized in Figure 3.
Figure 2.
Relative absolute abundance of representative MMP types and TIMPs were examined by immunoblotting or zymography in myocardium obtained from referent control animals or MI Only and CHAM injected animals at 8 weeks after MI. Samples were taken from the remote (R), borderzone (BZ), and MI regions. Levels of the collagenase MMP-13 in the remote and borderzone regions were returned toward the referent control value by CHAM treatment. MMP-7 increased in the MI Only group but decreased toward the referent control value with CHAM treatment. Absolute values are shown in Tables 2 and 3 and percent change from referent control values are shown in Figure 3.
Table 2.
Levels of myocardial MMPs and MMP activity at eight weeks after MI; Effects of biocomposite microsphere injection
| MMP Class | Referent Control |
Region | MI Only | CHAM# | |
|---|---|---|---|---|---|
| 1.3 mL | 2.6 mL | ||||
| Collagenases | |||||
| MMP-1 (IOD, pixels) |
484 ± 59 | MI | 107 ± 46* | 110 ± 33* | 213 ± 109 |
| BZ | 638 ± 135 | 326 ± 76 | 603 ± 197 | ||
| Remote | 1095 ± 178 | 661 ± 239 | 632 ± 194 | ||
| MMP-8 (IOD, pixels) |
176 ± 39 | MI | 475 ± 128 | 532 ± 162* | 584 ± 213* |
| BZ | 256 ± 19 | 423 ± 88* | 456 ± 94* | ||
| Remote | 351 ± 63 | 390 ± 95 | 346 ± 79 | ||
| MMP-13 (IOD, pixels) |
209 ± 29 | MI | 8538 ± 2901* | 560 ± 152 | 1310 ± 569 |
| BZ | 1621 ± 720 | 453 ± 367 | 157 ± 37 | ||
| Remote | 1644 ± 614* | 180 ± 35 | 301 ± 80 | ||
| Gelatinases | |||||
| MMP-2 (IOD, pixels) |
40752 ± 5152 | MI | 147374 ± 28310* | 226764 ± 43391* | 171429 ± 27589* |
| BZ | 70700 ± 20356 | 115240 ± 21609 | 100450 ± 47673 | ||
| Remote | 34762 ± 7343 | 56962 ± 3015 | 35965 ± 8778 | ||
| MMP-9 (IOD, pixels) |
975 ± 81 | MI | 155 ± 86* | 289 ± 252* | 206 ± 170* |
| BZ | 509 ± 107* | 883 ± 173 | 767 ± 178 | ||
| Remote | 827 ± 91 | 1049 ± 158 | 1005 ± 165 | ||
| Matrilysin | |||||
| MMP-7 (IOD, pixels) |
1527 ± 85 | MI | 2214 ± 328 | 1243 ± 267 | 1495 ± 446 |
| BZ | 2234 ± 321 | 1069 ± 234 | 1286 ± 129 | ||
| Remote | 3035 ± 466* | 1444 ± 300 | 1857 ± 192 | ||
| Membrane-type | |||||
| MT1-MMP (IOD, pixels) |
432 ± 69 | MI | 2352 ± 370* | 2251 ± 565* | 3065 ± 362* |
| BZ | 1085 ± 273* | 653 ± 199 | 743 ± 109 | ||
| Remote | 887 ± 114* | 815 ± 251 | 812 ± 106 | ||
| MMP Activity | |||||
| Activity (×10−7ng/mg/hr) |
12.2±2.2 | MI | 28.9±1.6* | 19.2±2.9* | 31.3±6.2* |
| BZ | 20.7±1.5* | 29.8±5.2* | 22.1±5.0* | ||
| Remote | 8.4±1.4 | 10.9±1.2 | 9.8±1.1 | ||
Values presented as mean±SEM.
p<0.05 vs. referent control.
= calcium hydroxyapatite microspheres (CHAM); IOD: Integrated optical density; BZ: Border zone
Sample sizes: Referent Control n=6, MI Only n=13, 1.3 mL n=6, 2.6 mL n=5
Table 3.
Levels of myocardial TIMPs at eight weeks after MI; Effects of biocomposite microsphere injection
| Referent Control |
Region | MI Only | CHAM# | ||
|---|---|---|---|---|---|
| 1.3 mL | 2.6 mL | ||||
| TIMP-1 (IOD, pixels) | 180 ± 42 | MI | 12 ± 2* | 5 ± 2* | 5 ± 2* |
| BZ | 557 ± 102 | 617 ± 182 | 572 ± 121 | ||
| Remote | 786 ± 113* | 939 ± 87* | 483 ± 58* | ||
| TIMP-2 (IOD, pixels) | 252 ± 29 | MI | 63 ± 22* | 98 ± 29* | 153 ± 67* |
| BZ | 200 ± 74 | 365 ± 123 | 927 ± 460 | ||
| Remote | 445 ± 157 | 1135 ± 442 | 963 ± 422 | ||
| TIMP-4 (IOD, pixels) | 977 ± 64 | MI | 323 ± 113* | 336 ± 225* | 389 ± 306 |
| BZ | 1102 ± 143 | 977 ± 187 | 1008 ± 227 | ||
| Remote | 1267 ± 109 | 1337 ± 67 | 1167 ± 99 | ||
Values presented as mean±SEM.
p<0.05 vs. referent control.
= calcium hydroxyapatite microspheres (CHAM); IOD: Integrated optical density; BZ: Border zone
Sample sizes: Referent Control n=6, MI Only n=13, 1.3 mL n=6, 2.6 mL n=5
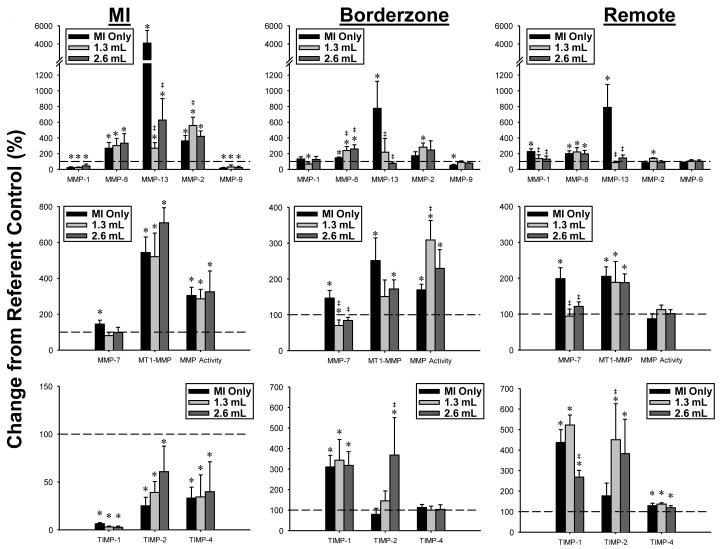
Figure 3.
Percent change from referent control values in the abundance of the matrix metalloproteinases (MMPs), MMP activity, and tissue inhibitors of metalloproteinases (TIMPs) in the MI, borderzone, and remote regions at 8 weeks after MI. The dashed line represents the referent control value set to 100%. MMP-13 was robustly increased in all regions of the MI Only group and returned toward referent control values with CHAM treatment. In the remote region, MMP-1 and MMP-7 were normalized toward control value by CHAM treatment. MMP activity determined by a fluorogenic assay approach, was similar between groups. Within the borderzone, MMP-13 levels and MMP-7 levels were lower, but again MMP activity was either similar or higher with CHAM treatment. * p<0.05 vs referent control, ‡ p<0.05 vs MI Only, § p<0.05 vs 1.3 mL treatment group. Treatment groups, Sample sizes: MI Only (n=13), 1.3 mL CHAM (n=6), 2.6 mL CHAM (n=5)
MI Region
MMP-1 was decreased by greater than 50% in all groups. MMP-8 was increased by greater than 200% in all groups. MMP-13 significantly increased in the MI Only group but fell by over 6-fold in both CHAM treatment groups. MMP-2 increased in all MI groups, and increased further in the low volume CHAM treatment group. MMP-9 fell by greater than 50% in all MI groups. MMP-7 increased in the MI Only group and was normalized in both CHAM groups. MT1-MMP increased by over 5-fold in all MI groups irrespective of treatment. All TIMPs decreased in the MI region in all groups and were not affected by treatment. While differential changes in MMP levels occurred with CHAM, MMP activity was increased in all post-MI groups to the same relative degree.
Borderzone Region
MMP-1 decreased in the low volume CHAM group only. MMP-8 was increased in all MI groups. MMP-13 increased by 7-fold in the MI Only group and was normalized in both CHAM groups. MMP-2 increased in the low volume CHAM group only. MMP-9 decreased in the MI Only group and was normalized in both of the CHAM groups. MMP-7 increased in the MI Only group and decreased from both referent control and MI only values in the low volume CHAM group. MT1-MMP increased in both the MI Only and the high volume CHAM group. TIMP-1 increased by greater than 3-fold in all MI groups. TIMP-2 increased over 3-fold in the high volume CHAM group as compared to the MI Only group. While MMP activity was increased in all post-MI groups, the highest relative levels occurred in the low volume CHAM group.
Remote Region
MMP-1 increased in the MI Only group and was normalized in both CHAM groups. MMP-8 increased in all MI groups. MMP-13 increased over 7-fold in the MI Only group and was normalized to referent control values in both of the CHAM groups. MMP-2 increased in the low volume CHAM group only. MMP-7 increased in the MI Only group and was normalized in both of the CHAM groups. MT1-MMP increased in the MI Only and high volume CHAM groups. TIMP-1 increased in all MI groups, with the low volume CHAM group further increased from the MI Only group. TIMP-1 in the high volume CHAM group decreased from the low volume CHAM group. TIMP-2 increased over 4-fold in the low volume CHAM group and was significantly elevated in comparison to the MI Only group. TIMP-4 increased in all MI groups with no change in MMP proteolytic activity.
CHAM and MMP Interactions
In order to examine the relationship between CHAM treatment and MI on the biologic MMP and TIMP response variable two-way ANOVA was performed. From this analysis a treatment and region dependent interaction was observed for MMP-1 (p=0.02). This indicates that MMP-1 was differentially affected within the different myocardial regions examined as well as by CHAM treatment. A univariate correlation analysis was performed to analyze the relationship between MMP and TIMP values and posterior wall thickness (i.e. MI thickness). The analysis demonstrated the change in MMP-7 was correlated with the change in MI thickness (r = −0.40, p = 0.05). Specifically, decreased MMP-7 levels were associated with increased MI thickness.
MMP/TIMP mRNA and Determinants of Fibrosis
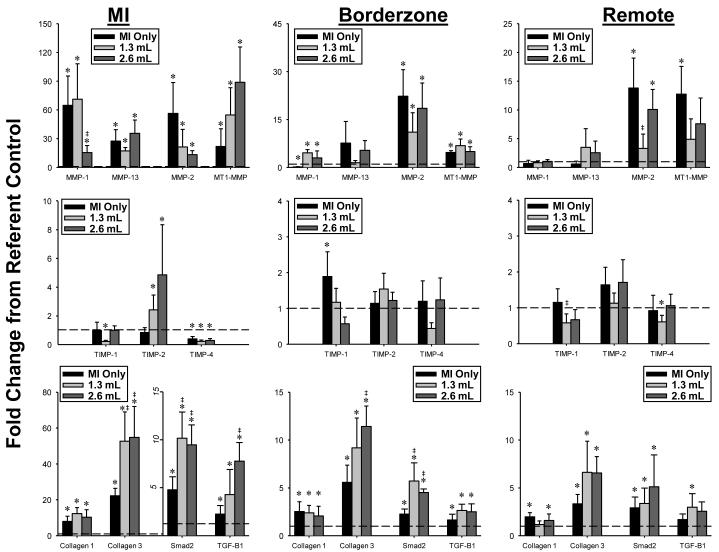
In order to determine whether the differential changes in MMP/TIMP protein abundance which occurred with CHAM may have been due to alternations in transcription, steady-state mRNA levels were determined and the results summarized in Figure 4. These results are presented as a function of normal referent control values and by region.
Figure 4.
The relative changes in mRNA levels for MMPs, TIMPs, fibrillar collagens, transforming growth factor-beta (TGF-B1) and the TGF intracellular signaling moiety, Smad-2 were determined by rtPCR, and computed as fold change from referent control values. The most notable changes in terms of CHAM treatment occurred within the MI region where an over 2-fold higher mRNA levels for collagen-III and Smad-2 occurred. [* p<0.05 vs referent control, ‡ p<0.05 vs MI Only. Sample sizes: MI Only (n=13), 1.3 mL CHAM (n=6), 2.6 mL CHAM (n=5)]
MI Region
MMP-1, -13, -2 and MT1-MMP mRNA levels were increased in all post-MI groups, where relative MMP-1 levels were reduced in the high dose CHAM group. Relative TIMP-1 mRNA levels were reduced in the low dose CHAM group. TIMP-2 mRNA levels were increased in both CHAM groups. TIMP-4 mRNA levels were reduced in all post-MI groups. Fibrillar collagen I and III mRNA levels were increased in all post-MI groups. Collagen III mRNA levels were significantly higher in both CHAM groups when compared to MI only. TGF and Smad-2 mRNA were increased in all post-MI groups, and were significantly increased from MI only values in both CHAM groups.
Borderzone Region
Relative MMP-1, MMMP-2 and MT1-MMP levels were increased to a similar degree in all post-MI groups. TIMP-1 mRNA levels were increased in the MI only group, but were reduced from this value in both CHAM groups. Collagen I and III mRNA levels were increased in all post-MI groups, with the highest collagen III mRNA levels occurring in the high dose CHAM group. TGF and Smad-2 mRNA levels were increased in all post-MI groups, with the highest Smad-2 levels measured in both CHAM groups.
Remote Region
MMP-2 mRNA levels were increased in all post-MI groups, whereas MT1-MMP mRNA levels were increase in the MI only group. Relative TIMP-1 and TIMP-4 mRNA levels were reduced in the low dose CHAM group. Collagen I mRNA levels were increased in the MI only and high dose CHAM groups, whereas collagen III mRNA levels were increased in all post-MI groups. TGF was increased in the low dose CHAM group, and Smad-2 mRNA was increased in all post-MI groups.
Effects of CHAM on LV Fibroblast MMP/TIMP Expression
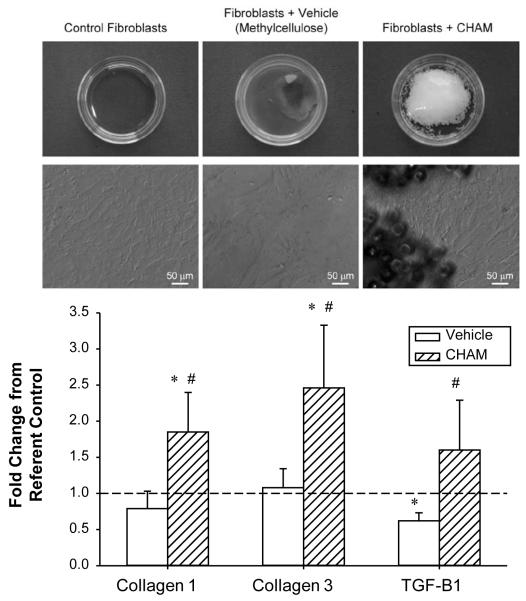
The targeted region for CHAM delivery was the MI region, and the predominant cell type within this region is the fibroblast.28, 29 LV myocardial fibroblast cultures were exposed to the vehicle utilized to deliver CHAM, as well as CHAM, and relative mRNA levels of the MMP/TIMP and determinants of the fibrotic cascade examined. Representative photomicrographs primary cultures of sheep LV myocardial fibroblasts utilized in these studies as well as the critical quantitative results are shown in Figure 5. There were no significant changes in relative MMP/TIMP mRNA levels in LV fibroblasts incubated with vehicle or CHAM, when compared to untreated values (data not shown). However, LV fibroblasts plated on CHAM causes a robust increase in collagen I, III and TGF mRNA levels when compared to untreated or vehicle values.
Figure 5.
Primary cultures of sheep LV myocardial fibroblasts were plated on the vehicle utilized for CHAM delivery, CHAM or in untreated culture dishes. TOP- low power views of the confluent LV fibroblast cultures and high power views demonstrating the typical morphometry and growth patterns. While plating LV fibroblasts on vehicle or CHAM did not appear to alter MMP/TIMP mRNA levels, collagen I and III as well as transforming growth factor-beta (TGF-B1) increased in the CHAM groups. All experiments performed in triplicate; * p<0.05 vs control, # p<0.05 vs vehicle.
Discussion
Recent cellular and genetic approaches to attenuate post-MI LV remodeling have focused upon targeted, localized delivery to the MI region, which can entail the use of a biocomposite matrix.30, 31 However, in the effects of the biocomposite material itself upon key determinants of LV remodeling remain poorly understood. Accordingly, the present study tested the hypothesis that targeted injections of calcium hydroxyapatite (CHAM) would alter proteolytic and profibrotic pathways, i.e. matrix metalloproteinases (MMPs), MMP tissue inhibitors (TIMPs), transforming growth factor-beta (TGF) and collagen expression, which in turn would alter parameters of LV remodeling in the post-MI context. The important findings of this study were 3-fold. First, CHAM injection within the MI region resulted in differential expression of MMPs and TIMPs, and these changes were related to increased MI thickness and reduced LV dilation. Second, CHAM injections altered levels of TGF and canonical intracellular signaling molecules (Smad-2), which in turn was associated with increased collagen expression, but not increased fibrosis. Third, LV myocardial fibroblasts exposed to CHAM altered critical determinants of matrix remodeling (collagen expression, TGF). Thus, biocomposite material delivered into the MI region affected determinants of post-MI remodeling. Future studies utilizing biocomposite materials as a matrix for cellular or pharmacologic therapies may require assessment of these independent effects when evaluating the relative effectiveness of such interventions on post-MI remodeling.
Past studies of biocompatible materials
Biocomposite materials such as alginate,32 fibrin glue,33 and collagen34 have been utilized as scaffolds for the delivery of cellular therapy post-MI in numerous studies. For example, in a small animal model, Dai et al. examined the functional effects of collagen delivered into an MI, reporting improved stroke volume and ejection fraction at 6 weeks post-MI.34 In a pig model Mukherjee et al. examined the functional effects of fibrin-alginate delivery into the MI, reporting fibrin-alginate decreased infarct expansion and increased LV wall thickness.20 However, a systematic examination of critical determinants of post-MI remodeling at the cellular or extracellular level, and whether and to what degree they contribute to the effects of such biocomposite materials, remains to be established. Accordingly, the focus of the present study was to examine the relationship of biocomposite material delivery to that of an important determinant of post-MI remodeling, such as MMP/TIMP profiles and the profibrotic molecule TGF. The results of this study build upon past reports by identifying specific MMPs which were modulated by CHAM, that CHAM likely induced the TGF signaling cascade, and was associated with heightened collagen expression. Thus, the use of biocomposite materials can impart effects on both proteolytic and profibrotic processes which can directly affect the course of post-MI remodeling.
CHAM placement and MMP/TIMP profiles
The MMPs are a broad family of proteases which has been divided into subgroups such as the interstitial collagenases, which include MMP-1, MMP-8, and MMP-13.3, 4 Specifically, the MMP-13 substrate portfolio is extensive and its functions are diverse, including activation of pro-MMP-1.3 MMP-13 induction has been implicated in a wide array of disease processes such as breast cancer metastasis, arthritic bone remodeling, and end-stage dilated cardiomyopathy.3, 21, 35 In the present study MMP-13 levels were decreased by CHAM treatment in all myocardial regions analyzed, which likely had a favorable effect on LV remodeling. In addition to MMP-13, MMP-1 was also decreased by CHAM treatment in the remote region. Overall, the decreased levels of MMP-13 and MMP-1 in the present study were associated with a favorable response with regards to post-MI LV remodeling. MMP-2 has been shown to be ubiquitously elevated in most myocardial remodeling processes.3-5, 12, 21 Consistent with these past reports, MMP-2 increased in the MI region of all groups and interestingly, was increased, albeit to a small degree in with low volume CHAM delivery. A unique member of the MMP family which has been documented to play a potentially important role in post-MI remodeling is the matrilysin MMP-7.3 MMP-7 is of a low molecular weight but has a diverse proteolytic profile, which includes cytokine processing and degradation of a number of extracellular matrix proteins.3, 36 Lindsey et al. reported transgenic mice with an MMP-7 deletion demonstrated favorable changes in LV remodeling.36 Furthermore, MMP-7 was demonstrated to alter the abundance and distribution of a critical conduction protein, connexin.36 In the present study, MMP-7 levels were increased post-MI but were normalized by CHAM treatment. Moreover, decreased MMP-7 levels were associated with increased MI thickness. Using a fluorogenic substrate, total MMP proteolytic activity was uniformly increased within the MI region, and unaffected by CHAM. Thus, while overall MMP proteolytic activity appeared similar, the MMP types which contributed to this net proteolytic activity were distinctly different with CHAM treatment. Due to the diversity in MMP substrates and biological activity, these observations hold relevance as to the mechanisms by which CHAM treatment altered post-MI remodeling. For example, the reduction in the contribution of MMP-13 to net MMP proteolytic activity likely altered matrix degradation, whereas the reduced contribution by MMP-7 likely altered cytokine processing. In addition, it has now been demonstrated that MMPs such as MT1-MMP may actually facilitate the profibrotic response such as TGF and ultimately collagen expression,23, 24 and the fact that CHAM treatment did not alter this particular MMP type likely holds relevance to matrix structure and function within the MI region. Specifically, the shift in net MMP proteolytic activity towards different MMP types, such as MT1-MMP would favor increased TGF signaling and in turn increased fibrillar collagen expression with CHAM treatment. The TIMPs form an important post-translational control point for MMP activity, but it is becoming recognized that TIMPs can affect a diverse number of biological processes such as fibroblast growth and viability.3 Consistent with past reports, the post-MI remodeling process is associated with a relative reduction in TIMP levels in particular TIMP-4.3, 5, 12 Unlike the differential effects of CHAM treatment on MMP profiles, there were no effects on relative TIMP levels within the MI region, with modest effects on TIMP-2 levels within the borderzone and remote regions. Taken together, these findings suggest that the primary effect of CHAM treatment was not through altering a post-translational control point of MMP activity (TIMPs), nor causing a net change in overall MMP activity, but rather through a specific switch in the MMP types which are active within the post-MI period.
Mechanisms of CHAM on MMP/TIMP profiles and profibrotic pathways
In the present study, CHAM selectively affected certain MMP types such as MMP-1, -13 and -7, whereas other MMP types such as MMP-2 were unaffected. In light of the fact that a control point for MMP expression is transcriptional regulation,3, 4, 7, 9 then the relative mRNA levels for targeted MMPs and TIMPs were examined. These set of studies revealed that the MMP/TIMP mRNA levels were not fully concordant with the respective protein measurements. For example, MMP-1 mRNA levels were increased within the MI region, but MMP-1 protein levels were reduced. These findings would suggest that there may be changes in mRNA stability, increased translational efficiency, and/or increased MMP-1 protein turnover. One important post-transcriptional regulatory point that holds significant relevance to matrix remodeling is through the expression and interaction of microRNAs.37-39 These microRNAs selectively bind to mRNA transcripts which in turn will impair the initiation and efficiency of translation, and thereby constitute an important pathway by which a significant disparity between mRNA and protein levels can occur. Increased expression of microRNAs such as miR-29a and miR-133a have been shown to directly alter the degree of myocardial matrix accumulation and fibroblast function.38, 39 Moreover, altered levels of microRNAs such as miR-29a and miR-133a have been identified in the context of MI.40 However, whether and to what degree CHAM treatment may alter this post-transcriptional process remains speculative. Another important regulatory pathway in terms of matrix structure and function is through the activation and expression of the profibrotic molecule TGF, whereby past in-vitro and in-vivo studies have demonstrated that changes in mechanical stress-strain patterns can evoke the production of TGF.41 In the present study, CHAM caused a robust increase in TGF expression following MI which was paralleled by a concordant increase in the canonical intracellular TGF signaling protein Smad-2, and finally increased fibrillar collagen expression. Taken together, these measurements would suggest that CHAM injections enhanced TGF signaling which in turn would improve matrix stability within the MI region. In absolute terms, collagen content within the MI region was not increased with CHAM, and in the low volume group was actually reduced. There are several likely reasons for this observation. First, fibrillar collagen mRNA is also under post-transcriptional control through microRNAs,37-39 and therefore an absolute increase in collagen mRNA will not necessarily result in increased fibrillar collagen content. Second, a number of post-translational steps are required for final collagen fibril assembly whereby the nascent fibrils may be particularly susceptible to MMP mediated degradation.3 While CHAM treatment did not increase the amount of collagen in absolute terms (per gram wet weight), MI thickness was increased with CHAM treatment and therefore the total amount of collagen within the entire MI region would be higher. Whether and to what degree these changes in TGF signaling and collagen content and composition with CHAM treatment would alter inherent material properties within the MI region remain to be examined.
There are several lines of evidence to suggest that CHAM treatment was not associated with a chronic inflammatory response. First, while MMP-8 increased within the MI region, MMP-8 levels were unaffected by CHAM treatment. Second, consistent with prior reports,5, 12 MMP-9 levels in the present study were relatively unchanged as compared to referent control levels at 8 weeks post-MI, indicative of resolution of the acute inflammatory phase at this specific time point. CHAM treatment did not significantly alter MMP-9 levels within the MI region. MMP-8 is localized to cells indicative of inflammation, i.e. neutrophils and macrophages, and MMP-9 is most notably produced by neutrophils and is often associated inflammation.3, 4 Therefore, the placement of the biocomposite material CHAM was not associated with an exacerbated chronic and proteolytic inflammatory response in the context of post-MI remodeling. However, CHAM treatment likely influenced the major cell type within the MI region, the fibroblast.28, 29 Through a set of in-vitro experiments using LV myocardial fibroblasts, the present study demonstrated that the vehicle utilized in the CHAM formulation did not affect determinants of matrix synthesis or degradation. However, LV fibroblasts grown on CHAM resulted in increased TGF and collagen expression. These results suggest that a cellular mechanism by which CHAM treatment caused changes in the TGF and collagen expression in-vivo, were through direct effects on resident LV fibroblasts within the MI region.
Summary
The present study is the first to examine the effects of CHAM on critical determinants of matrix synthetic and proteolytic pathways and the relation to LV remodeling post-MI. However, this study is not without limitations. The results presented are from only one time point and therefore conclusions about the effects of this biocomposite material on post-MI remodeling at earlier or later time points cannot be made. Also, the method in which CHAM was delivered to the myocardium in this study represents only one of many potential delivery methods, which could influence the effects of CHAM on LV remodeling. Nonetheless, this study did attempt to examine volume dependent and region dependent effects of a biocomposite material delivered to the MI. These findings suggest that localized, targeted injections of a biocomposite material can favorably affect the post-MI remodeling process and therefore hold promise as a treatment strategy in and of itself, or as a matrix with potentially synergistic effects with localized pharmacologic or cellular therapies.
Supplementary Material
Acknowledgments
Funding Sources This research was supported by NIH grants HL57952, HL59165, HL95608, HL78825, HL63954, HL73021, and a Merit Award from the Veterans’ Affairs Health Administration.
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jackson BM, Gorman JH, Moainie SL, Guy TS, Narula N, Narula J, John-Sutton MG, Edmunds LH, Jr., Gorman RC. Extension of borderzone myocardium in postinfarction dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1160–1167. doi: 10.1016/s0735-1097(02)02121-6. discussion 1168-1171. [DOI] [PubMed] [Google Scholar]
- 2.Gupta KB, Ratcliffe MB, Fallert MA, Edmunds LH, Jr., Bogen DK. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation. 1994;89:2315–2326. doi: 10.1161/01.cir.89.5.2315. [DOI] [PubMed] [Google Scholar]
- 3.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 4.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: A temporal and spatial window. Cardiovasc Res. 2006;69:604–613. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation. 2003;107:618–625. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- 6.Spinale FG, Mukherjee R, Zavadzkas JA, Koval CN, Bouges S, Stroud RE, Dobrucki LW, Sinusas AJ. Cardiac restricted overexpression of membrane type-1 matrix metalloproteinase causes adverse myocardial remodeling following myocardial infarction. J Biol Chem. 2010;285:30316–30327. doi: 10.1074/jbc.M110.158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruddy JM, Jones JA, Stroud RE, Mukherjee R, Spinale FG, Ikonomidis JS. Differential effects of mechanical and biological stimuli on matrix metalloproteinase promoter activation in the thoracic aorta. Circulation. 2009;120:S262–268. doi: 10.1161/CIRCULATIONAHA.108.843581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charoonpatrapong-Panyayong K, Shah R, Yang J, Alvarez M, Pavalko FM, Gerard-O’Riley R, Robling AG, Templeton E, Bidwell JP. Nmp4/ciz contributes to fluid shear stress induced mmp-13 gene induction in osteoblasts. J Cell Biochem. 2007;102:1202–1213. doi: 10.1002/jcb.21349. [DOI] [PubMed] [Google Scholar]
- 9.Wang TL, Yang YH, Chang H, Hung CR. Angiotensin ii signals mechanical stretch-induced cardiac matrix metalloproteinase expression via jak-stat pathway. J Mol Cell Cardiol. 2004;37:785–794. doi: 10.1016/j.yjmcc.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Ross JJ. Mechanical consequences of regional myocardial ischemia. In: Fozzard HA, Haber E, Jennings RB, Katz AM, Morgan HE, editors. The heart and cardiovascular system. Raven Press; New York, NY: 1991. pp. 1997–2014. [Google Scholar]
- 11.Lieberman AN, Weiss JL, Jugdutt BI, Becker LC, Bulkley BH, Garrison JG, Hutchins GM, Kallman CA, Weisfeldt ML. Two-dimensional echocardiography and infarct size: Relationship of regional wall motion and thickening to the extent of myocardial infarction in the dog. Circulation. 1981;63:739–746. doi: 10.1161/01.cir.63.4.739. [DOI] [PubMed] [Google Scholar]
- 12.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, 3rd, Edmunds LH, Jr., Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107:2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 13.Dixon JA, Gaillard WF, 2nd, Rivers WT, Koval CN, Stroud RE, Mukherjee R, Spinale FG. Heterogeneity in mt1-mmp activity with ischemia-reperfusion and previous myocardial infarction: Relation to regional myocardial function. Am J Physiol Heart Circ Physiol. 2010;299:H1947–1958. doi: 10.1152/ajpheart.00314.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilla JJ, Blom AS, Gorman JH, 3rd, Brockman DJ, Affuso J, Parish LM, Sakamoto H, Jackson BM, Acker MA, Gorman RC. Early postinfarction ventricular restraint improves borderzone wall thickening dynamics during remodeling. Ann Thorac Surg. 2005;80:2257–2262. doi: 10.1016/j.athoracsur.2005.05.089. [DOI] [PubMed] [Google Scholar]
- 15.Enomoto Y, Gorman JH, 3rd, Moainie SL, Jackson BM, Parish LM, Plappert T, Zeeshan A, St John-Sutton MG, Gorman RC. Early ventricular restraint after myocardial infarction: Extent of the wrap determines the outcome of remodeling. Ann Thorac Surg. 2005;79:881–887. doi: 10.1016/j.athoracsur.2004.05.072. discussion 881-887. [DOI] [PubMed] [Google Scholar]
- 16.Kelley ST, Malekan R, Gorman JH, 3rd, Jackson BM, Gorman RC, Suzuki Y, Plappert T, Bogen DK, Sutton MG, Edmunds LH., Jr. Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation. 1999;99:135–142. doi: 10.1161/01.cir.99.1.135. [DOI] [PubMed] [Google Scholar]
- 17.Cheng A, Nguyen TC, Malinowski M, Langer F, Liang D, Daughters GT, Ingels NB, Jr., Miller DC. Passive ventricular constraint prevents transmural shear strain progression in left ventricle remodeling. Circulation. 2006;114:I79–86. doi: 10.1161/CIRCULATIONAHA.105.001578. [DOI] [PubMed] [Google Scholar]
- 18.Blom AS, Pilla JJ, Gorman RC, 3rd, Gorman JH, Mukherjee R, Spinale FG, Acker MA. Infarct size reduction and attenuation of global left ventricular remodeling with the corcap cardiac support device following acute myocardial infarction in sheep. Heart Fail Rev. 2005;10:125–139. doi: 10.1007/s10741-005-4640-2. [DOI] [PubMed] [Google Scholar]
- 19.Ryan LP, Matsuzaki K, Noma M, Jackson BM, Eperjesi TJ, Plappert TJ, St John-Sutton MG, Gorman JH, 3rd, Gorman RC. Dermal filler injection: A novel approach for limiting infarct expansion. Ann Thorac Surg. 2009;87:148–155. doi: 10.1016/j.athoracsur.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee R, Zavadzkas JA, Saunders SM, McLean JE, Jeffords LB, Beck C, Stroud RE, Leone AM, Koval CN, Rivers WT, Basu S, Sheehy A, Michal G, Spinale FG. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann Thorac Surg. 2008;86:1268–1276. doi: 10.1016/j.athoracsur.2008.04.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000;102:1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 22.Spinale FG, Koval CN, Deschamps AM, Stroud RE, Ikonomidis JS. Dynamic changes in matrix metalloprotienase activity within the human myocardial interstitium during myocardial arrest and reperfusion. Circulation. 2008;118:S16–23. doi: 10.1161/CIRCULATIONAHA.108.786640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leask A. Potential therapeutic targets for cardiac fibrosis: Tgfbeta, angiotensin, endothelin, ccn2, and pdgf, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. Tgf-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Spruill LS, Lowry AS, Stroud RE, Squires CE, Mains IM, Flack EC, Beck C, Ikonomidis JS, Crumbley AJ, McDermott PJ, Spinale FG. Membrane-type-1 matrix metalloproteinase transcription and translation in myocardial fibroblasts from patients with normal left ventricular function and from patients with cardiomyopathy. Am J Physiol Cell Physiol. 2007;293:C1362–1373. doi: 10.1152/ajpcell.00545.2006. [DOI] [PubMed] [Google Scholar]
- 26.Bumham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach. Springer-Verlag; New York: 2002. [Google Scholar]
- 27.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 28.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: The role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 29.Porter KE, Turner NA. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Poller W, Hajjar R, Schultheiss HP, Fechner H. Cardiac-targeted delivery of regulatory rna molecules and genes for the treatment of heart failure. Cardiovasc Res. 2010;86:353–364. doi: 10.1093/cvr/cvq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: A critical appraisal. Nat Rev Cardiol. 2010;7:204–215. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- 32.Hao X, Silva EA, Mansson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, Wardell E, Brodin LA, Mooney DJ, Sylven C. Angiogenic effects of sequential release of vegf-a165 and pdgf-bb with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75:178–185. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 33.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 34.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: A novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 35.Selvamurugan N, Partridge NC. Constitutive expression and regulation of collagenase-3 in human breast cancer cells. Mol Cell Biol Res Commun. 2000;3:218–223. doi: 10.1006/mcbr.2000.0215. [DOI] [PubMed] [Google Scholar]
- 36.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW, Gourdie RG, Matrisian LM, Spinale FG. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006;113:2919–2928. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 37.Divakaran V, Mann DL. The emerging role of micrornas in cardiac remodeling and heart failure. Circ Res. 2008;103:1072–1083. doi: 10.1161/CIRCRESAHA.108.183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. Mir-133 and mir-30 regulate connective tissue growth factor: Implications for a role of micrornas in myocardial matrix remodeling. Circ Res. 2009;104:170–178. doi: 10.1161/CIRCRESAHA.108.182535. 176p following 178. [DOI] [PubMed] [Google Scholar]
- 39.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of micrornas after myocardial infarction reveals a role of mir-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating micrornas in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 41.O’Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: Role of tgf-beta(1) Hypertension. 2000;36:319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.