Abstract
OBJECTIVE
Alzheimer’s disease (AD), cerebral vascular brain injury (VBI), and isocortical Lewy body (LB) disease (LBD) are the major contributors to dementia in community- or population-based studies: Adult Changes in Thought (ACT) study, Honolulu-Asia Aging Study (HAAS), Nun Study (NS), and Oregon Brain Aging Study (OBAS). However, the prevalence of clinically silent forms of these diseases in cognitively normal (CN) adults is less clear.
DESIGN and SETTING
We evaluated 1672 brain autopsies from ACT, HAAS, NS, and OBAS of which 424 met criteria for CN.
MAIN OUTCOME MEASURES
Of these, 336 cases had a comprehensive neuropathologic examination of neuritic plaque (NP) density, Braak stage for neurofibrillary tangles (NFTs), Lewy body (LB) distribution, and number of cerebral microinfarcts (CMIs).
RESULTS
47% of CN cases had moderate or frequent NP density; of these 6% also had Braak stage V or VI for NFTs. 15% of CN cases had medullary LBD; 8% also had nigral and 4% isocortical LBD. The presence of any CMIs was identified in 33% and high level CMIs in 10% of CN individuals. Overall burden of lesions in each individual and their co-morbidity varied widely within each study but were similar among studies.
CONCLUSIONS
These data show an individually varying complex convergence of subclinical diseases in the brain of older CN adults. Appreciating this ecology should help guide future biomarker or neuroimaging studies as well as clinical trials that focus on community- or population-based cohorts.
Search terms: Alzheimer’s disease, vascular brain injury, Lewy body disease, cognitive aging
INTRODUCTION
Recent neuroimaging and cerebrospinal fluid biomarker studies have indicated that fibrillar amyloid (A) β accumulation in cerebrum occurs in approximately one-quarter to one-half of cognitively normal (CN) older adults, and likely represents latent Alzheimer’s disease (AD).1, 2 These findings are consonant with a large body of literature from neuropathologic studies that have characterized features of AD, including neuritic plaque (NP) formation, in cognitively normal older adults.3–13
Most of the above studies have investigated research clinic cohorts that commonly use clinical criteria to enrich for subjects with probable AD and often exclude subjects with a history of cerebrovascular disease or its risk factors, thereby limiting the representation of other common diseases that can contribute to dementia.14, 15 Indeed, investigations of community- or population-based cohorts for brain aging and dementia repeatedly have observed three common contributors to dementia: AD, vascular brain injury (VBI), and Lewy body (LB) disease (LBD).16, 17 While there are accepted histopathologic criteria for assessing the lesions of AD or LBD, there are no widely employed criteria for VBI; however, two of the population-based studies included here (HAAS and ACT) have independently shown that microvascular brain injury (μVBI) as assessed by systematic screening for cerebral microinfarcts (CMIs) carries a relative risk for dementia similar to AD.18, 19 While changes of AD, μVBI, and LBD are the most prevalent structural correlates of dementia in community- or population-based cohorts, we hasten to add that there are other diseases that cause dementia and produce distinctive lesions in brain; however, these are relatively uncommon and not captured efficiently in community- or population-based studies of elderly individuals.15, 19
Since there are as yet no validated neuroimaging or biomarker protocols for μVBI or LBD, current information on clinically silent forms of these diseases is available only from autopsy-based studies. Some community- or population-based studies of brain aging and dementia with autopsy have investigated clinically silent disease;3, 4, 14, 15, 20–22 however, all but one15 have been focused on a single cohort resulting in a relatively low number of cases and potentially restricted generalization. Moreover, differences in the methods for histologic assessment or the definition of CN have varied among these studies and might account for some of the apparently discrepant outcomes. Here we address these limitations with a harmonized histologic assessment of AD, μVBI, and LBD in participants with uniformly defined cognitive function from the Adult Changes in Thought (ACT) study, the Honolulu-Asia Aging Study (HAAS), the Nun Study (NS), and the Oregon Brain Aging Study (OBAS).
METHODS
ACT, HAAS, NS, and OBAS were approved by the respective Institutional Review Boards. All cases were drawn from the existing databases for brain autopsies from these four studies as of January 2010.
All cohorts shared the same general characteristics. Each was recruited as a participant for a longitudinal study of brain aging from a defined community or population. Subjects did not present for study because of concerns about memory or cognitive function. The NS cohort is composed of American sisters of the School Sisters of Notre Dame religious congregation born before 1917 who volunteered to join a longitudinal study of aging and Alzheimer’s disease;23 the HAAS cohort is a subset of the Honolulu Heart Program followed for cognitive and aging function;24 the ACT study cohort is composed of individuals 65 years or older who were members of the Group Health Cooperative, a managed care organization in the Seattle area;25 the OBAS study cohort is composed of initially healthy seniors recruited from the community via advertising and senior congregate site canvassing for studies focused on the oldest old.26
Clinical Assessments
Only those individuals whose last clinical evaluation was within 2 years of death were included in this study. At the time of last evaluation, study participants were diagnosed with “dementia” or “not dementia” based on Diagnostic Statistical Manual (DSM) criteria: DSM-IIIR for HAAS and DSM-IV for ACT, NS, and OBAS. Individuals who were diagnosed as “not dementia” were further stratified based on performance on a cognitive screening test: the Mini-Mental State Examination (MMSE) for NS and OBAS, and the Cognitive Assessment Screening Instrument (CASI) for ACT and HAAS. To minimize misclassification we further restricted analysis to subjects whose scores on their respective cognitive screening test at last evaluation was in the upper four quintiles for the entire cohort; this cut-off was MMSE ≥24 for NS, MMSE ≥28 for OBAS, CASI ≥83 for HAAS and CASI >90 for ACT (Table 1).
Table 1.
Participant Characteristics.
| ACT | HAAS | NS | OBAS | Total | ||
|---|---|---|---|---|---|---|
| Total autopsies (n) | 344 | 446 | 548 | 334 | 1672 | |
| & last evaluation within 2 yr of death (n) | 299 | 262 | 475 | 280 | 1316 | |
| & “Not Dementia” by DSM+ at last evaluation (n) | 182 | 126 | 182 | 166 | 656 | |
| Eligible Cases | & in upper four quintiles of cognition function for entire cohort# (n) | 116 | 59 | 162 | 87 | 424 |
| complete neuropathologic examination (n) | 116 | 59 | 106* | 55^ | 336 | |
| Age at death (yr, mean ± SD) | 84 ± 6 | 83 ± 5 | 89 ± 5 | 91 ± 5 | 87 ± 4 | |
| M:F (n) | 52:64 | 59:0 | 0:106 | 23:32 | 1:1.5 | |
| Last MMSE (median, range) | 28, 25 to 30 | 27, 23 to 29 | 28, 24 to 30 | 29, 28 to 30 | ---- | |
| Last CASI (mean ± SD) | 95 ± 3 | 89 ± 4 | ---- | ---- | ---- | |
| Brain weight (g; mean ± SD) | 1178 ± 143 | 1292 ± 101 | 1143 ± 122 | 1222 ± 125 | 1209 ± 64 |
DSM-IIIR for HAAS and DSM-IV for NS and ACT.
MMSE for NS (≥24) and OBAS (≥28), and CASI for HAAS (≥83) and ACT (≥91).
Neuropathologic examination for OBAS switched to a protocol identical to ACT in 2004; all eligible cases accrued past this date had comparable neuropathologic examinations and were included.
Only NS cases without missing Braak or CMI scores were included (n=106).
Histopathologic Scoring
CERAD score for NPs was determined as described previously and recorded as part of the primary data for ACT, OBAS, and NS using modified Bielchowsky stain.27 HAAS used the same method but recorded data as density of NP in different regions of cerebral cortex from which the CERAD NP score was derived. Braak staging for NFTs was determined by the same method in all four studies.28 CMIs were determined in all four studies by the same method of screening hematoxylin & eosin (H&E)-stained sections of cerebrum for infarcts that were not observed grossly.19 HAAS screened more regions of cerebrum for microinfarcts than the other studies; however, all data presented here are limited to the same twelve sections from the same six bilateral cerebral regions as previously described.19 Immunohistochemistry for α-synuclein was used to highlight LBs in all studies.29 The regions evaluated for LBs overlapped among all studies only for frontal and temporal cortex. ACT and NS also evaluated LBs in medulla, and HAAS, ACT, and OBAS assessed LBs in the substantia nigra (SN).
We have previously used a summary neuropathology score as a convenient metric to capture the magnitude of AD, μVBI, and LBD in each individual in the ACT cohort.30 As previously published, this summary neuropathology score derives from the sum of subsocres for each of the main axes: (i) AD subscore (Braak stage for NFTs expressed as number and then divided by 2 and thus ranging from 0 to 3), (ii) CMI subscore (number of CMI with ≥ 3 CMI by screening protocol assigned a value of 3 and thus ranging from 0 to 3), and (iii) LBD subscore (0 for none, 1 for medullary LBD, 2 for SN LBD, and 3 for isocortical LBD in frontal or temporal cortex).
Statistical analyses were performed using GraphPad Prism (San Diego, CA)
RESULTS
We pursued a uniform analysis of cases from ACT, HAAS, NS, and OBAS with the goal of obtaining comparable datasets from these community- or population-based studies of brain aging and dementia. Table 1 presents our selection criteria and the eligible cases from each cohort. Overall, this study derived from 1672 individuals who participated in one of these four studies and who consented to brain autopsy as part of a research study. Of these, 424 people were cognitively normal (CN) as defined by: (i) their last clinical evaluation being within two years of death (average = 369 d), (ii) at that time they were diagnosed as not dementia, and (iii) at that time their cognitive testing battery results were in the upper four quintiles for the entire cohort in which they participated. Of these 424 CN cases, complete neuropathologic evaluation was performed on 336; missing CMI data being the most frequent cause of an incomplete evaluation. These 336 autopsies with complete neuropathologic examination from CN older adults who participated in population- or community-based studies form the basis of the present study (Table 1).
We chose to reference all other lesions to NP score because this was the most prevalent lesion among eligible cases. However, CERAD NP has potential challenges as a measure of fibrillar Aβ. For this reason we performed two experiments to assess the limits of this measure. First, since ACT also collects rapidly frozen tissue, we compared tissue burden of detergent-insoluble Aβ42 with CERAD NP score in cortex from middle frontal gyrus and superior and middle temporal gyri: CERAD NP “none” (n=5) = 0.3 ± 0.1 and 0.2 ± 0.1, “sparse” (n=10) = 2.0 ± 0.1 and 1.5 ± 0.5, and “moderate” (n=4) = 13 ± 4.1 and 9.6 ± 4.6 ng/g detergent-soluble Aβ42 (Spearman ranked correlation P < 0.001 in both cerebral cortical regions). Second, analogous to a method commonly used in transgenic mice that express mutant human APP,31 we measured percent gray matter area occupied by Aβ immunoreative peptide in temporal cortices from randomly selected ACT cases and correlated these results with CERAD NP score from the contralateral temporal cortex; again, these two measures were highly significantly correlated (n = 86; Spearman ranked correlation r = 0.50, P < 0.0001). These results indicate that CERAD NP score, a measure that was applied across all four studies, is highly significantly correlated with cerebral cortical burden of plaque-associated Aβ peptides as determined by immunohistochemistry with antibody 6E10 and with cerebral cortical detergent-insoluble Aβ42.
Table 2 stratifies CERAD NP score by Braak stage for NFTs in CN adults in the four studies. Approximately 47% of CN older individuals had moderate or frequent NP scores across all Braak stages. However only 6% of these individuals also had Braak stage V or VI for NFTs. Thus, the substantial cerebral cortical accumulation of NPs that was observed in approximately one-half of CN older adults was not usually accompanied by high Braak stage for NFTs. Interpretation of these necessarily cross-sectional data is limited but does suggest that, while accumulation of cerebral cortical Aβ is common, the full spectrum of pathologic changes of AD is relatively uncommon in CN older adults.
Table 2. AD Pathologic Changes.
Stratification of the 336 eligible participants with complete neuropathologic examination from the four studies into CERAD neuritic plaque (NP) score and Braak stage for neurofibrillary tangles (NFTs) groups. Percent of individuals from each study was calculated separately; data are average percentages (SD) for the four studies. Braak stage for NFTs was stratified as “None to II” to correspond with later analyses but also as “(None)” to highlight those individuals without NFTs.
| Braak NFT Stage | Total | ||||
|---|---|---|---|---|---|
| None to II (None) | III or IV | V or VI | |||
| CERAD NP Score | None | 23 ± 6 (5 ± 1) | 6 ± 4 | 0 ± 1 | 30 ± 8 |
| Sparse | 15 ± 5 (3 ± 2) | 7 ± 4 | 1 ± 1 | 23 ± 8 | |
| Moderate | 13 ± 4 (1 ± 1) | 8 ± 3 | 3 ± 2 | 24 ± 6 | |
| Frequent | 12 ± 4 (0 ± 1) | 9 ± 3 | 3 ± 1 | 23 ± 7 | |
| Total | 63 ± 6 (9 ± 3) | 30 ± 5 | 7 ± 8 | 100 | |
Table 3 presents a similar stratification for LBD among the four studies. Because data for LBD were not collected in the same manner across all studies, data from only two studies could be combined for medulla and from three studies for substantia nigra; all four studies collected comparable data for LBD in isocortex. LBD in CN older adults followed an apparent anatomic progression with 15% showing medullary LBD; a subset of these (8%) also had LBD in substantia nigra, while a further subset (4%) also had isocortical LBD, similar to observations made by others.32
Table 3. Lewy Body Disease.
Stratification of the 336 eligible participants with complete neuropathologic examination by CERAD neuritic plaque (NP) score and Lewy Body Disease (LBD). Percent of individuals from each study was calculated separately; data are average percentages (SD) for the four studies.
| LBD | ||||
|---|---|---|---|---|
| Medulla (ACT & NS) | and SN (ACT, HAAS, & OBAS) | and Isocortical (all four studies) | ||
| CERAD NP Score | None | 6 ± 1 | 1 ± 1 | 0 ± 0 |
| Sparse | 4 ± 3 | 3 ± 2 | 2 ± 2 | |
| Moderate | 3 ± 3 | 3 ± 1 | 1 ± 1 | |
| Frequent | 2 ± 2 | 1 ± 2 | 1 ± 1 | |
| Total | 15 ± 2 | 8 ± 3 | 4 ± 2 | |
Regional LBD was assessed differently in the four studies. ACT and NS (NS) screened for medullary LBs using SNCA IHC; HAAS and OBAS did not have data on medullary LBs. ACT, HAAS, and OBAS screened for LBs in the substantia nigra (SN) using H&E stained slides. All studies used SNCA IHC in frontal cortex or temporal cortex.
Although several groups have reported on subclinical NP, NFT, and LB in cognitively normal individuals from research cohorts, there have been relatively little data on μVBI in CN older adults. Table 4 presents CMI data from CN individuals in our four studies stratified by CERAD NP score. There was no significant correlation between CERAD NP score and the categories for CMI as presented in Table 4. Overall, approximately one-third of CN older adults had CMI captured by our screening protocol; of these 10% had ≥ 3 CMI, a level of disease that is associated with increased risk of clinically evident dementia.19
Table 4. Cerebral Microinfarcts.
Stratification of the 336 eligible participants with complete neuropathologic examination by number of cerebral microinfarcts (CMI) and CERAD neuritic plaque (NP) score. Percent of individuals from each study was calculated separately; data are average percentages (SD) for the three studies.
| CMI | Total | ||||
|---|---|---|---|---|---|
| None | 1 or 2 | ≥ 3 | |||
| CERAD NP Score | None | 22 ± 12 | 6 ± 2 | 3 ± 1 | 31 ± 11 |
| Sparse | 13 ± 5 | 6 ± 1 | 3 ± 1 | 22 ± 6 | |
| Moderate | 14 ± 7 | 5 ± 2 | 1 ± 1 | 20 ± 6 | |
| Frequent | 18 ± 4 | 6 ± 4 | 3 ± 3 | 27 ± 8 | |
| Total | 67 ± 8 | 23 ± 6 | 10 ± 3 | 100 | |
There were no instances of other less common diseases that can cause dementia in this cohort of CN individuals, e.g., fronto-temporal lobar degeneration or prion disease. Three instances of hippocampal sclerosis were observed (one each in ACT, HAAS and NS); each was co-morbid with AD and/or μVBI. Two of 116 CN cases from the ACT cohort had amygdala-only LBD; this was not evaluated in the other cohorts.
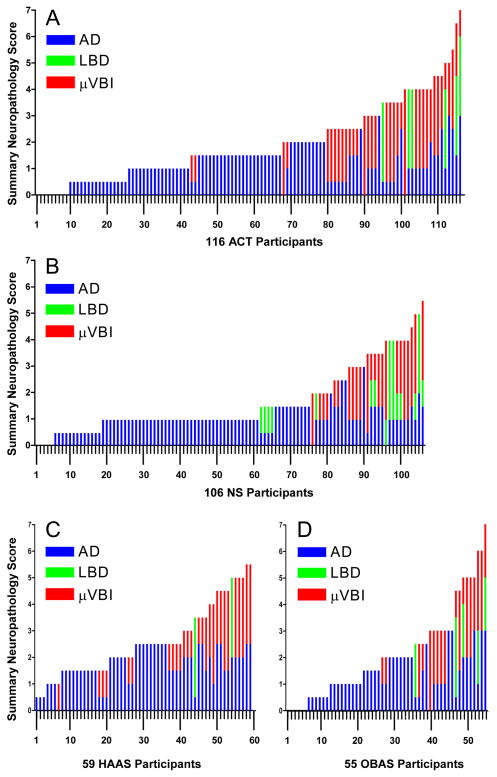
Figures 1A through 1D present each CN individual’s summary neuropathology score as a bar divided into its corresponding subscores for AD, μVBI, and LBD; these data have been arranged by summary neuropathology score (lowest to highest), then ranked by AD subscore, and then ranked by μVBI subscore with the individual bars opposed so they appear (appropriately) as a continuum. Results from ACT (Figure 1A), HAAS (Figure 1B), NS (Figure 1C), and OBAS (Figure 1D) are similar and show that it is uncommon for CN older individuals to have no neuropathologic evidence of disease(s) that can cause dementia (Table 5). Moreover, the burden of diseases ranged widely within each study and showed substantial individually varying co-morbidity. While we can only speculate on the extent of diseases that existed at the time of cognitive testing, on average approximately one year before neuropathologic evaluation, our cross-validating results strongly suggest that CN older individuals have widely varying burdens of disease(s) that can cause dementia, and that there is highly individually varying co-morbidity among these three common diseases.
Figure 1.
Brain autopsy results from cognitively normal (CN) individuals expressed as Summary Neuropathology Score (range 0 to 9) ranked from lowest to highest. Each stacked bar shows an individual’s burden of AD (blue), LBD (green), and μVBI (red). (A) 116 Adult Changes in Thought (ACT) study participants, (B) 106 Nun Study (NS) participants, (C) 59 Honolulu Asian Aging Study (HAAS) participants, and (D) 55 Oregon Health & Science (OBAS) study participants.
Table 5. Comorbidity.
Percentage of the 336 eligible participants with complete neuropathologic examination from the four studies who had no pathologic changes of Alzheimer’s disease (AD), microvascular brain injury (μVBI), or Lewy body disease (LBD), or who had different combinations of these three diseases. “No pathologic changes” indicates no NPs, NFTs, LBs, or CMIs. Average percent (SD) of the results from all four studies is shown.
| ACT | HAAS | NS | OBAS | Average (SD) | |
|---|---|---|---|---|---|
| No pathologic changes | 8 % | 0 % | 3 % | 6 % | 4 ± 3 % |
| AD only | 58 % | 54 % | 67 % | 56 % | 59 ± 6 % |
| AD plus μVBI or LBD | 34 % | 46 % | 30 % | 36 % | 37 ± 7 % |
| μVBI only | 0 % | 0 % | 0 % | 2 % | 1 ± 1 % |
| LBD only | 0 % | 0 % | 0 % | 0 % | 0 % |
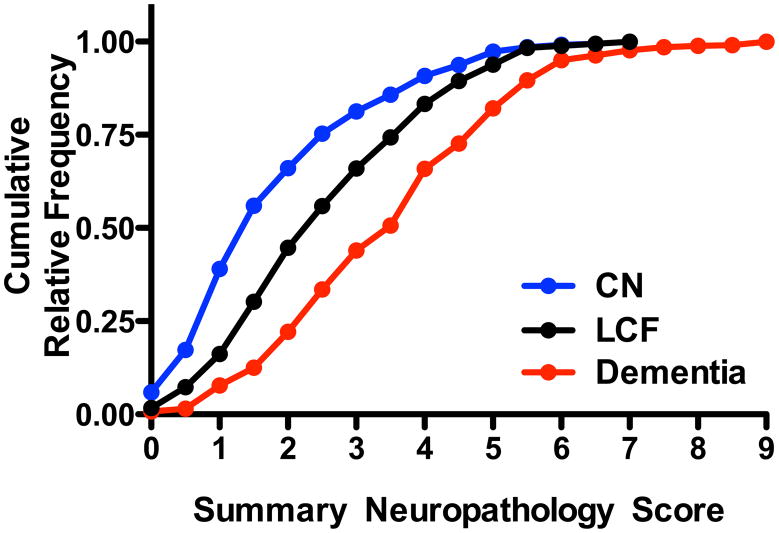
Figure 2 presents the cumulative relative frequency for the summary neuropathology score for individuals in each study. In addition to CN older adults who have been the focus of the preceding analyses, we have included for comparison data on individuals from each study who were last seen by study investigators within two years of death and were diagnosed as not demented, but performed in the lowest quintile on the cognitive screening test (lower cognitive function or LCF), and those who were diagnosed with dementia; to be eligible all cases were required to have undergone complete neuropathologic examination. These data support the conclusion that individuals with LCF or dementia carry progressively greater average burden of lesions from AD, μVBI, and/or LBD. Indeed, when we applied non-parametric analysis of variance to the data presented in Figure 2, the overall result of Kruskal-Wallis test was significant (P < 0.0001) for the three groups, as was Dunn’s corrected paired post tests (P < 0.0001) for each of the three paired comparisons. These results suggest a high degree of correlation between structure and function when a broad perspective is brought to evaluating the three diseases that commonly cause dementia in older individuals.
Figure 2.
Cumulative relative frequency of Summary Neuropathology Score for those autopsied individuals with complete neuropathologic examination from the four studies who were CN as defined in Table 1 (n=336), those with lower cognitive function (LCF) defined as those individuals last evaluated within two years of death at which time they were diagnosed as not dementia but scored in the lowest quintile on the cognitive screening test (n=189), and those who were diagnosed with dementia (n=522). Non-parametric analysis of variance comparing the Summary Neuroapthology Score among the three groups had P < 0.0001 and Dunn’s corrected multiple paired comparisons had P < 0.0001 for CN vs. LCF, LCF vs. Dementia, and CN vs. Dementia.
In our final analysis we correlated summary neuropathology score with age at death. Results from this analysis were mixed. When all four cohorts were combined, there was a weak correlation between summary neuropathology score and age (Spearman ranked correlation P < 0.05); however, this was driven by the relatively younger ACT cohort of men and women (Spearman ranked correlation P < 0.01) but none of the other three cohorts (Spearman ranked correlation P > 0.05), which may be due in part to their smaller sample size (HAAS and OBAS), average older age (OBAS), or women only (NS). Correlation of the AD or μVBI subscores with age yielded somewhat clearer results; AD score, which is simply Braak stage divided by 2, was significantly associated with increasing age in both of the larger cohorts (P < 0.01 for ACT and NS) while μVBI score was weakly associated with age only in the ACT cohort (P < 0.05).
DISCUSSION
Building on the efforts of the late Dr. William Markesbery, we have been able to apply similar protocols in ACT, HAAS, NS, and OBAS for evaluation of NPs, NFTs, LBs, and CMIs; however, to date databasing and analyses have been done independently among these studies. Since these lesions are the standard means to assess the most common diseases that contribute to dementia in community- or population-based cohorts, we undertook an evaluation in CN individuals to estimate the occurrence and interaction of clinically silent diseases in cohorts drawn from multiple regions of the US.
Our resource for this analysis was 1672 brain autopsies from participants in ACT, HAAS, NS, and OBAS from which we selected 424 CN individuals whose last clinical examination was performed on average about one year before death; average age at death was in the mid 80’s. Since not all studies used comprehensive neuropathologic examinations from their inception, 336 brain autopsies from among these 424 CN cases were eligible to be included. Our major conclusions are (i) AD, μVBI, and LBD were prevalent among CN older adults, (ii) the burden of these three diseases varied widely, and (iii) co-morbidity among these three diseases is quite variable among individuals. Many others have reported on neuropathologic evidence of usually one or two of these diseases, mostly in research clinic cohorts, and less commonly in community- or population-based cohorts. Any interval between last clinical evaluation and autopsy creates a potential for misclassification, especially since progression to dementia typically seems to be preceded by a prodrome.33 We made an effort to minimize inclusion of people with prodromal dementia by selecting only those performing within the upper four quintiles on a cognitive screening test at last evaluation, rather than simply individuals without a diagnosis of dementia. To our knowledge, ours is the first to report evidence of clinically silent dementia-associated neuropathologic changes among CN individuals from multiple independent community- or population-based cohorts. Our results show an intricate complexity among clinically silent diseases. We hope these data on the ecology of aging brain will be useful in guiding expectations of future neuroimaging or biomarker studies as well as clinical trials with cognitively normal older individuals in the community setting.
Using similar but not directly comparable methods of histopathologic assessment, others have reported on clinically silent lesions of AD, μVBI, and LBD in older individuals without dementia in the Religious Orders Study (ROS) and Memory and Aging Project (MAP) (n = 202, average age = 84 years) and observed prevalence of these lesions similar to our cohorts, although with somewhat less co-morbidity.15 Indeed, the concordance among the four cohorts analyzed in this study as well as the ROS and MAP mitigates the serious concern about generalizability among cohorts that focused on specific populations such as men or women in religious orders, or ethnic Japanese on Oahu; indeed the neuropathologic outcomes from all these diverse studies are remarkably similar. Nevertheless, we should be cautious in extrapolating these validated findings in non-demented or CN individuals to other ethnic or racial groups or other societies.
Cerebral NP accumulation was the most common disease process among CN older adults. The proportion of CN older adults with moderate or frequent CERAD NP score, a metric significantly correlated with Aβ peptide-immunoreactive plaque area and detergent-insoluble Aβ42 accumulation, matches well with reports using fibrillar Aβ PET imaging in cognitively normal individuals. Indeed, these neuroimaging reports from CN older individuals observe a prevalence of PET-positive fibrillar Aβ ranging from about one-fifth to about one-half, the variance related at least in part to age, APOE, and socio-economic status, with the latter perhaps related to education level or cognitive reserve.34, 35
CMIs were the next most prevalent clinically silent lesion in our cohorts, present in about one-third of CN older adults. High burden of CMIs, meaning lesions at the magnitide significantly associated with dementia,19 was present in approximately one in ten CN older adults. This screening protocol for CMIs has been shown independently in HAAS and ACT to be correlated with others forms of VBI, such as lacunes and territorial infarcts. However, among these forms of VBI, increased CMIs are most strongly and independently associated with increased risk of dementia.18, 19 These results are consistent with neuroimaging estimates of white matter hyperintensities, imaging abnormalities that appear to derive at least in part from pathogenic mechanisms overlapping with those that produce CMIs (reviewed in 36).
LBD was the least prevalent clinically silent disease in our cohorts. Our data suggest that future imaging modalities for LBD should expect to detect medullary LBD in approximately one in six CN individuals; about one-half of these also will have nigral LBD, and about one-half of those with midbrain LBD will have isocortical LBD. Amygdala-only LBD appears to be uncommon in CN individuals as was hippocampal sclerosis. Finally, it is important to stress that while we are evaluating lesions that are diagnostic for specific underlying diseases, our neuropathologic protocols likely have varying sensitivities and this should be remembered when considering our estimates of disease burden. Nevertheless, evaluating these histologic lesions currently remains the only means of which we are aware to assess simultaneously the presence and extent of AD, μVBI, and LBD.
Many of the studies referenced above have noted extensive co-morbidity among AD, μVBI, and/or LBD, especially with advancing age, but with somewhat different protocols for histologic assessment. Our results validate this very important point in multiple population- or community-based cohorts from different regions of the US. Importantly, we add to this body of work by showing that the extent of co-morbidity was remarkably similar in CN older adults among the four studies. In further accord with work of others, including work from our individual cohorts, we observed that approximately 10 to 15% of CN individuals across the four studies harbored clinically silent AD, μVBI, and/or LBD at levels that equaled or exceeded the average burden of lesions in individuals diagnosed with dementia, a finding that may be interpreted as suggesting some form of compensation or reserve capacity. Although not a focus of this analysis, it is at least interesting to note that a similarly sized subset of individuals with dementia have a burden of lesions in cerebrum that is less than the average burden in CN individuals. Since this is an autopsy study and not limited by technologies focused on a particular disease process, we have demonstrated that these individuals with dementia and low burden of AD, μVBI, and/or LBD do not instead have less common diseases that also can cause dementia, such as fronto-temporal lobar degeneration or prion disease. These are the opposite of the “cognitive reserve” group and raise questions about heightened susceptibility or additional contributing disease mechanisms not captured by neuropathologic examination in a small subset of patients that meet clinical criteria for dementia.
Our results from four independent community- or population-based studies of brain aging and dementia demonstrate that clinically silent AD, μVBI, and LBD are prevalent and commonly co-morbid but with extensive individual variation. While it is a challenge to generalize from any research study to the primary medical setting, these results at least should guide expectations for neuroimaging and biomarker studies as well as clinical trials focused on disease prevention that will be populated with community-dwelling CN subjects. Moreover, our results suggest that the aging brain is experiencing multiple simultaneous stressors, injuries, and responses to injury that likely interact with each other to generate a complex convergent environment. Importantly, our results also suggest that there are yet-to-be identified factors that in some individuals may suppress and in others promote clinical expression of disease. Although autopsy-based studies provide important insight, further studies of the ecology of aging brain will require intra vitam disease- and mechanism-specific markers that will be critical to the efficient conduct of research focused on disease prevention or neuroprotection.
Acknowledgments
This work was supported by NIH grants AG05136, NS62684, AG06781, AG008017, AG11378, Minnesota Medical Foundation, University of Minnesota Institute for Translational Neuroscience, and the Nancy and Buster Alvord Endowment.
Industry sponsorship: None
Footnotes
Disclosures: Drs. Sonnen, Santa Cruz, Hemmy, Woltjer, Leverenz, Kathleen Montine, Jack, Kaye, Lim, Larson, White, and Thomas Montine report no conflicts of interest.
References
- 1.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 3.Iacono D, Markesbery WR, Gross M, et al. The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology. 2009;73(9):665–673. doi: 10.1212/WNL.0b013e3181b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyas SL, Snowdon DA, Desrosiers MF, Riley KP, Markesbery WR. Healthy ageing in the Nun Study: definition and neuropathologic correlates. Age Ageing. 2007;36(6):650–655. doi: 10.1093/ageing/afm120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51(5):567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 6.Troncoso JC, Cataldo AM, Nixon RA, et al. Neuropathology of preclinical and clinical late–onset Alzheimer’s disease. Ann Neurol. 1998;43(5):673–676. doi: 10.1002/ana.410430519. [DOI] [PubMed] [Google Scholar]
- 7.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62(11):1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 8.Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12(4):295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55(3):370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 10.Price JL, McKeel DW, Jr, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30(7):1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57(12):1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC, Storandt M, McKeel DW, Jr, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46(3):707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 13.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Massoud F, Devi G, Stern Y, et al. A clinicopathological comparison of community-based and clinic-based cohorts of patients with dementia. Arch Neurol. 1999;56(11):1368–1373. doi: 10.1001/archneur.56.11.1368. [DOI] [PubMed] [Google Scholar]
- 15.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18(3):691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montine TJ, Larson EB. Late-life dementias: does this unyielding global challenge require a broader view? Jama. 2009;302(23):2593–2594. doi: 10.1001/jama.2009.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montine TJ, Sonnen JA. Mini-Forum on Clinical-Pathologic Correlations in Population- and Community-Based Studies of Brain Aging. Journal of Alzheimer’s disease. 2009;18:643–725. [Google Scholar]
- 18.White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 19.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62(4):406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 20.Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol. 2009;68(7):816–822. doi: 10.1097/NEN.0b013e3181ac10a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White L, Small BJ, Petrovitch H, et al. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol. 2005;18(4):224–227. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- 22.Erten-Lyons D, Woltjer RL, Dodge H, et al. Factors associated with resistance to dementia despite high Alzheimer disease pathology. Neurology. 2009;72(4):354–360. doi: 10.1212/01.wnl.0000341273.18141.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. Jama. 1996;275(7):528–532. [PubMed] [Google Scholar]
- 24.White L, Petrovitch H, Ross GW, et al. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. Jama. 1996;276(12):955–960. [PubMed] [Google Scholar]
- 25.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 26.Gonzales Mc Neal M, Zareparsi S, Camicioli R, et al. Predictors of healthy brain aging. J Gerontol A Biol Sci Med Sci. 2001;56(7):B294–301. doi: 10.1093/gerona/56.7.b294. [DOI] [PubMed] [Google Scholar]
- 27.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 28.Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm Suppl. 1998;53:127–140. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- 29.Leverenz JB, Hamilton R, Tsuang DW, et al. Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol. 2008;18(2):220–224. doi: 10.1111/j.1750-3639.2007.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montine T, Sonnen J, Montine K, Crane P, Larson E. Adult Changes in Thought Study: Dementia is an Individually Varying Convergent Syndrome with Prevalent Clinically Silent Diseases that may be Modified by Some Commonly Used Therapeutics. Current Alzheimer’s Research. 2010 doi: 10.2174/156720512801322555. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keene CD, Chang RC, Lopez-Yglesias AH, et al. Suppressed accumulation of cerebral amyloid {beta} peptides in aged transgenic alzheimer’s disease mice by transplantation with wild-type or prostaglandin E2 receptor subtype 2-null bone marrow. Am J Pathol. 2010;177(1):346–354. doi: 10.2353/ajpath.2010.090840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 33.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71(12):903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gouw AA, Seewann A, van der Flier WM, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2010 doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]