Abstract
Natural killer (NK) cells can kill transformed cells and represent a promising tool for the treatment of cancer. Their function is governed by a balance of stimulatory and inhibitory signals triggered by surface receptors. Advances in NK cell therapy require the development of dependable methods for obtaining an adequate number of effector cells; additional activation or genetic modification may further increase their anti-cancer capacity. A method for NK cell expansion used in our laboratory relies on a genetically modified form of the K562 myeloid leukemia cell line, engineered to express a membrane-bound form of interleukin-15 and the ligand for the costimulatory molecule 4-1BB (CD137). Expanded NK cells can be transduced with genes encoding chimeric antigen receptors that stimulate tumor cell-specific cytotoxicity. These methods for NK cell expansion and genetic modification have been adapted to large-scale, clinical-grade, Current Good Manufacturing Practices conditions and support two active clinical trials. Summarized are current efforts for NK cell immunotherapy for cancer and future perspectives.
Keywords: NK cells, cell therapy, immunotherapy, chimeric receptors
Introduction
Natural killer (NK) cells represent 5% to 20% of human lymphocytes (1). Morphologically, they are large granular lymphocytes, and immunophenotypically they are defined by the expression of CD56 and the lack of CD3 and T-cell receptor proteins (2). NK cells have a unique function, in that they can lyse virally-infected or transformed cells without previous sensitization (3). However, their immunological role now appears more complex, extending beyond direct cell lysis: NK cells have also been implicated in several adaptive immune responses through interaction with other cells such as T lymphocytes and dendritic cells (1),(4).
Most peripheral blood NK cells express relatively low levels of CD56 and high levels of CD16, the FcγRIII receptor that binds the Fc portion of Ig (3). The primary function of this CD56dim population appears to be direct cytotoxicity, either by inducing target cell apoptosis via the perforin-granzyme pathway, and/or ligation of death-receptors through expression of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), or the Fas ligand (5). Killing by these NK cells can also be triggered by the binding of the Fc portion of antibodies to CD16, resulting in antibody-dependent cellular cytotoxicity (ADCC) (6). Approximately 10% of NK cells in peripheral blood express very high levels of CD56 and have dim CD16 expression (3). These CD56bright cells predominate in secondary lymphoid tissue (7) and are believed to have an immunoregulatory role, which is exerted by cytokine and chemokine secretion in order to propagate inflammation and recruit additional immune cells (8).
NK cells develop from CD34+ hematopoietic progenitors, first in the fetal liver, then in bone marrow and lymph nodes under the influence of the cytokine IL-15 (9),(10),(11). IL-2 also likely contributes to NK cell differentiation and receptor acquisition, although its contribution to normal development has not been entirely elucidated (11). Many NK cell surface receptors engage major histocompatibility complex (MHC) class I and MHC class I–like molecules. A concept that has dominated contemporary NK cell biology studies is the functional NK inhibition by MHC or human leukocyte antigen (HLA) class I molecules expressed on the surface of putative target cells (12). NK cells express killer-cell immunoglobulin-like receptors (KIRs), most of which recognize specific corresponding target HLA class I molecules and deliver inhibitory signals which suppress NK cell function (13). This negative signaling helps promote self-tolerance. More recently, it has been shown that interaction between NK cells and HLA molecules might also be important for full functional competency - a process which has been termed “licensing” (4), (14).
Interest in NK cells for immunotherapy of cancer has increased commensurately with a better understanding of their role in tumor immunity as well as an improvement in the methods for their characterization, purification and expansion (15). Considerable potential exists in the exploitation of current technology in both cell processing and cell engineering. Reviewed here is selected experience and future directions for NK cell therapy for cancer. This includes efforts to generate larger numbers of NK cells, enhance cytotoxicity, and improve specificity.
NK Cell Isolation and Expansion
NK cells can be specifically isolated from peripheral blood with methods that have been adapted to large-scale, clinical conditions. Typically, a leukapheresis product is subjected to immunomagnetic bead selection to obtain near pure populations of CD56+ CD3- cells (16). Effective NK cell functional activity depends on adequate effector: target ratios. Because NK cells comprise less than 20% of peripheral blood lymphocytes, it may be difficult to obtain adequate numbers for effective NK cell-mediated immunotherapy. Moreover, freshly isolated NK cells are typically in a resting state and subsequently require multiple, tightly-regulated activation steps before target engagement, formation of a cytolytic synapse and cell killing (17).
A continuously growing NK cell line, NK-92, has been explored as an alternative to primary NK cells, as they easily expand and are active against multiple cancer targets (18). Although this approach is appealing in its simplicity, NK-92 is a transformed cell line and requires lethal irradiation before infusion to prevent growth in vivo which may limit the anti-tumor activity of these cells and consequently, the effectiveness of this technology. Methods to increase numbers of non-transformed NK cells following isolation include cytokine stimulation as well as use of accessory cells.
Cytokines
NK cells generally do not undergo prolonged proliferation when exposed to cytokines (19). IL-2 can transiently stimulate their proliferation while enhancing their cytotoxic capacity (19), but only a small subpopulation of NK cells proliferates for a sustained period (19),(20). The addition of an anti-CD3 antibody to cultures including T lymphocytes reportedly increases IL-2-driven NK cell expansion in healthy donors as well as cancer patients (21). IL-15, a cytokine that is essential for NK cell development and survival, produces minimal NK cell expansion, even when used in conjunction with IL-2 (5),(22),(23). Other cytokines, such as IL-1, IL-4, IL-7, IL-12, and TNF may augment expansion driven by other signals but appear to be insufficient on their own (24), (25).
Expansion of NK cells with accessory cells
The addition of an exogenous cell population to NK cell cultures can provide additional signals to promote NK cell proliferation through mechanisms that are incompletely understood (26),(27). Reportedly, these methods have resulted in expansions ranging from 30-fold to several hundred-fold over several weeks, depending on cell type and culture conditions (26), (28), (29). Berg et al. described nearly 500- fold induced expansion after 2 weeks following contact with irradiated EBV-transformed lymphoblastoid cells (30). Similar levels of expansion have been achieved with HFWT, a Wilm's tumor-derived cell line (31). The BCR-ABL1 chronic myelogenous leukemia cell line, K562 also induces NK cells to expand and increases the known proliferative response to IL-15 (31),(27),(23).
Our laboratory engineered K562 cells to express two NK stimulatory molecules after retroviral transduction. First, K562 cells were transduced with a construct containing the human IL-15 gene fused to the gene encoding the human CD8α transmembrane domain, an approach inspired by the superior proliferative signals delivered by membrane-associated IL-15 as compared to soluble IL-15 (32),(33). Secondly, the K562 cells were transduced with the gene encoding the ligand of the NK costimulatory surface molecule 4-1BB (CD137) which sends activation signals (34). By expressing both membrane-bound IL-15 and 4-1BBL, the signals could act synergistically, and the resultant cell line (K562-mb15-41BBL) induced a 21.6-fold median NK cell expansion in one week of culture with 10 IU/mL IL-2 (23). Expansion continues beyond 7 days, and by increasing the IL-2 concentration to 100 IU/mL after one week, NK cell expansion by K562-mb15-41BBL was driven even further- with expansions greater than 1000-fold in three weeks. Importantly, there was little to no expansion of CD3+ T-lymphocytes (23),(35).
Augmenting NK Cell Functional Activity
Improved survival and function
Among the complex immunoregulatory signals that govern NK cells, IL-2 and IL-15 are known to have important overlapping yet distinct actions (11). In vitro supplementation of IL-2 to NK cell cultures can significantly increase their cytotoxicity (11). IL-2 administration also sustains NK cells survival in immunodeficient mice engrafted with human NK cells (36), and it is used in patients receiving NK cell infusions (37),(38). However, off-target side effects of IL-2 can be problematic, including a potentially life-threatening vascular leak syndrome caused by stimulation through IL-2 receptors expressed by endothelial cells (39). Micromolar amounts of IL-15, a cytokine which shares a receptor component with IL-2, can markedly prolong NK survival, even in absence of serum (22). Although therapeutic studies with recombinant IL-15 are only beginning phase I testing, animal data suggests a potential for myelosuppression as well as constitutional side effects (40).
Although the risk of adverse events may be low at the cytokine dosages required to sustain NK cell survival, genetic modifications generating autocrine cytokine signals may be a mechanism to avoid the consequences of systemic administration. IL-2 has been successfully transduced into NK cell lines, resulting in increased cytotoxicity as well as proliferation independent of supplementation (41). IL-15 transduction also increases natural cytotoxicity and survival (42). Thus far, there is limited described experience with cytokine transduction into primary or expanded NK cells (16), but such modifications could allow increased NK cell survival or proliferation without the restrictions associated with using transformed cell lines.
Potentiation of cytotoxicity
NK cells discriminate between healthy, self-derived cells from those that are transformed or infected by a delicate balance of inhibitory and activating signals via surface molecules (2). Individual target cells are repeatedly engaged by NK cells, and the relative strength of an activating signal as compared to competing negative signals determines the fate of the target cell (12). Activation molecules on NK cells include the natural cytotoxicity receptors, NKp46 (CD335), NKp44 (CD336), and NKp30 (CD337) as well as NKG2D (CD314) and some activating co-receptors including 2B4 (CD244) and DNAM-1 (CD226) (13).
Cytokine stimulation with IL-2 will increase the density of surface expression of activation molecules and consequently NK cell cytotoxicity (35). Driving overexpression of these molecules through cytokine gene transduction may increase cytolytic activity (41), (42). Additionally, NK cells that have been expanded by K562-mb15-41BBL stimulation have higher surface expression of these molecules as well as considerably higher cytotoxicity against acute myeloid leukemia (AML) cells than IL-2-stimulated cells (35). These expanded NK cells also acquire cytotoxicity against cell lines derived from patients with Ewing sarcoma (Fig. 1), rhabdomyosarcoma and neuroblastoma (43).
Figure 1.
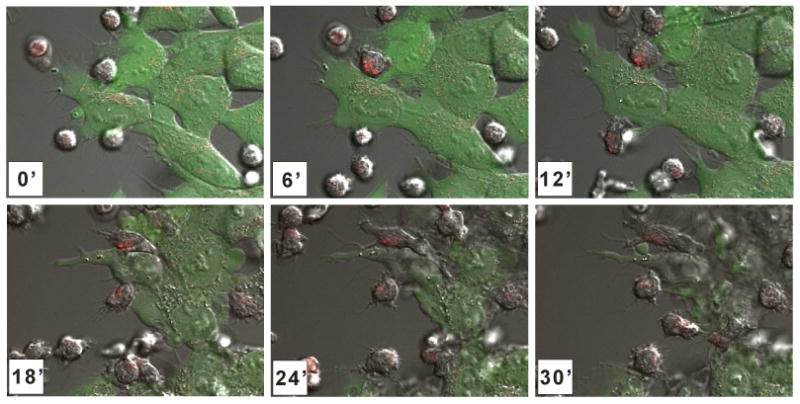
Killing of Ewing sarcoma cells by expanded activated NK cells. Time lapse confocal microscopy of expanded activated NK cells pre-incubated with LysoTracker Red (Invitrogen), co-cultured with GFP-expressing Ewing sarcoma cells. The time (minutes) after the beginning of the coculture at which each photograph was taken is indicated.
Beyond augmenting activating signals, the down-regulation of inhibitory signals represents an alternative approach to biologic modification. The primary negative regulator of NK cell cytotoxicity is mediated through the engagement of MHC class I and MHC class I–like molecules (44). Most KIRs expressed on human NK cells recognize specific corresponding HLA class I molecules on target cells, and deliver inhibitory signals (45). These inhibitory signals from HLA can override activating signals and suppress the function of NK cells. The human monoclonal anti-KIR antibody 1-7F9 prevents inhibitory signaling through KIR 2DL1,-2, and -3 and has been shown to increase killing of HLA-matched AML blasts in vitro and in vivo (45). This agent is currently under clinical investigation. The NK surface molecule NKG2A (CD159a) recognizes the HLA class Ib molecule HLA-E, and its signal significantly inhibits NK cell cytotoxicity (44). Experiments investigating RNA interference against NKG2A demonstrated a 40% increase in NK cell killing (46). Table 1 summarizes the activating and inhibitory signaling molecules that have been targeted for modulation in NK cell therapy.
Table 1. NK cell activating and inhibitory signaling molecules and possible ways to modulate their function.
| Signaling molecule (Known ligand) | Method of modulation | References |
|---|---|---|
| Activating | ||
| NKG2D/CD314 (MICA/B; ULBPs) NKp46/CD335 NKp44/CD336 NKp30/CD337 |
Upregulation of expression with:
|
(35, 42, 60) |
| DNAM-1/CD226 (PVR/CD155; Nectin-2/CD112) | Upregulation of expression with:
|
(52), (60) |
| CD3ζ 4-1BB/CD137 (4-1BBL) 2B4/CD244 (CD48) |
Molecule incorporation into chimeric receptors | (23), 49) |
| Inhibitory | ||
| 2DL1,-2, and -3 (HLA Class I) | Inhibition of interaction with ligand by monoclonal anti-KIR antibody | (45) |
| NKG2A/CD159a (HLA-E) | Suppression of inhibitory signals by RNA interference targeting NKG2A | (46) |
Abbreviations: KIR, Killer-Cell immunoglobulin-like receptors; HLA, human leukocyte antigen
Redirecting NK Cell Specificity
Cell therapies based on the broad cytotoxic activity of NK cells can be increased by generating chimeric antigen receptors, thereby facilitating the killing of previously resistant targets. Our laboratory demonstrated proof of this concept by transducing NK cells expanded by K562-mb15-41BBL stimulation with an artificial receptor against CD19 (23). CD19 is expressed by acute lymphoblastic leukemia and B-cell malignancies, which are only minimally sensitive to NK cell attack. Transduction with the anti-CD19 receptor gave the NK cells activity against previously resistant patient leukemia samples. This cytotoxic signal could be further augmented by the addition of both CD3ζ and the 4-1BB costimulatory molecules. Of note, the anti-CD19-41BB-CD3ζ receptor developed in our laboratory, when expressed in T lymphocytes, powerfully stimulates their cytotoxicity against CD19-positive tumor cells (47), and it was recently shown to provoke remarkable anti-leukemic activity in patients with B-cell chronic lymphocytic leukemia (48, 49). Importantly, other groups have demonstrated that similar receptors can be generated by replacing the anti-CD19 scFv with the scFv of another antibody, such as anti-CD20, -ErbB2, -GD2, and -HER-2, effectively redirecting the specificity of NK cells (50),(51),(52),(53).
Application to Clinical Practice
In 2005, Miller et al. reported that allogeneic NK cells infused into patients with high-risk malignancies who had received immunosuppressive but not myeloablative chemotherapy could expand and have measureable anti-leukemic activity (37). In this study, there were 19 adult patients with high-risk AML who received cyclophosphamide and fludarabine followed by infusion of an NK cell-enriched product (approximately 40% NK cells) which was prepared by T-cell depletion and IL-2 (1000 IU/mL) activation. The patients also received scheduled IL-2 injections in efforts to improve NK cell survival. Eight of 15 evaluable AML patients showed ≥1% donor engraftment after 7 days. In addition, five patients achieved a morphologic complete remission. The number of circulating NK cells was significantly greater in the patients who achieved remission than those who did not. In a more recent study performed at St. Jude Children's Research Hospital (NKAML), 10 pediatric patients with AML in first remission received a single infusion of KIR-mismatched, haploidentical CD56+ CD3- NK cells which were highly purified using the Miltenyi CliniMACS system (38). The conditioning was similar to the one used by Miller et al. and the patients also received six doses of IL-2 at 1 million units/m2. This therapy was well tolerated with no evidence of graft versus host disease, and all children remain in remission.
The NK activation and expansion system using the K562-mb15-41BBL cells developed in our laboratory has also been adapted for clinical protocols using cGMP guidelines (35) and supports two active Phase I cell dose escalation clinical trials at St. Jude (Fig. 2). Both protocols use a cyclophosphamide and fludarabine-based conditioning regimen as well as the scheduled IL-2 dosing based on Miller et al. (37) and tested in pediatric patients in the NKAML protocol. The cell product is initially prepared by peripheral blood apheresis collection from a haploidentical donor followed by mononuclear cell separation by Ficoll. The K562-mb15-41BBL cells (from a Master Cell Bank) are then irradiated (100 Gy) and added at a ratio of 1 CD56+ CD3− cell to 10 K562-mb15-41BBL cells. Cell cultures are performed in a closed system and fed twice with fresh IL2-containing media (SCGM; CellGenix, Antioch, IL) during the expansion phase. Expansion of NK cells is substantial under these conditions, with median 90.5-fold after 7 days (35). In the NKEXP protocol, the cells are harvested after one week, depleted of residual T cells using the Miltenyi CliniMACS system, then washed and resuspended prior to patient infusion. In the NKCD19 protocol, the expanded NK cells undergo two days of viral transduction with an anti-CD19 chimeric antigen receptor followed by four additional days of culture. After confirmation of cell viability and receptor expression, the cells are infused into the patient (summarized in Fig 2).
Figure 2.
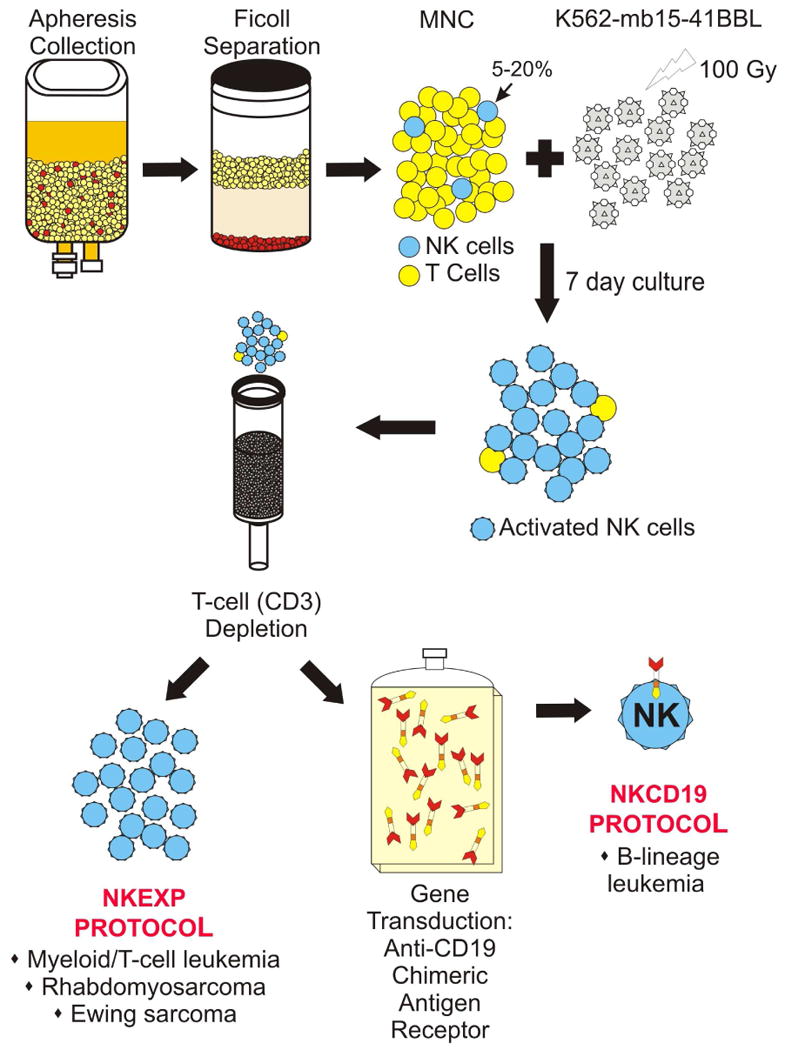
Schematic representation of the NK cell expansion and genetic modification protocols currently ongoing at St. Jude Children's Research Hospital.
Beyond adoptive immunotherapy, hematopoietic stem-cell transplantation practices may also benefit from advances in NK cell engineering. NK cells are the first lymphocyte subset to recover following transplantation and are thus likely responsible for early graft vs. tumor effect (54). Increased numbers of NK cells is associated with improved transplant survival and decreased relapse and acute graft versus host disease (15). Moreover, it is also known that donor NK cells can exert an effective anti-leukemia effect if they do not express KIRs reacting with the HLA class I epitope expressed by the patient's leukemia cells (55). The generation of these KIR-HLA mismatched NK cells is important for anti-leukemia alloreactivity and is increasingly recognized as important for donor selection in haploidentical stem cell transplantation, where NK cell graft versus leukemia effect is most essential (56). Autologous KIR mismatching can also occur and correlates with response to autologous transplantation for pediatric solid tumors (57),(58). Increased number of NK cells or alteration in their function could, in turn, have important consequences to transplantation outcomes.
Future work will focus on NK cell engineering to generate cells with improved activity or survival. One limitation to current approaches to genetic modification is the reliance on gene transfer primarily by retroviral transduction. Electroporation offers a mechanism of transient expression without the time, expense or risks of clinical-grade viral transduction (59).
Acknowledgments
This work was supported by grants CA113482 and CA21765 from the National Cancer Institute, grants from the Assisi Foundation and from the Fondation de Gouverneurs de l'Espoir, and by the American Lebanese Syrian Associated Charities (ALSAC)
References
- 1.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–61. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 2.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–37. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava S, Lundqvist A, Childs RW. Natural killer cell immunotherapy for cancer: a new hope. Cytotherapy. 2008;10:775–83. doi: 10.1080/14653240802648181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becknell B, Caligiuri MA. Natural killer cells in innate immunity and cancer. J Immunother. 2008;31:685–92. doi: 10.1097/CJI.0b013e318182de23. [DOI] [PubMed] [Google Scholar]
- 7.Fehniger TA, Cooper MA, Nuovo GJ, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 8.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci U S A. 108:728–32. doi: 10.1073/pnas.1012356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaleco AC, Blom B, Res P, et al. Fetal liver contains committed NK progenitors, but is not a site for development of CD34+ cells into T cells. J Immunol. 1997;159:694–702. [PubMed] [Google Scholar]
- 10.Spits H, Lanier LL, Phillips JH. Development of human T and natural killer cells. Blood. 1995;85:2654–70. [PubMed] [Google Scholar]
- 11.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–39. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 12.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 13.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–9. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 15.Lee DA, Verneris MR, Campana D. Acquisition, preparation, and functional assessment of human NK cells for adoptive immunotherapy. Methods Mol Biol. 2010;651:61–77. doi: 10.1007/978-1-60761-786-0_4. [DOI] [PubMed] [Google Scholar]
- 16.Alici E, Sutlu T, Sirac Dilber M. Retroviral gene transfer into primary human natural killer cells. Methods Mol Biol. 2009;506:127–37. doi: 10.1007/978-1-59745-409-4_10. [DOI] [PubMed] [Google Scholar]
- 17.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–25. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam YK, Martinson JA, Doligosa K, Klingemann HG. Ex vivo expansion of the highly cytotoxic human natural killer-92 cell-line under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy. 2003;5:259–72. doi: 10.1080/14653240310001523. [DOI] [PubMed] [Google Scholar]
- 19.Trinchieri G, Matsumoto-Kobayashi M, Clark SC, Seehra J, London L, Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984;160:1147–69. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.London L, Perussia B, Trinchieri G. Induction of proliferation in vitro of resting human natural killer cells: IL 2 induces into cell cycle most peripheral blood NK cells, but only a minor subset of low density T cells. J Immunol. 1986;137:3845–54. [PubMed] [Google Scholar]
- 21.Alici E, Sutlu T, Bjorkstrand B, et al. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111:3155–62. doi: 10.1182/blood-2007-09-110312. [DOI] [PubMed] [Google Scholar]
- 22.Carson WE, Fehniger TA, Haldar S, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–43. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–83. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson MJ, Manley TJ, Donahue C, Levine H, Ritz J. Costimulatory signals are required for optimal proliferation of human natural killer cells. J Immunol. 1993;150:1705–14. [PubMed] [Google Scholar]
- 25.Naume B, Gately M, Espevik T. A comparative study of IL-12 (cytotoxic lymphocyte maturation factor)-, IL-2-, and IL-7-induced effects on immunomagnetically purified CD56+ NK cells. J Immunol. 1992;148:2429–36. [PubMed] [Google Scholar]
- 26.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–9. [PubMed] [Google Scholar]
- 27.Robertson MJ, Cameron C, Lazo S, Cochran KJ, Voss SD, Ritz J. Costimulation of human natural killer cell proliferation: role of accessory cytokines and cell contact-dependent signals. Nat Immun. 1996;15:213–26. [PubMed] [Google Scholar]
- 28.Luhm J, Brand JM, Koritke P, Hoppner M, Kirchner H, Frohn C. Large-scale generation of natural killer lymphocytes for clinical application. J Hematother Stem Cell Res. 2002;11:651–7. doi: 10.1089/15258160260194794. [DOI] [PubMed] [Google Scholar]
- 29.Rabinowich H, Sedlmayr P, Herberman RB, Whiteside TL. Increased proliferation, lytic activity, and purity of human natural killer cells cocultured with mitogen-activated feeder cells. Cell Immunol. 1991;135:454–70. doi: 10.1016/0008-8749(91)90290-r. [DOI] [PubMed] [Google Scholar]
- 30.Berg M, Lundqvist A, McCoy P, Jr, et al. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009;11:341–55. doi: 10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada H, Watanabe S, Saijo K, Ishiwata I, Ohno T. A Wilms tumor cell line, HFWT, can greatly stimulate proliferation of CD56+ human natural killer cells and their novel precursors in blood mononuclear cells. Exp Hematol. 2004;32:614–21. doi: 10.1016/j.exphem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Musso T, Calosso L, Zucca M, et al. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999;93:3531–9. [PubMed] [Google Scholar]
- 33.Kobayashi H, Dubois S, Sato N, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105:721–7. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 34.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–72. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 35.Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–7. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujisaki H, Kakuda H, Imai C, Mullighan CG, Campana D. Replicative potential of human natural killer cells. Br J Haematol. 2009;145:606–13. doi: 10.1111/j.1365-2141.2009.07667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 38.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–9. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krieg C, Letourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A. 107:11906–11. doi: 10.1073/pnas.1002569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fewkes NM, Mackall CL. Novel gamma-chain cytokines as candidate immune modulators in immune therapies for cancer. Cancer J. 16:392–8. doi: 10.1097/PPO.0b013e3181eacbc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagashima S, Mailliard R, Kashii Y, et al. Stable transduction of the interleukin-2 gene into human natural killer cell lines and their phenotypic and functional characterization in vitro and in vivo. Blood. 1998;91:3850–61. [PubMed] [Google Scholar]
- 42.Zhang J, Sun R, Wei H, Tian Z. Characterization of interleukin-15 gene-modified human natural killer cells: implications for adoptive cellular immunotherapy. Haematologica. 2004;89:338–47. [PubMed] [Google Scholar]
- 43.Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of Activated Natural Killer Cells Against Pediatric Solid Tumors. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borrego F, Kabat J, Kim DK, et al. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637–60. doi: 10.1016/s0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 45.Romagne F, Andre P, Spee P, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–77. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Figueiredo C, Seltsam A, Blasczyk R. Permanent silencing of NKG2A expression for cell-based therapeutics. J Mol Med. 2009;87:199–210. doi: 10.1007/s00109-008-0417-0. [DOI] [PubMed] [Google Scholar]
- 47.Imai C, Campana D. Genetic modification of T cells for cancer therapy. J Biol Regul Homeost Agents. 2004;18:62–71. [PubMed] [Google Scholar]
- 48.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced Leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller T, Uherek C, Maki G, et al. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother. 2008;57:411–23. doi: 10.1007/s00262-007-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pegram HJ, Jackson JT, Smyth MJ, Kershaw MH, Darcy PK. Adoptive transfer of gene-modified primary NK cells can specifically inhibit tumor progression in vivo. J Immunol. 2008;181:3449–55. doi: 10.4049/jimmunol.181.5.3449. [DOI] [PubMed] [Google Scholar]
- 52.Altvater B, Landmeier S, Pscherer S, et al. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res. 2009;15:4857–66. doi: 10.1158/1078-0432.CCR-08-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kruschinski A, Moosmann A, Poschke I, et al. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci U S A. 2008;105:17481–6. doi: 10.1073/pnas.0804788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savani BN, Mielke S, Adams S, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21:2145–52. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 55.Leung W, Iyengar R, Turner V, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–50. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 56.Moretta L, Locatelli F, Pende D, Marcenaro E, Mingari MC, Moretta A. Killer Ig-like receptor-mediated control of natural killer cell alloreactivity in haploidentical hematopoietic stem cell transplantation. Blood. 2011;117:764–71. doi: 10.1182/blood-2010-08-264085. [DOI] [PubMed] [Google Scholar]
- 57.Leung W, Handgretinger R, Iyengar R, Turner V, Holladay MS, Hale GA. Inhibitory KIR-HLA receptor-ligand mismatch in autologous haematopoietic stem cell transplantation for solid tumour and lymphoma. Br J Cancer. 2007;97:539–42. doi: 10.1038/sj.bjc.6603913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Venstrom JM, Zheng J, Noor N, et al. KIR and HLA genotypes are associated with disease progression and survival following autologous hematopoietic stem cell transplantation for high-risk neuroblastoma. Clin Cancer Res. 2009;15:7330–4. doi: 10.1158/1078-0432.CCR-09-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li L, Liu LN, Feller S, et al. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther. 2010;17:147–54. doi: 10.1038/cgt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyiadzis M, Memon S, Carson J, et al. Up-regulation of NK cell activating receptors following allogeneic hematopoietic stem cell transplantation under a lymphodepleting reduced intensity regimen is associated with elevated IL-15 levels. Biol Blood Marrow Transplant. 2008;14:290–300. doi: 10.1016/j.bbmt.2007.12.490. [DOI] [PubMed] [Google Scholar]


