Abstract
Objective
Evaluate neuropsychological functioning in children with non-syndromic cleft of the lip and/or palate (NSCL/P) through profile variance within type of cleft and comparisons to controls.
Methods
Children ages 7 to 17 years participated; 66 had a diagnosis of NSCL/P and 87 were healthy controls. Neuropsychological tests of language, visual-perceptual, executive functioning, and memory skills were administered. Between- and within-group differences were assessed.
Results
Within cleft types, children with NSCLP had an even profile with equal Verbal and Performance IQ (VIQ and PIQ, respectively). Children with non-syndromic cleft palate only (NSCP) had significantly lower VIQ than PIQ, while children with non-syndromic cleft lip only (NSCL) showed a nonsignificant trend for higher VIQ than PIQ. Overall, subjects with NSCL/P performed lower on measures of expressive language and verbal memory than controls.
Conclusions
While deficits in verbal and memory skills for children with NSCL/P remain apparent, there is still uncertainty around the possible influence of cleft type on the pattern of deficits.
Keywords: Cleft, Children, Cognition, Verbal skill, Non-syndromic
Oral clefts are one of the most common congenital malformations (Swanenburg de Veye, Beemer, Mellenbergh, Wolters, & Heineman-de Boer, 2003). About 30% are associated with a known genetic syndrome, but the remaining 70% of clefts occur in isolation or without a known syndrome identified (Jones, 1988). The cause and sequelae of non-syndromic cleft of the lip and/or palate (NSCL/P) remain unclear.
Clefts occur during neonatal development at 5 to 8 weeks postconception when the neural crest cells (building blocks for brain and facial tissue) differentiate from the neural tube and migrate to the facial region. A problem occurs in the differentiation, proliferation (growth), and/or migration of these neural crest cells. The problem may be related to chromosomes, genes, proteins, or environmental teratogens (Burdi, 2006). The resulting oral clefts differ by location (i.e., lip, palate, or both; unilateral or bilateral) and extent (i.e., complete or incomplete; soft palate only or soft and hard palate (Berkowitz, 2006).
Depending on the type and extent of cleft, various concerns for the child may arise. The most common sequelae that require monitoring and/or treatment include the physical cleft, feeding, hearing, dental growth, and speech development (Moller, Starr, & Johnson, 1990). Another area of concern is cognitive development and language skills. The majority of research has found that while, overall, children perform within the average range of intelligence (Broder, Richman, & Matheson, 1998; Richman & Eliason, 2001), deficits are found in specific learning areas. While rates of mental impairment in children with NSCL/P are 4% to 6 % (Endriga & Kapp-Simon, 1999) the prevalence of learning disabilities (specifically reading) has been estimated to be between 30% and 40% (Richman & Ryan, 2003). One study on the prevalence of learning disabilities in children with NSCL/P from two Craniofacial Centers found that 46% of the combined sample had been diagnosed with a learning disability. Further analyses revealed that this rate was significantly higher for males with cleft palate only (NSCP; Broder et al., 1998).
Language skills, including expressive and receptive vocabulary, visual memory, and reading, are most commonly noted (Brennan & Cullinan, 1974; Broder et al., 1998; Endriga & Kapp-Simon, 1999; Richman, 1980; Richman & Eliason, 1984; Richman, Eliason, & Lindgren, 1988; Richman & Ryan, 2003; Richman, Wilgenbusch, & Hall, 2005; Scherer & D’Antonio, 1995). Early work found that measures of expressive language are the strongest predictor in determining presence or absence of a cleft (Fox, Lynch, & Brookshire, 1978) and the risk for language disorders increases with the number of other congenital abnormalities present (McWilliams & Matthews, 1979). Little research on cognitive functioning in this special population has been done since the late 1980s. This research found differential patterns of deficits for children with cleft palate only (CP) and cleft of lip and palate (CLP) have emerged. Children with CLP have demonstrated patterns of a Verbal Expressive Disorder (VED; low verbal expressive skill and average associative language skill) while children with CP have shown patterns of a more General or Mixed Language Disorder (low associative and expressive language skills). These differences resulted in higher rate of reading disorders in children with CP (Richman, 1980; Richman & Eliason, 1984, 2001; Richman et al., 1988).
There has been less focus on visual perception, executive functioning, and memory skills among children with cleft. Most work has established perceptual and visual/spatial skills as an area of relative strength, with average performance (Eliason, 1990; McWilliams & Matthews, 1979; Richman, 1980). For example, Nopoulos and colleagues (2002) found no deficits in areas of motor skill/dexterity, or visual processing in adult males with NSCL/P. Although, some work has found early deficits (at 5 months of age) in motor skill that improved over time (Neiman & Savage, 1997). Executive functioning skills (attention, organization, monitoring, and initiation) have not been assessed in children with cleft. The one study found on executive functioning in adult males with NSCL/P found a trend of poorer performance on the Stroop task, indicating more difficulty with set-shifting (moving attention between two different stimuli). Recent work does suggest that frontal and pre-frontal functions may be impaired in a considerable portion of children with cleft, suggesting the need to further examine executive functions (Richman & Nopoulos, 2008). Finally, the research done in memory has shown higher rates of visual (Richman et al., 2005) and verbal (Smith & McWilliams, 1968) deficits. Visual memory deficits have been correlated to poor verbal labeling and reading problems (Richman et al., 2005).
The current study sought to update and extend the research on cognitive deficits in children and adolescents with NSCL/P by utilizing neuropsychological assessments to compare cognitive skills to a control group. Further, profiles of strengths and weaknesses will be compared within cleft types. As previously discussed, some differences in level of impairment have been found based on cleft type (Richman, 1980; Richman & Eliason, 1984, 2001; Richman et al., 1988). It is hypothesized that while subjects with cleft will perform within the average range on most tests, their performance will be lower than controls in areas of verbal and executive functioning. By obtaining an understanding of the patterns of cognitive deficits in children with clefts, improvements can be made in early identification and remediation of learning disorders.
METHODS
Procedure
Subjects in this study were a subsample from a larger study evaluating brain structure and function differences in children with non-syndromic oral clefts compared to controls (Nopoulos, Langbehn, Canady, Magnotta, & Richman, 2007). Children with oral clefts were identified from the Cleft Palate Clinic in a Midwestern university hospital. Children with a diagnosed genetic syndrome and/or more than mild hearing loss (> 30 db threshold at 500 to 1000 cycles per second) in the worse ear were excluded. From the larger study with 102 participants, 66 (42 male and 24 female; ages 7 to 17 years) were in the current subsample. Of these, 14 had non-syndromic Cleft Lip Only (NSCL), 22 had non-syndromic Cleft Palate Only (NSCP), and 30 had non-syndromic Cleft Lip and Palate (NSCLP). The subsample (M = 12.55 years, SD = 3.46) was slightly older than those not included in analysis (M = 9.64 years, SD = 1.60), F(1, 88) = 0.742, p = .391, and no differences were found in socioeconomic status or Full Scale IQ, F(1, 88) = 0.742, p = .391; and F(1, 88) = 0.331, p = .566, respectively.
An additional 87 (43 male and 44 female; matched by age) participants without cleft were recruited from the community through advertisements. These children were screened for learning, attention, and health problems. Screening entailed asking the parent/guardian if there was a history of the aforementioned and those with a positive history per parental report were excluded.
This study was approved by the hospital’s Institutional Review Board, and all parents signed consents and subjects signed assent documents to participate. Following scheduling, subjects came with their parents to the hospital research clinic where they spent the morning completing cognitive and behavioral testing (this visit was not a part of any clinical evaluation). Testing occurred from December of 2002 through June of 2007 and each child was seen once. All subjects completed a cognitive test battery administered by the Research Assistant (a psychologist trained in neuropsychological assessment) and children with cleft also underwent a speech assessment. Children were compensated monetarily for their participation. Due to the physical nature of clefting, the examiner was not blinded to the group status of each child. Tests were not counterbalanced due to scheduling restrictions but were presented in the order typical of any clinical case. Parents completed a demographic questionnaire.
Child Test Battery
The test battery was designed to assess overall intelligence and functioning in four cognitive domains (i.e., language, visual perception, executive functioning, and memory; see Table 1). The neuropsychological tests chosen for each cognitive domain represent tests widely used in clinical and experimental research studies on neuropsychological patterns in children (Spreen, Risser, & Edgell, 1995; Spreen & Strauss, 1998; Teeter & Semrud-Clikeman, 1997). Furthermore, these tests have been successful in determining specific neuropsychological patterns in children with language-learning disorders (Richman, 2000; Richman & Wood, 1999) and in children with cleft lip and palate (Richman, Ryan, Wilgenbusch, & Millard, 2004; Richman et al., 2005).
Table 1.
Cognitive Domains Assessed by each Measure.
| Measure | Domain | Ages |
|---|---|---|
| WISC-III/WAIS-III | ||
| Vocabulary (Voc) | Verbal IQ | 7–17 years |
| Similarities (Sim) | Verbal IQ | 7–17 years |
| Block Design (BD) | Perceptual IQ | 7–17 years |
| Picture Completion (PC) | Perceptual IQ | 7–17 years |
| NEPSY | ||
| Speeded Naming | Rapid Labeling | 7–12 years |
| Verbal Fluency | Fluency/Generation | 7–12 years |
| Tower | Executive Functioning | 7–12 years |
| Sentence Repetition | Memory | 7–12 years |
| MAE | ||
| Controlled Word Association | Fluency/Generation | 13–17 years |
| Sentence Repetition | Memory | 13–17 years |
| Boston Naming | Naming | 7–17 years |
| RAN (Colors and Objects) | Rapid Labeling | 7–17 years |
Wechsler Intelligence Scale for Children, 3rd edition (WISC-III)
The WISC-III (Wechsler, 1991) was the most recent edition of the Wechsler children’s scales when this study was begun. It was designed to assess the intellectual abilities of children ages 6 to 16 years. Scales include Verbal IQ, Performance IQ, and Full Scale IQ. Children were administered the Vocabulary, Similarities, Block Design, and Picture Completion sub-tests. This is a well-established test of intellectual functioning and the subtests administered have high reliability (internal consistency: r = .87, .81, .87, and .77, respectively).
Wechsler Adult Intelligence Scale, 3rd edition (WAIS-III)
The WAIS-III (Wechsler, 1997) is a well-known test of adult intelligence. The subtests and scales are parallel to those in the child form. Adolescents who were 17 years old were administered this subtest because norms on the WISC-III only go up to 16 years. The WISC-III and WAIS-III have high correlations between FSIQ (.88), VIQ (.88), and PIQ (.78), suggesting that scores on each are fairly equivalent.
NEPSY
This test was designed to assess development of children ages 3 to 12 years on five domains (Executive Function, Language, Memory and Learning, Sensorimotor, and Visuospatial; Korkman, Kirk, & Kemp, 1998). Its name is an acronym for neurology and psychology. Children (ages 7 to 12 years) were administered the Tower, Speeded Naming, Verbal Fluency, and Sentence Repetition tests, which have demonstrated high internal consistency (r = .82, .74, .74, and .81, respectively). Adolescents (ages 13 to 17 years) were only administered the Tower and Speeded Naming tests.
Controlled Oral Word Association (COWA)
Adolescents over the age of 12 years were administered this subtest from the Multilingual Aphasia Examination (Benton & Hamsher, 1983) in place of the NEPSY Verbal Fluency subtest. Adolescents are requested to name as many words they can think of that start with a specific letter in 60 seconds. Three phonetic trials are conducted and their score is the total of these trials.
Sentence Repetition
This is a memory subtest from the Multilingual Aphasia Examination (Benton & Hamsher, 1983). It consists of 14 sentences ranging in length from 3 to 16 words. Children are given one point for every sentence they repeat correctly. This was administered to children over 12 years old to replace the Sentence Repetition test from the NEPSY.
Boston Naming Test
All children were administered this test of naming vocabulary. Each subject looked at pictures ranging from easy to difficult recognition and had to name the item pictured (Kaplan, Goodglass, & Weintraub, 1983). Stimulus and phonemic cues were provided, but only total score without cues was used for analyses.
Rapid Automatized Naming (RAN; colors and objects)
This rapid naming test was developed by Denckla and Rudel (1976). Children are required to rapidly verbally name a page of colors and a page of common objects. The score derived for each subtest is the time required to label all of the colors or objects. Scores on this test have been previously associated with reading disabilities in cleft populations (Richman & Ryan, 2003).
Speech Assessment
Subjects with NSCL/P underwent speech and resonance assessments including standardized articulation testing via the Goldman-Fristoe Test of Articulation, Second Edition (GFTA-2; Goldman & Fristoe, 2000). Percent consonants correct was calculated for the single word production subtest. Using structured conversational speech, perceptual judgments were also made regarding presence and degree of hypernasal resonance, hyponasal resonance, nasal emission, overall velopharyngeal function, articulation, overall speech intelligibility and presence of compensatory articulation. For the perceptual rating scale on the preceding measures: 1 = normal, 2 = mild, 3 = mild-moderate, 4 = moderate, 5 = moderate-severe, and 6 = severe. A Nasometer developed by Kay Elemetrics (now KayPENTAX; Adams, 1988) was also used to assess average nasalance. All speech assessments were conducted by a Speech-Language Pathologist with over 10 years of experience in evaluation of children with CL/P.
RESULTS
Demographics
The average ages of the group with NSCL/P (M = 12.55 years, SD = 3.46) and Controls (M = 12.51 years, SD = 3.06) were not significantly different, F(1, 151) = 0.007, p = .933. Gender ratios were not significantly different between groups, χ2(1, n = 153) = 3.070, p = .080. The majority of both groups were of Caucasian ethnicity (82% for NSCL/P and 95% for Controls), consistent with demographics in the region. The Control group had a significantly higher ratio of individuals with Caucasian ethnicity, χ2(1, n = 152) = 7.592, p = .006. Social class was significantly higher for the control group (M = 2.30 versus 2.67, respectively), F(1, 151) = 15.679, p < .001; see Table 2, based on ratings made by parents (Hollingshead, 1975), where a lower number indicated higher socioeconomic status (SES). For this reason, SES was covaried in the analyses.
Table 2.
Demographic Measures.
| Controla |
NSCL/Pb |
p value | Power | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Age | 12.51 | 3.06 | 12.55 | 3.46 | .933 | .051 |
| SES | 2.30 | 0.57 | 2.67 | 0.56 | < .001 | .976 |
|
| ||||||
| N (%) | N (%) | χ2 | p value | |||
|
| ||||||
| Gender | 3.070 | .080 | ||||
| Boys | 43 (49) | 42 (64) | ||||
| Girls | 44 (51) | 24 (36) | ||||
| Ethnicity | 7.592 | .006 | ||||
| Caucasian | 83 (95) | 53 (80) | ||||
| Asian-American | 1 (2) | 7 (11) | ||||
| Hispanic | – | 2 (3) | ||||
| Multi-Racial | 3 (3) | 3 (5) | ||||
| Unknown | – | 1 (2) | ||||
Note. Non-syndromic cleft of lip and/or palate (NSCL/P), socioeconomic status (SES), Full Scale IQ (FSIQ).
n = 87.
n = 66.
Obtaining Z Scores for Comparison
To standardize those subtests that did not have age-appropriate norms, raw scores from the control group were used to create a mean and standard deviation for each subtest. These values were applied to the raw score of every subject on each test, resulting in z scores for the following subtests: Tower, Speeded Naming (time to complete), Verbal Fluency (phonetic trial), COWA, both Sentence Repetition subtests, Boston Naming, and RAN Colors and Objects (time to complete). Because scores were now on the same scale, tests measuring the same construct (but given to different age groups) were combined. Specifically, scores for child NEPSY Verbal Fluency and adolescent COWA (Word Fluency) were assessed as the single variable: Combined Verbal Fluency. Similarly, the Sentence Repetition subtests were assessed as the single variable: Combined Sentence Repetition. Finally, Wechsler scales from the WISC-III and WAIS-III were also combined. From this point on, the terms Verbal Fluency, Sentence Repetition, Similarities, Picture Completion, Block Design, and Vocabulary refer to the combined variables.
Cognitive Functioning of Subjects with Cleft vs. Controls
Wechsler IQ scales
An analysis of variance was performed with group type (NSCL/P vs. Control) as the independent variable and Full Scale IQ (FSIQ) as the dependent variable. SES was included as a covariate and significantly increased with FSIQ, F(1, 150) = 27.793, p < .001. Differences between the two groups were not significant, F(1, 150) = 2.394, p = .124; see Table 3.
Table 3.
Means of Wechsler IQ Scales and Neuropsychological Battery.
| Controla |
NSCL/Pb |
NSCLcf |
NSCPdg |
NSCLPe |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | |
| Wechsler Scales | ||||||||||
| FSIQ | 114.22 | 17.65 | 105.82 | 15.60 | 105.57 | 16.13 | 103.77 | 18.66 | 107.43 | 13.06 |
| VIQh | 112.29 | 15.69 | 103.02 | 14.14 | 107.36 | 15.54 | *97.77 | 15.45 | 104.83 | 11.54 |
| PIQ | 110.89 | 16.58 | 105.02 | 16.52 | 99.57 | 15.36 | 107.18 | 18.32 | 105.97 | 15.62 |
| Neuropsychological Battery | ||||||||||
| Tower | .21 | 0.81 | −.20 | 1.51 | −.13 | 1.73 | −.53 | 1.38 | .01 | 1.51 |
| Sp. Namingi | **.12 | 0.81 | −.25 | 1.06 | −.32 | 0.95 | −.36 | 1.15 | −.15 | 1.06 |
| Verbal Fluency | .02 | 1.04 | −.19 | 1.10 | −.37 | 1.10 | −.51 | 0.89 | .12 | 1.18 |
| Sent. Rep.i | **−.04 | 1.01 | −.72 | 1.28 | −.29 | 1.27 | −.97 | 1.17 | −.74 | 1.34 |
| Boston Namingi | **.08 | 1.03 | −.48 | 1.21 | −.34 | 1.22 | −.75 | 1.16 | −.34 | 1.25 |
| RAN Colors | .11 | 1.01 | −.20 | 1.18 | −.14 | 0.88 | −.54 | 1.58 | .03 | 0.91 |
| RAN Objectsi | *.12 | 0.74 | −.13 | 0.99 | −.13 | 0.74 | −.34 | 1.30 | .02 | 0.81 |
Note. Non-syndromic cleft of lip and/or palate (NSCL/P), Non-syndromic cleft lip only (NSCL), Non-syndromic cleft palate only (NSCP), Non-syndromic cleft lip and palate (NSCLP), Full Scale IQ (FSIQ), Verbal IQ (VIQ), Performance IQ (PIQ), Rapid Automized Naming (RAN).
n = 87.
n = 66.
n = 14.
n = 22.
n = 30.
VIQ > PIQ, trend (p = .095).
VIQ < PIQ (p = .011).
NSCP < Control.
NSCL/P < Control.
p < .05.
p < .01.
To evaluate potential differences in groups and scales, a 2 × 2 factorial analysis was performed with group type as the between-subjects variable, and Wechsler Scale (i.e., VIQ and PIQ) as the within-subject variable. SES was included as a covariate. Major assumptions of this test were met, including homogeneity of variance-covariance [Box’s M (7.006) F(3, 7278088) = 2.301, p = .075] and homogeneity of variance [Levene’s Test F(1, 151) = 0.493, p = 0.484 and F(1, 151) = 0.010, p = .484; for VIQ and PIQ, respectively]. The interaction of group type and IQ scale was not significant, F(1, 150) = 1.589, p = .209; see Table 3.
Wechsler IQ scales by cleft type
Because previous research has found differences between children based on cleft type, a second series of analyses were performed with cleft type ( i.e., NSCLP, NSCL, NSCP, Control) as the independent variable. In the first analysis of variance Full Scale IQ (FSIQ) was the dependent variable and SES was a covariate. SES significantly increased with FSIQ, F(3, 148) = 27.076, p < .001. Differences between the four groups on FSIQ were not significant, F(3, 148) = 0.890, p = .448; see Table 3.
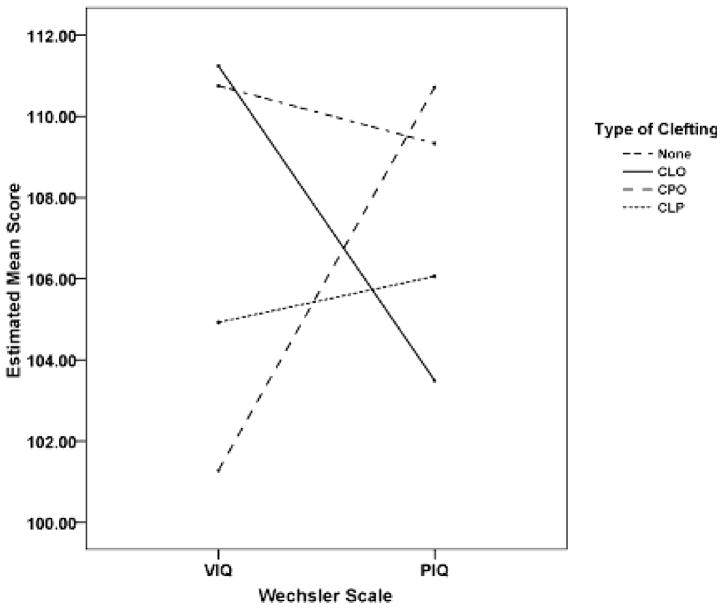
To evaluate potential differences in groups and scales, a 4 × 2 factorial analysis was performed with cleft type as the between-subjects variable, and Wechsler Scale (VIQ and PIQ) as the within-subject variable. SES was included as a covariate. Again, major assumptions of this test were met, including homogeneity of variance-covariance [Box’s M (10.664) F(9, 20167.618) = 1.138, p = .332] and homogeneity of variance [Levene’s Test F(3, 149) = 0.799, p = .496 and F(1, 151) = 0.021, p = .996; for VIQ and PIQ, respectively]. The interaction of group type and IQ scale was significant for differences, F(3, 148) = 4.220, p = .007; see Table 3. Post hoc analyses indicated that children with NSCP had significantly higher PIQ than VIQ, F(1, 20) = 7.862, p = .011, and there was a trend for children with NSCL to have higher VIQ than PIQ, F(1, 12) = 3.290, p = .095. Differences between VIQ and PIQ were not significantly different for children with NSCLP or Controls (see Figure 1). Further post hoc analysis comparing levels of VIQ and PIQ between the four groups was significant, F(6, 296) = 2.823, p = .001. Children with NSCP had lower VIQ than controls, after controlling for SES (Mean Difference = 9.472, p = .045).
Figure 1.
Mean Verbal IQ and Performance IQ scores for each cleft type.
Neuropsychological battery
Finally, a separate multivariate analysis was performed with group type (NSCL/P vs. Control) as the independent variable and the following cognitive subtests as the dependent variables: Tower, Speeded Naming, Verbal Fluency, Sentence Repetition, Boston Naming, RAN Colors, and RAN Objects. Age, SES, and FSIQ were included as covariates and a Bonferroni correction was applied to post hoc tests. Of the covariates, only age and FSIQ were significant, F(7, 141) = 44.108, p < .001 and F(7, 141) = 8.660, p < .001, respectively, where scores improved with higher age and FSIQ. There was a main effect for group type, F(7,141) = 2.570, p = .016. Control subjects performed higher than subjects with NSCL/P on: Speeded Naming, F(1, 147) = 8.582, p = .004, Sentence Repetition, F(1, 147) = 7.549, p = .007, Boston Naming, F(1, 147) = 9.072, p = .003, and RAN Objects, F(1, 147) = 4.960, p = .027. The remaining variables did not have any significant between-group differences. To determine if differences in performance existed between different types of clefting, this analysis was also performed with cleft type as the independent variable and the same dependent variables and covariates. Differences did not reach significance, F(21, 423) = 1.446, p = .092; see Table 3.
Post Hoc Speech Assessment
To evaluate the potential effect of speech dysfunction on verbal skills, analysis was conducted on a subset of the current sample of children with cleft (n = 53) who also participated in a speech assessment. There were 33 males and 20 females. Eleven children had NSCL, 17 had NSCP, and 25 had NSCLP. The average age of this subsample was 13.07 years (SD = 3.58) and average SES was 2.60 (SD = 0.53). Distributional information for the speech assessment is provided in Table 4.
Table 4.
Distribution of Scores for Speech Assessment in Children with NSCL/P (n = 53).
| Measure | Frequency of Score
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Hypernasality | 74% | 19% | 7% | – |
| Hyponasality | 89% | 11% | – | – |
| Audible Nasal Emission | 62% | 32% | 6% | |
| Velopharyngeal Function | 74% | 26% | – | – |
| Articulation Proficiency | 75% | 19% | 4% | 2% |
| Overall Intelligibility | 85% | 11% | 4% | – |
| Compensatory Articulation | 96% | – | 4% | – |
| Voice Quality | 98% | – | 2% | – |
|
| ||||
| M | SD | % in Normal Range | ||
|
| ||||
| Percent Consonants Correct | .98 | .05 | n/a | |
| Nasalance | ||||
| High Oral Pressure | .22 | .14 | 73% | |
| Low Pressure | .25 | .16 | 75% | |
| Nasal Consonants | .59 | .11 | 87% | |
Note. Non-syndromic cleft lip and/or palate (NSCL/P). For the first eight variables: 1 = normal, 2 = mild, 3 = mild-moderate, 4 = moderate, 5 = moderate-severe, and 6 = severe. For Percent Consonants Correct, 1.00 is considered a perfect score. For the nasalance measures, scores > .30 indicate hypernasality for High Oral Pressure and Low Pressure, scores < .50 indicate Hyponasality for Nasal Consonants.
To assess if there was a difference on performance between the children with NSCL/P who received a speech assessment and those who did not, a MANOVA was conducted with VIQ, PIQ, and the neuropsychological subtests as dependent variables; age and SES were covariates and a Bonferroni correction applied to post hoc tests. There was no significant difference on subtest performance for those who received a speech evaluation and those who did not, F(9, 54) = 0.802, p = .616.
Next, a partial correlation (controlling for age and SES) was run between the 11 speech measures (i.e., Hypernasality, Hyponasality, Audible Nasal Emission, Velopharyngeal Function, Articulation Proficiency, Overall Intelligibility, Compensatory Articulation, Percent Consonants Correct, and three measures of nasalance) and seven verbal measures (i.e., VIQ, Speeded Naming, Verbal Fluency, Sentence Repetition, Boston Naming, RAN Colors, and RAN Objects). Of the 77 calculated correlations only 3 were significant, and after controlling for multiple comparisons, there were no significant correlations. Therefore, no clear relationship between measures of speech dysfunction and performance of verbal skills was found. This supports the notion that the verbal skills deficits identified by neuropsychological tests in the current study are not in any substantial way related to abnormalities in speech.
DISCUSSION
Cognitive Functioning of Subjects with NSCL/P vs. Controls
Wechsler IQ scales
As a whole, subjects with NSCL/P did not demonstrate significant differences in Full Scale, Verbal, or Performance IQ from healthy controls. Although, differences emerged when children with different types of cleft (i.e., NSCP, NSCL, and NSCLP) were viewed separately and within-subject profiles of Verbal and Performance IQ were evaluated.
Previous research has shown children with CL/P to have greater discrepancies between their Verbal and Performance IQ scores. In the current study, children with NSCLP did not have significantly discrepant Verbal and Performance IQs. However, a new pattern of differences between subjects with NSCP and NSCL was found. Subjects with NSCP had Verbal IQs less than the Performance IQ (as predicted), while subjects with NSCL had Performance IQs somewhat, yet nonsignificantly, lower than Verbal IQs. The finding that participants with NSCL had lower Performance IQ is particularly interesting since it shows a relative strength in an area (Verbal IQ) that has previously been reported as a relative weakness. The majority of research has focused on children with NSCLP or NSCP clefting, and it is possible that such differences may have been previously overlooked in the research. It is also possible that the smaller number of children with NSCL (n = 14) could have lowered the variability of scores and the power of the test.
More research comparing children across the cleft types would be required to fully assess the validity of this finding. Because children with NSCL show deficits in perceptual or nonverbal areas of functioning, further study should include specific measures of visual-perceptual, visual-motor, and visual-abstract reasoning skills. It will be important to find out if this finding is truly due to differential deficit patterns between types of cleft.
Neuropsychological skills
Subjects with NSCL/P performed lower on the majority of verbal skill measures than the control group, even when accounting for age, SES, and Full Scale IQ. Significant discrepancies were found on tests of rapid verbal labeling (i.e., Speeded Naming and RAN Objects), Verbal Fluency (Boston Naming), and Verbal Memory (Sentence Repetition). These findings are consistent with previous research (Nopoulos et al., 2000; Richman & Ryan, 2003; Richman et al., 2005).
There was not a great difference between subjects with NSCL/P and the controls on perceptual or nonverbal measures. The absence of overall perceptual deficit is concurrent with the literature.
Subjects were administered two tests of executive function: Tower (planning and organization) and Verbal Fluency (initiation). Neither was significant for differences between children with cleft and matched controls. These two measures only tap into a few aspects of executive functioning. Further assessment utilizing more precise measures of these functions, such as the Delis-Kaplan Executive Function System (Delis, Kaplan, & Kramer, 2001) might be useful in future research.
Finally, the measure of memory (Sentence Repetition) given to subjects in this study did yield significant results, with the subjects with NSCL/P performing lower than those without cleft. Previous work by Smith and McWilliams (1968) has shown verbal memory deficits in children with cleft and is supported by these findings.
Why are there Differences?
Various hypotheses have arisen to explain these differences. Some theorize that the medical, physical, and psychological aspects of cleft and its treatment may cause some difficulties. For example, higher rates of otitis media and subsequent hearing loss in children with cleft in addition to physical problems with speech related to the cleft itself may cause sequelae of lower functioning on verbally based tasks (Riski, 2006). Studies have shown an inverse relationship of more hearing problems correlating to lower performance on cognitive tasks in children with cleft (Broen, Devers, Doyle, Prouty, & Moller, 1998; Jocelyn, Penko, & Rode, 1996; Speltz et al., 2000).
In contrast, the current study controlled for hearing loss and did not find significant correlations between speech and verbal measures, suggesting there may be more behind the observed verbal deficits. Another theory is that abnormal brain development due to cell migration problems is the primary cause of the cleft and identified learning problems (Eliason, 1990; Nopoulos, Berg, Canady, et al., 2002). Research by Nopoulos and colleagues have documented abnormal brain structures (pathological volumetric differences in the cerebellum and cerebrum) in children, adolescents, and adult males with NSCL/P (Nopoulos, Berg, Canady, et al., 2002; Nopoulos et al., 2001; Nopoulos et al., 2007). They have also correlated the abnormal brain structures in adult males to lower cognitive scores (Nopoulos, Berg, VanDemark, et al., 2002). Goldsberry, O’Leary, Hichwa, and Nopoulos (2006) examined PET images taken in adult males with NSCLP while reading and found similar patterns to persons with dyslexia and significant differences from the controls assessed. Further, a recent study found functional measures of the superior temporal plane (a region associated with language deficits) to correlate to IQ and language test scores in adult males with NSCLP, but test scores did not correlate to measures of childhood hearing deficits (Shriver, Canady, Richman, Andreasen, & Nopoulos, 2006).
Limitations
The power of this study is limited by two factors. There was a lower proportion of females with cleft (as is seen in the general cleft population) and a low number of subjects within each cleft type (NSCL, NSCP, and NSCLP). The small numbers within each cleft type should be considered when interpreting results of analyses comparing these groups.
The distributions of scores for the speech assessment were skewed with the majority of children performing within the average range. This restriction of range may have limited the power in the correlation analysis. However, this does not diminish the finding that children with NSCL/P in this study showed greater signs of verbal deficits, despite average speech quality among the majority of participants.
Given the high rate of Caucasian children in the study, generalizing the results to other ethnic groups should be done with caution. Larger studies recruiting a greater variety of ethnic groups are needed to determine if these findings apply to other cultural groups.
Future Directions
These findings further support the hypothesis of lower verbal skills in children and adolescents with cleft and the need to monitor them as they enter school age. It is in the elementary school years that even mild language disorders can result in reading or other learning disorders. Routine evaluation by a psychologist and monitoring of school activities by parents and teachers can help in early identification of a learning disorder. When remediation can be applied earlier, the benefits are often greater for the child. Children with cleft, as a whole, tend to perform lower on skills of rapid labeling, visual memory, and expressive vocabulary. Common remediation strategies for these weaknesses include phonics, memory strategies, and practice. Children with more severe deficits (e.g., Mixed Expressive-Receptive Language Disorder) may require more specialized and ongoing language therapy. This study also introduced the potential for differential deficits dependent upon cleft type. If further research continues to support the pattern of higher verbal and lower performance skills in children with NSCL, alternative interventions and remediation would been needed for this population.
The emerging research on brain structure and function in populations with non-syndromic clefts is intriguing. As recently mentioned by Cunningham (2007), advancements in brain imaging and genetics are moving quickly, and what we now deem as a “non-syndromic cleft” today may be identified as a syndrome in the future. Although, researchers were advised to remember that sometimes a structural deficit may lead to neuropathology rather than vice versa. To answer this chicken or the egg question, future work needs to focus on longitudinal assessments of cognition with measures of the brain (structural and functional). This is planned in future studies.
References
- Adams LE. Nasometer 6200-2 Instruction Manual. Pine Brooks, NJ: Kay Elemetrics Corp; 1988. [Google Scholar]
- Benton AL, Hamsher KD. Multilingual Aphasia Examination. Iowa City: Department of Neurology, University of Iowa Hospitals and Clinics; 1983. [Google Scholar]
- Berkowitz S. The effect of clefting of the lip and palate and the palatal arch form. In: Berkowitz S, editor. Cleft lip and palate. 2. New York: Springer; 2006. pp. 43–54. [Google Scholar]
- Brennan DG, Cullinan WL. Object identification and naming in cleft palate children. Cleft Palate Journal. 1974;11:188–195. [PubMed] [Google Scholar]
- Broder HL, Richman LC, Matheson PB. Learning disability, school achievement, and grade retention among children with cleft: A two-center study. Cleft Palate Craniofacial Journal. 1998;35(2):127–131. doi: 10.1597/1545-1569_1998_035_0127_ldsaag_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Broen PA, Devers MC, Doyle SS, Prouty JM, Moller KT. Acquisition of linguistic and cognitive skills by children with cleft palate. Journal of Speech, Language, and Hearing Research. 1998;41(3):676–687. doi: 10.1044/jslhr.4103.676. [DOI] [PubMed] [Google Scholar]
- Burdi AR. Developmental biology and morphogenesis of the face, lip, and palate. In: Berkowitz S, editor. Cleft lip and palate. 2. New York: Springer; 2006. pp. 3–12. [Google Scholar]
- Cunningham ML. Is cleft lip and palate ever isolated? Phenotype is in the eye of the beholder. Archives of Pediatrics and Adolescent Medicine. 2007;161(8):811–812. doi: 10.1001/archpedi.161.8.811. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- Denckla MB, Rudel RG. Rapid “Automatized” Naming (R.A.N): Dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976;14(4):471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Eliason MJ. Neuropsychological perspectives of cleft lip and palate. In: Bardach J, Morris H, editors. Multidisciplinary management of cleft lip and palate. Philadelphia: W. B. Saunders Company; 1990. pp. 825–831. [Google Scholar]
- Endriga MC, Kapp-Simon KA. Psychological issues in craniofacial care: State of the art. Cleft Palate Craniofacial Journal. 1999;36(1):3–11. doi: 10.1597/1545-1569_1999_036_0001_piiccs2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Fox D, Lynch J, Brookshire B. Selected developmental factors of cleft palate children between two and thirty-three months of age. Cleft Palate Journal. 1978;15(3):239–245. [PubMed] [Google Scholar]
- Goldman R, Fristoe M. Goldman-Fristoe Test of Articulation. 2. Bloomington, MN: Pearson Assessments; 2000. [Google Scholar]
- Goldsberry G, O’Leary D, Hichwa R, Nopoulos P. Functional abnormalities in the neural circuitry of reading in men with nonsyndromic clefts of the lip or palate. Cleft Palate Craniofacial Journal. 2006;43(6):683–690. doi: 10.1597/05-043. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. New Haven: Department of Sociology, Yale University; 1975. [Google Scholar]
- Jocelyn LJ, Penko MA, Rode HL. Cognition, communication, and hearing in young children with cleft lip and palate and in control children: A longitudinal study. Pediatrics. 1996;97(4):529–534. [PubMed] [Google Scholar]
- Jones MC. Etiology of facial clefts: Prospective evaluation of 428 patients. Cleft Palate Journal. 1988;25(1):16–20. [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY: A developmental neuropsychological assessment. Washington, DC: The Psychological Corp; 1998. [Google Scholar]
- McWilliams BJ, Matthews HP. A comparison of intelligence and social maturity in children with unilateral complete clefts and those with isolated cleft palates. Cleft Palate Journal. 1979;16(4):363–372. [PubMed] [Google Scholar]
- Moller KT, Starr CD, Johnson SA. A parent’s guide to cleft lip and palate. Minneapolis: University of Minnesota Press; 1990. [Google Scholar]
- Neiman GS, Savage HE. Development of infants and toddlers with clefts from birth to three years of age. Cleft Palate Craniofacial Journal. 1997;34(3):218–225. doi: 10.1597/1545-1569_1997_034_0218_doiatw_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, Canady J, Richman L, Van Demark D, Andreasen NC. Abnormal brain morphology in patients with isolated cleft lip, cleft palate, or both: A preliminary analysis. Cleft Palate Craniofacial Journal. 2000;37(5):441–446. doi: 10.1597/1545-1569_2000_037_0441_abmipw_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, Canady J, Richman L, Van Demark D, Andreasen NC. Structural brain abnormalities in adult males with clefts of the lip and/or palate. Genetics in Medicine. 2002;4(1):1–9. doi: 10.1097/00125817-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, VanDemark D, Richman L, Canady J, Andreasen NC. Increased incidence of a midline brain anomaly in patients with nonsyndromic clefts of the lip and/or palate. Journal of Neuroimaging. 2001;11(4):418–424. doi: 10.1111/j.1552-6569.2001.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, VanDemark D, Richman L, Canady J, Andreasen NC. Cognitive dysfunction in adult males with non-syndromic clefts of the lip and/or palate. Neuropsychologia. 2002;40(12):2178–2184. doi: 10.1016/s0028-3932(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Langbehn DR, Canady J, Magnotta V, Richman L. Abnormal brain structure in children with isolated clefts of the lip or palate. Archives of Pediatrics and Adolescent Medicine. 2007;161(8):753–758. doi: 10.1001/archpedi.161.8.753. [DOI] [PubMed] [Google Scholar]
- Richman LC. Cognitive patterns and learning disabilities of cleft palate children with verbal deficits. Journal of Speech and Hearing Research. 1980;23(2):447–456. doi: 10.1044/jshr.2302.447. [DOI] [PubMed] [Google Scholar]
- Richman LC. Speech and language disorders. In: Association AP, editor. Encyclopedia of psychology. New York: Oxford University Press; 2000. [Google Scholar]
- Richman LC, Eliason M. Type of reading disability related to cleft type and neuropsychological patterns. Cleft Palate Journal. 1984;21(1):1–6. [PubMed] [Google Scholar]
- Richman LC, Eliason MJ. Disorders of communications: Developmental language disorders and cleft palate. In: Walker CE, Roberts M, editors. Handbook of child clinical psychology. 3. New York: John Wiley & Sons; 2001. pp. 603–617. [Google Scholar]
- Richman LC, Eliason MJ, Lindgren SD. Reading disability in children with clefts. Cleft Palate Journal. 1988;25(1):21–25. [PubMed] [Google Scholar]
- Richman LC, Nopoulos P. Neuropsychological and neuroimaging aspects of clefting. In: Losee JE, Kirschner RE, editors. Comprehensive cleft care. New York: Springer; 2008. [Google Scholar]
- Richman LC, Ryan SM. Do the reading disabilities of children with cleft fit into current models of developmental dyslexia? Cleft Palate Craniofacial Journal. 2003;40(2):154–157. doi: 10.1597/1545-1569_2003_040_0154_dtrdoc_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Richman LC, Ryan S, Wilgenbusch T, Millard T. Overdiagnosis and medication for attention-deficit hyperactivity disorder in children with cleft: Diagnostic examination and follow-up. Cleft Palate Craniofacial Journal. 2004;41(4):351–354. doi: 10.1597/03-047.1. [DOI] [PubMed] [Google Scholar]
- Richman LC, Wilgenbusch T, Hall T. Spontaneous verbal labeling: Visual memory and reading ability in children with cleft. Cleft Palate Craniofacial Journal. 2005;42(5):565–569. doi: 10.1597/04-128r.1. [DOI] [PubMed] [Google Scholar]
- Richman LC, Wood KM. Psychological assessment and treatment of communication disorders: Childhood language subtypes. In: Netheron SD, Holmes D, Walker CF, editors. Child and adolescent psychological disorders: A comprehensive text book. New York: Oxford University Press; 1999. pp. 51–75. [Google Scholar]
- Riski JE. Speech, language, and velopharyngeal dysfunction: Management throughout the life of an individual with cleft palate. In: Berkowitz S, editor. Cleft lip and palate. 2. New York: Springer; 2006. pp. 705–718. [Google Scholar]
- Scherer NJ, D’Antonio LL. Parent questionnaire for screening early language development in children with cleft palate. Cleft Palate Craniofacial Journal. 1995;32(1):7–13. doi: 10.1597/1545-1569_1995_032_0007_pqfsel_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Shriver AS, Canady J, Richman L, Andreasen NC, Nopoulos P. Structure and function of the superior temporal plane in adult males with cleft lip and palate: Pathologic enlargement with no relationship to childhood hearing deficits. Journal of Child Psychology and Psychiatry. 2006;47(10):994–1002. doi: 10.1111/j.1469-7610.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Smith RM, McWilliams BJ. Psycholinguistic considerations in the management of children with cleft palate. Journal of Speech Hearing Disorders. 1968;33(1):26–32. doi: 10.1044/jshd.3301.26. [DOI] [PubMed] [Google Scholar]
- Speltz ML, Endriga MC, Hill S, Maris CL, Jones K, Omnell ML. Cognitive and psychomotor development of infants with orofacial clefts. Journal of Pediatric Psychology. 2000;25(3):185–190. doi: 10.1093/jpepsy/25.3.185. [DOI] [PubMed] [Google Scholar]
- Spreen O, Risser AT, Edgell D. Developmental neuropsychology. New York: Oxford University Press; 1995. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests. 2. New York: Oxford University Press; 1998. [Google Scholar]
- Swanenburg de Veye HF, Beemer FA, Mellenbergh GJ, Wolters WH, Heineman-de Boer JA. An investigation of the relationship between associated congenital malformations and the mental and psychomotor development of children with clefts. Cleft Palate Craniofacial Journal. 2003;40(3):297–303. doi: 10.1597/1545-1569_2003_040_0297_aiotrb_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Teeter PA, Semrud-Clikeman M. Child neuropsychology: Assessment and interventions for developmental disorders. Boston: Allyn and Bacon; 1997. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children, manual. 3. Washington, DC: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale, manual. 3. Washington, DC: The Psychological Corporation; 1997. [Google Scholar]