Abstract
Studies in humans and animal models link maternal infection and imbalanced levels of inflammatory mediators in the foetal brain to the aetiology of neuropsychiatric disorders. In a number of animal models, it was shown that exposure to viral or bacterial agents during a period that corresponds to the second trimester in human gestation triggers brain and behavioural abnormalities in the offspring. However, little is known about the early cellular and molecular events elicited by inflammation in the foetal brain shortly after maternal infection has occurred. In this study, maternal infection was mimicked by two consecutive intraperitoneal injections of 200 μg of LPS (lipopolysaccharide)/kg to timed-pregnant rats at GD15 (gestational day 15) and GD16. Increased thickness of the CP (cortical plate) and hippocampus together with abnormal distribution of immature neuronal markers and decreased expression of markers for neural progenitors were observed in the LPS-exposed foetal forebrains at GD18. Such effects were accompanied by decreased levels of reelin and the radial glial marker GLAST (glial glutamate transporter), and elevated levels of pro-inflammatory cytokines in maternal serum and foetal forebrains. Foetal inflammation elicited by maternal injections of LPS has discrete detrimental effects on brain development. The early biochemical and morphological changes described in this work begin to explain the sequelae of early events that underlie the neurobehavioural deficits reported in humans and animals exposed to prenatal insults.
Keywords: prenatal inflammation, lipopolysaccharide (LPS), brain development, cytokine, maternal infection, neurodevelopmental disorder
Abbreviations: Arc, activity-regulated cytoskeletal-associated protein; CNS, central nervous system; CP, cortical plate; DAPI, 4′,6-diamidino-2-phenylindole; GD, gestational day; GFAP, glial fibrillary acidic protein; GLAST, glial glutamate transporter; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; H&E, haematoxylin and eosin; IEG, immediate early gene; IL, interleukin; IZ, intermediate zone; LPS, lipopolysaccharide; MgZ, marginal zone; PFA, paraformaldehyde; poly(I:C), polyriboinosinic-polyribocytidilic acid; qRT–PCR, quantitative real-time PCR; SVZ/VZ, subventricular zone/ventricular zone; TNFα, tumour necrosis factor α
INTRODUCTION
Previous studies suggest that modifications of the ‘in utero’ environment due to maternal bacterial or viral infections can have disturbing effects on foetal brain development resulting in lifelong intellectual and behavioural disorders, such as schizophrenia and cerebral palsy (Rees and Harding, 2004; Hagberg and Mallard, 2005; Rees et al., 2008; Fatemi and Folsom, 2009; Meyer et al., 2009b; Patterson, 2009; Watanabe et al., 2010). Animal models have been developed to study the link(s) between functional deficits and cellular and morphological changes in the offspring's brain following prenatal exposure to agents known to stimulate the immune system, such as LPS (lipopolysaccharide) or poly(I:C) (polyriboinosinic-polyribocytidilic acid) (Nawa and Takei, 2006; Meyer et al., 2009a; Boksa, 2010). These studies support the notion that some gestational periods (e.g. early versus late pregnancy) offer a higher risk for developing behavioural dysfunction following maternal infections (Meyer et al., 2007). The foregoing results are obviously dependent upon the neural cell types maturing during the gestational window that would be targeted by the events elicited during maternal immune activation.
The mechanism(s) that mediates the effects of maternal infection on the developing brain has not been yet identified. Data from different groups have linked elevated cytokine levels triggered by maternal infection to altered gene expression and function in the maternal/foetal environments, including the foetal brain, suggesting that inflammation and its mediators interfere with normal development. In fact, within hours (1–24 h) of LPS or poly(I:C) administration to pregnant rodents, elevated expression levels of pro-inflammatory cytokines were reported in the placenta and the amniotic fluid as well as in the foetal plasma, liver and brain (Urakubo et al., 2001; Bell et al., 2004; Ashdown et al., 2006; Jonakait, 2007; Boksa, 2010). Cytokines have a wide range of roles in the innate and adaptive immune system and influence various neurodevelopmental processes, including cell differentiation, maturation and survival (Zhao and Schwartz, 1998; Deverman and Patterson, 2009; Watanabe et al., 2010). Hence, fluctuations in their maternal and foetal levels, due for instance to a maternal infection, signify a disturbance that can impede the ongoing neurodevelopmental processes, and subsequently affect proper neural cell maturation (Jonakait, 2007; Meyer et al., 2009a, 2009b). In support of a role for pro-inflammatory cytokines in the brain, increased gliosis and apoptosis, and a loss of pyramidal cells in the hippocampus were also triggered by direct injection of the pro-inflammatory cytokine IL-6 (interleukin-6) to the mother (Smith et al., 2007). Overall these findings support the contention that immune activation at specific gestational times may have diverse effects on the development of brain regions and further support the hypothesis that some of the disturbances on brain development are mediated by an increase in cytokines.
Notwithstanding major advances made on the long-term effects of prenatal inflammation on postnatal brain functions (Boksa, 2010), very few reports have investigated changes occurring at prenatal stages of brain development shortly after a maternal challenge (Meyer et al., 2008a; Cui et al., 2009). Since these changes are more proximal than those observed postnatally, studies of foetal brains exposed in utero to maternal infection should be suited to identify the upstream molecular events of brain pathology and may eventually help to determine the underlying cause(s) of brain malfunction later in young adults. The present study was designed to investigate effects on the development of immature neurons as well as neural progenitors associated with foetal inflammation, and occurring shortly after maternal immune system stimulation at mid–late gestation. Maternal infection was mimicked by two consecutive intraperitoneal injections of LPS (200 μg/kg) to timed-pregnant rats at GD15 (gestational day 15) and GD16, such that the foetuses were exposed to increased concentrations of inflammatory mediators soon after the peak of neurogenesis (Sauvageot and Stiles, 2002). We show that maternal injections of LPS at mid–late gestation induced an increase in cytokines in the foetal brain as well as changes in neural cell maturation and patterning. These effects may be relevant to defects in intellectual and behavioural functions described in adult animals following prenatal exposure to inflammation.
MATERIALS AND METHODS
Animals and treatment
Timed pregnant Sprague–Dawley rats were purchased from Charles River and housed in AALAC-approved clean animal facilities with a 12 h light/12 h dark regime. The animals were divided into two groups: control (saline-treated) and LPS-treated. The dams were injected intraperitoneally with either saline or 200 μg of LPS/kg from Escherichia coli 055:B5 (List Biological Laboratories) at GD15 and GD16. The dams were observed daily to detect signs of distress and killed at different times after the first or second injection of LPS, or allowed to give birth. The studies were performed in accordance with the NIH guidelines for the Care and Use of Laboratory Animals, and approved by the UCLA Chancellor's Animal Research Committee.
ELISA assay in maternal serum
To measure the levels of pro-inflammatory cytokines in maternal serum, dams were injected intraperitoneally with either saline or LPS and killed 4 h after the first injection (saline n = 5; LPS n = 9) at GD15, 4 h after the second injection at GD16 (saline n = 6; LPS n = 10) or 2 days after the second injection at GD18 (saline n = 9 or LPS n = 9). The amounts of pro-inflammatory cytokines were assessed as previously reported (Juarranz et al., 2005) using murine IL-6, IL-1β and TNFα (tumour necrosis factor α) ELISA Development Kits (Peprotech). Absorbance was measured at 450 nm on a microplate reader (SPECTRA max M2; Molecular Devices).
Antibodies
The following mouse monoclonal antibodies were purchased from the vendors indicated in parentheses: against Nestin and Arc (activity-regulated cytoskeletal-associated protein; BD Biosciences), against α-internexin (Millipore), against βIII-tubulin (Covance) and against β-actin (Sigma). A mouse anti-rat monoclonal against CD68 (clone ED1) was purchased from Accurate Chemical and Scientific Corporation. The following rabbit polyclonal antibodies were used: against doublecortin (Cell Signaling), against α-internexin and reelin (Clones G10 and 142; Millipore), against βIII-tubulin (Covance), against GFAP (glial fibrillary acidic protein; Dako). A guinea pig polyclonal antibody against GLAST (glial glutamate transporter) was purchased from Millipore. Details are given in Supplementary Table S1 (available at http://www.asnneuro.org/an/003/an003e068add.htm).
Immunohistochemistry
Pregnant rats were killed at GD18, 2 days after the second injection of saline or LPS. The foetuses were rapidly dissected out, perfused with PBS and fixed overnight in 4% (w/v) PFA (paraformaldehyde) at 4°C. Newborn pups [postnatal day (P)1] were perfused intracardially with 4% PFA. Brains were dissected out, post-fixed in 4% PFA at 4°C overnight, cryoprotected in 15% sucrose and then embedded in Tissue Tek OCT compound. Immunolabelling of frozen sections (20 μm) was performed as previously described (Mattan et al., 2010). Briefly, sections were fixed in 4% PFA for 15 min, blocked in carrier solution (1% BSA and 0.3% Triton X-100) containing 20% normal goat serum for 1 h and incubated overnight at 4°C with primary antibodies diluted in carrier solution containing 5% normal goat serum. Sections were incubated with the appropriate secondary antibodies conjugated to Cy3 (Jackson ImmunoResearch Laboratories) or Alexa Fluor® 488 (Molecular Probes), and mounted in Vectashield mounting medium with DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories). Immunostained sections were visualized using a Zeiss Axio Imager 2 with an AxioCam MRm and the ApoTome imaging system or an Olympus IX81 microscope equipped with a Hamamatsu ORCA-ER CCD camera.
H&E (haematoxylin and eosin) staining and measurements
GD18 and P1 brain structures were stained with H&E as previously reported (Mattan et al., 2010) and inspected using the Axiovision software on a Zeiss Axioskop with an Axiocam. Measurements were performed on the third sagittal brain slice, typically 1.5 mm from the midline, of three or four consecutive slides from three to six animals. Neocortical measurements were obtained from presumptive motor areas slightly caudal to the anterior commissure, and near the level of the posterior genu of the corpus callosum. Total neocortical and laminar thickness were measured along a line (eyepiece micrometer) orthogonal to the superficial pial and callosal surfaces, and averaged across cases for comparisons between groups. Hippocampal measurements were obtained in a comparable fashion from presumptive CA1 area, as evidenced by its distinct pyramidal cell layer, immediately rostral and superior to its transition from the dorsal subiculum.
Western-blot analysis
Rat cerebral cortices from GD18 foetuses were rapidly dissected, and the two halves were frozen separately and used for Western-blot analysis or qRT–PCR (quantitative real-time PCR). Cortices were homogenized in lysis buffer containing 50 mM Tris/HCl, 0.25% (w/v) DOC (sodium deoxycholate), 150 mM NaCl, 1 mM EDTA, 1% (w/v) Triton X-100, 0.1% (w/v) SDS, 1 mM Na3VO4 (sodium orthovanadate), 1 mM AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride], 10 µg/ml aprotinin, 10 µg/ml leupeptin, 10 µg/ml pepstatin and 4 µM sodium fluoride. Western blottings were performed as previously described (Ghiani and Gallo, 2001; Ghiani et al., 2010). Then 25–35 μg of total proteins were loaded on to a 4–20% Tris-glycine gel (Invitrogen). Protein bands were detected by chemiluminescence using the Amersham ECL kit (GE Healthcare) with HRP (horseradish peroxidase)-conjugated secondary antibodies (Cell Signaling). Relative intensities of the protein bands were quantified by scanning densitometry using the NIH Image Software (Image J, http://rsb.info.nih.gov/ij/). Equal protein loading was verified by Ponceau S solution (Sigma) reversible staining of the blots, and each extract was also analysed for relative protein levels of β-actin. For the comparison of relative protein levels in GD18 cerebral cortices of foetuses from saline- and LPS-injected dams, each background-corrected value was normalized according to the relative β-actin level of the sample, and then referred to the average of the saline values calculated from the same immunoblot image.
Real-time RT–PCR
Total RNA was extracted using TRIzol® (Invitrogen), following the manufacturer's protocol. Samples were further purified by treatment with TURBO DNA-free™ (Ambion), followed by a second extraction with phenol/chloroform. RNA was reverse transcribed using iScript cDNA Synthesis Kit (Bio-Rad Laboratories) then analysed for various transcript expressions (see Supplementary Table S2 available at http://www.asnneuro.org/an/003/an003e068add.htm). The primers for Egr-1 were part of the Qiagen QuantiTect® Primer Assay (Qiagen). qRT–PCR was set up using iQ SYBR® Green Supermix (Bio-Rad Laboratories) and performed in triplicate as previously described (Ghiani et al., 2006; Mattan et al., 2010) on an iCycler MyiQ Real Time PCR machine (Bio-Rad Laboratories). Negative controls (samples in which reverse transcriptase was omitted) were amplified individually using the same primer sets (Supplementary Table S2) to ensure the absence of genomic DNA contamination. PCR amplification resulted in the generation of single bands. Amplification specificity was assessed by melting curve and standard curves made from serial dilutions of control RNA were used for quantification. Data were normalized to the internal control GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 4.01 (GraphPad Software) by Student's t test or one-way ANOVA followed by Bonferroni's multiple comparison test when three or more experimental groups were compared.
RESULTS
Maternal injections of LPS elicited an inflammatory response in the foetal brain
Maternal bacterial infection was modelled in timed-pregnant rats at GD15 and GD16 by administering two consecutive injections of LPS (200 μg/kg intraperitoneally) to study the impact of the maternal and foetal inflammatory response on brain development. The dose was chosen based on previous reports showing a minimal rate of foetal re-absorption (Bell and Hallenbeck, 2002). Such a model is known to induce cellular and behavioural deficits in adult rodents, although little is known about the cellular and molecular events that take place in the foetal brain shortly after activation of the maternal immune system has occurred (Jonakait, 2007; Meyer et al., 2008b, 2009a; Boksa, 2010).
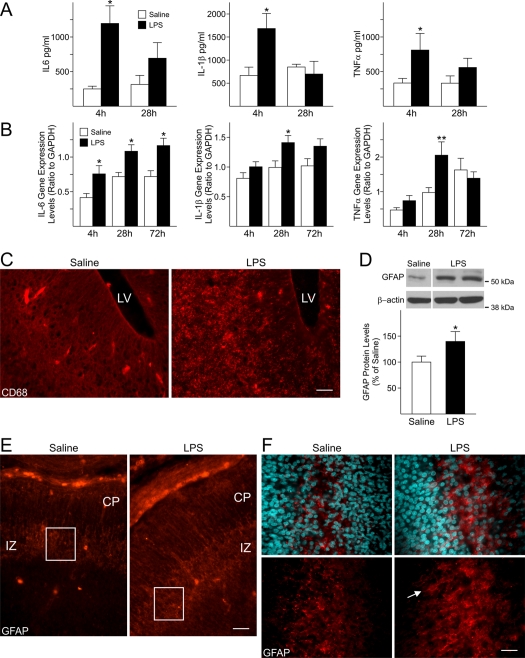
In order to determine if administration of LPS elicited a response in the dams, the levels of three pro-inflammatory cytokines (IL-6, IL-1β and TNFα), previously associated with the maternal and foetal response triggered by activators of the immune system, were analysed by ELISA in the maternal serum. The levels of these cytokines were significantly increased in the serum of LPS-injected dams (Figure 1A) 4 h after the first LPS-injection at GD15 as compared with saline-injected dams. Similar changes in cytokine gene expression were observed in the foetal forebrains at corresponding times by qRT–PCR (Figure 1B), indicating that a foetal inflammatory response was taking place in response to maternal injections of LPS. In particular, IL-6 gene expression displayed a time-dependent increase. Its levels were significantly increased not only 4 h after the first and the second maternal injections (28 h), but were still greatly elevated 72 h after the first injection, i.e. on GD18, which suggested that high levels of this inflammatory mediator persisted in the foetal forebrain (Figure 1B), albeit no changes in the maternal serum IL-6 were seen at this time (results not shown). Gene expression levels of IL-1β and TNFα in the foetal cerebral cortex were significantly increased 4 h after the second maternal injection of LPS (28 h; Figure 1B), but not 4 h after the first LPS injection. TNFα levels in experimental animals reverted to saline levels by 72 h (GD18), whereas IL-1β gene expression levels were still elevated, although this increase was not statistically significant (Figure 1B). In agreement with an up-regulation of the expression levels of inflammatory-related genes in the forebrain of foetuses from LPS-injected dams, increased immunoreactivity for CD68, a marker for microglia/macrophages, was seen 72 h after maternal injections of LPS (GD18; Figure 1C). These data appear to indicate that an extended foetal response was induced, as maternal levels of reactive cytokines decreased rapidly, while foetal levels remained elevated well after exposure to LPS.
Figure 1. Maternal injections of LPS elicited an inflammatory response in the foetal forebrain.
(A) Cytokine levels were significantly increased in maternal serum 4 h after the first injection of LPS (200 μg/kg, intraperitoneally) Dams were injected at GD15 and GD16 and killed 4 h after the first (4 h) or the second LPS-injection (28 h). Results were plotted as the means±S.E.M. for five or six saline-injected dams and nine or ten LPS-injected dams per time point. *P<0.05 versus respective control (saline-injected dams), One-way ANOVA followed by Bonferroni's multiple comparison test. (B) Pro-inflammatory cytokine expression levels were increased in the foetal forebrain at different time-points after maternal injections of LPS. Cytokine expression levels were measured in the foetal cerebral cortex by qRT–PCR at GD15, 4 h after the first (4 h) maternal injection of LPS or saline; at GD16, 4 h after the second injection (28 h), and at GD18, 48 h after the second maternal injection of LPS or saline (72 h). At least three foetuses/dam/group/time-point were analysed. Levels were normalized to GAPDH. Histograms represent the means±S.E.M. of 9–30 foetuses from five or six saline-injected dams and eight to ten LPS-injected dams per time point. *P<0.05, **P<0.01 versus respective saline-exposed foetuses, One-way ANOVA followed by Bonferroni's multiple comparison test. (C–F) Dams received two consecutive injections of LPS (200 μg/kg, intraperitoneally) at GD15 and GD16 and were killed at GD18. (C) The microglial marker CD68 was strongly expressed in the LPS-exposed foetal forebrain at GD18. LV, lateral ventricle. Scale bar = 50 μm. (D) A significant increase in GFAP protein levels was found at GD18 in LPS-exposed foetal cerebral cortex. Values derived from the densitometric analysis were corrected for the background, normalized to β-actin and are shown as a percentage of the value for saline-exposed foetuses. The histogram shows represents the means±S.E.M. for 11–12 foetuses from seven saline-injected and seven LPS-injected dams. *P<0.05 versus saline-exposed foetuses, Student's t test. (E) GFAP immunoreactivity was mainly localized in the IZ in both LPS- and saline-exposed foetuses. Scale bar = 50 μm. (F) Higher magnification of the insets in (B) shows that GFAP-positive cells display a reactive phenotype in the IZ of LPS-exposed foetuses (arrow). Nuclei were identified by DAPI staining. Scale bar = 20 μm.
A hallmark of the response of the CNS (central nervous system) to injury or inflammation is the presence of reactive astrocytes, characterized by increased expression of the specific marker GFAP. Western-blot analysis of GD18 saline- and LPS-exposed foetal cerebral cortices at 72 h revealed a 40% increase in GFAP protein levels in the latter group (Figure 1D). Furthermore, even though GFAP immunoreactivity could be seen in both the forebrains of LPS-exposed and control groups, and was mainly localized in the IZ (intermediate zone), a stronger signal was present in the LPS-exposed forebrains (Figure 1E), where GFAP-positive cells displayed the characteristic morphology of reactive astrocytes, which appear as hypertrophic process-bearing cells (Figure 1F, arrow).
Maternal injections of LPS impaired brain morphogenesis
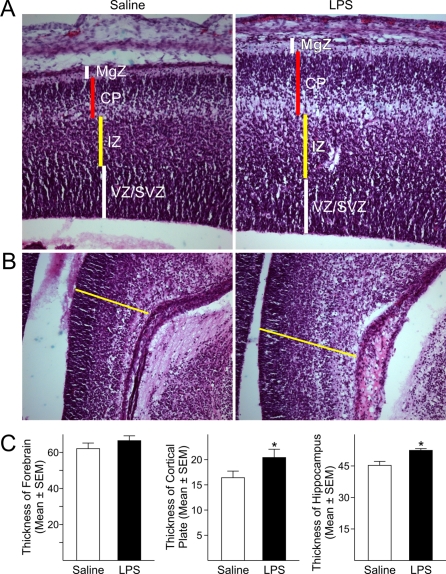
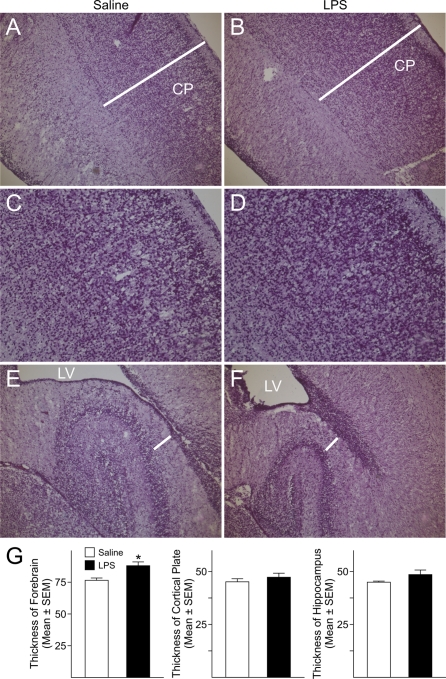
To determine whether activation of the maternal immune system triggered by LPS affected the cellular and laminar organization of the neocortex, coronal sections from LPS-exposed and control foetal forebrains collected at GD18 were analysed using H&E staining. Despite the fact that the total thickness of the forebrains, measured at the level of the parietal neocortex, was not significantly different between LPS-treated and control foetuses, analysis of the various laminae of the foetal cerebral cortex revealed a significant enlargement (24%) of the CP (cortical plate) in the neocortex of LPS-exposed foetuses compared with saline-injected animals (Figures 2A and 2C). No significant changes were seen in the MgZ (marginal zone), IZ or SVZ/VZ (subventricular zone/ventricular zone). Additionally, LPS-exposed foetuses displayed significantly thickened hippocampi (15%) as compared with controls animals (Figures 2B and 2C). These data suggest that activation of the maternal immune response affected development of foetal brain cytoarchitecture.
Figure 2. Maternal injections of LPS hindered the development of brain structures.
(A) H&E staining of GD18 foetal forebrain showed a significant enlargement of the CP in the forming cerebral cortex of LPS-exposed foetuses compared with saline-exposed foetuses. No significant differences were found in the thickness of other laminae of the cerebral cortex or in the total thickness of the cortex. (B) The hippocampus of LPS-exposed foetuses was significantly larger than in control animals. (C) Quantifications of the differences in the thickness of the forebrain, CP and hippocampus. Histograms represent the means±S.E.M. for six rat foetuses from six saline-injected and six LPS-injected dams. *P<0.05 versus saline-exposed foetuses, Student's t test. MgZ, marginal zone; IZ, intermediate zone; VZ/SVZ, ventricular zone/subventricular zone.
Maternal injections of LPS altered the expression pattern of immature neuronal markers
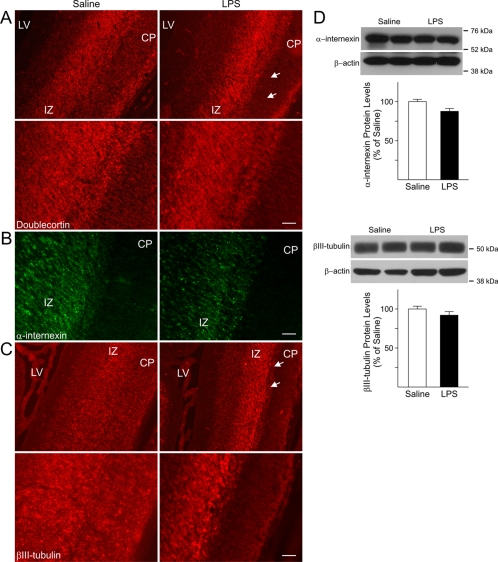
In rats, cortical neurogenesis begins around GD11 and GD12, peaks at GD14, and declines through the remainder of gestation into the postnatal period (Bayer and Altman, 1991; Sauvageot and Stiles, 2002). At the time of the LPS injections, GD15 and GD16, neurons destined to settle in the infragranular layers VI and V are being generated followed by neurons destined to settle in the supragranular layers IV–II at later stages (GD17–21) (Bayer and Altman, 1991). We surmised that foetal inflammation could affect those neurons whose generation and migration coincided with the time of the injections or soon after and were ordained to settle in the supragranular layers. To address this possibility, the expression of doublecortin, a microtubule-associated protein expressed in immature neurons and involved in the regulation of neuronal migration, was investigated. In control foetuses, doublecortin positive cells were detected in both the IZ and CP (Figure 3A). In contrast, in the foetal forebrains from LPS-injected dams, doublecortin-positive cells were predominantly found in the IZ and were nearly absent in the CP (Figure 3A). Likewise abnormal distributions of cells that expressed the immature neuronal markers α-internexin and βIII-tubulin were seen in the neocortex (Figures 3B and 3C, respectively). In fact, similar to the expression pattern of doublecortin (Figure 3A), α-internexin- and βIII-tubulin-positive cells (Figures 3B and 3C, respectively) populated both the CP and the IZ in the forming the neocortex of foetuses from saline-injected dams. In the forebrains of LPS-exposed foetuses, cells positive for these markers were mainly concentrated in the IZ with notably fewer immunoreactive cells detected in the CP (Figures 3B and 3C). Nonetheless, as shown in Figure 3(D), the total protein levels of α-internexin and βIII-tubulin were not significantly changed, suggesting that foetal inflammation predominantly affected the distribution pattern of neuronal cells.
Figure 3. Abnormal distribution of markers for immature neurons in the foetal forebrain after maternal injections of LPS.
(A) Doublecortin immunoreactivity was seen in both the CP and IZ in GD18 foetuses from saline-injected dams. Conversely, doublecortin-positive cells were mostly detected in the IZ of age-matched foetuses exposed to LPS, while the CP displayed lower immunoreactivity. Lower panels are higher magnifications of the area marked by the two arrows showing the atypical distribution of doublecortin positive cells in the CP and IZ of LPS-exposed animals. (B) Expression of the immature neuronal marker α-internexin could be seen in the IZ of GD18 LPS-exposed foetuses and was almost absent in the CP as compared with age-matched control (Saline). (C) Expression of βIII-tubulin could be observed throughout the cerebral cortex in GD18 saline-exposed foetuses. In the cerebral cortex of LPS-exposed foetuses, immunoreactivity for this marker was mainly found in the IZ, while it was almost absent in the CP. Lower panels are higher magnifications of the area marked by the two arrows. Scale bar = 50 μm. LV, lateral ventricle. (D) No differences were found in the protein levels of α-internexin and βIII-tubulin measured in whole tissue lysates prepared from cerebral cortices of GD18 saline- and LPS-exposed foetuses. Values derived from the densitometric analysis were corrected for the background, normalized to β-actin and are shown as a percentage of the value for saline-exposed animals. Histograms are the means±S.E.M. for 17 foetuses from seven dams injected with saline and 18 foetuses from eight LPS-injected dams.
The cellular and molecular machinery involved in neuronal migration is perturbed in LPS-exposed foetal forebrains
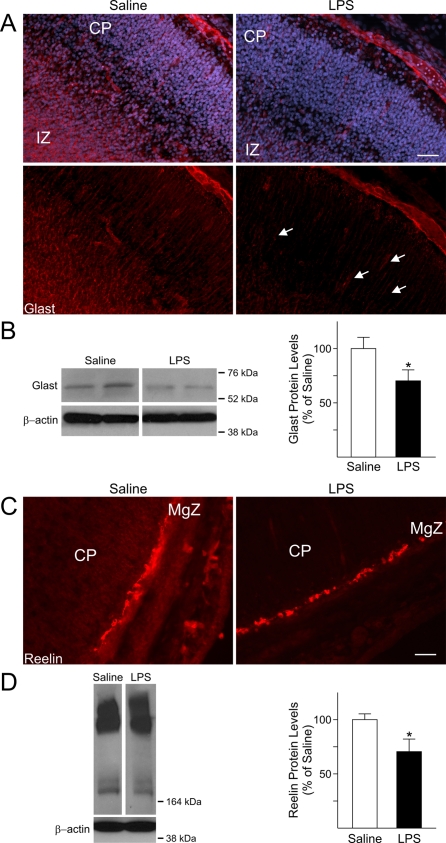
Proper migration of immature neurons to their final destination in the CP is essential for the development of a functioning CNS. Neuronal migration is a complex process that occurs with a defined temporal pattern and is regulated by soluble cues as well as interactions between migratory neurons and other cell types (Gupta et al., 2002; Nadarajah and Parnavelas, 2002). During this period in brain development, the majority of the neurons, destined to form the different layers of the CP migrate along specialized cells named radial glia. At GD9 and GD10 radial glial processes span the cortical wall from the VZ to the pial surface. Radial glial cells serve as both progenitor cells and a primitive migratory scaffold for post-mitotic neurons (Campbell and Gotz, 2002). To further ascertain the cellular changes occurring in GD18 LPS-exposed foetal forebrains that might underlie the defect in neuronal patterning, we examined the expression levels and distribution of GLAST, a marker for radial glia. In the developing neocortex of control foetuses, cell fibres positive for GLAST extended throughout the IZ and the CP, up to the pial surface (Figure 4A). GLAST immunoreactivity displayed a marked decrease in the IZ of LPS-exposed foetuses and was practically absent in the CP, where fewer positive processes could be seen (Figure 4A, arrows in left lower panel). Furthermore, GLAST protein levels showed a 30% decrease in the forebrain of GD18 foetuses from LPS-injected dams as compared with saline-injected foetuses (Figure 4B), potentially suggesting a weakening of the migratory scaffold formed by radial glia.
Figure 4. Altered expression levels of GLAST and Reelin in the foetal forebrain after maternal immune system activation with LPS.
(A) Processes immunopositive for GLAST were observed throughout the IZ and the CP of GD18 saline-exposed foetuses, whereas they were evidently decreased in the corresponding areas of GD18 LPS-exposed animals. Nuclei were identified by DAPI staining. (B) The total protein levels of GLAST were significantly decreased in the cerebral cortex of GD18 LPS-exposed foetuses compared with control. Values derived from the densitometric analysis were corrected for the background, normalized to β-actin, and are shown as a percentage of the value for saline-exposed animals. Histograms are the means±S.E.M. for 12 foetuses from seven dams injected with saline and 15 foetuses from eight LPS-injected dams. *P<0.05 versus saline-exposed foetuses, Student's t test. (C) Immunoreactivity of the glycoprotein protein reelin was decreased in the Cajal-Retzius cells in the MgZ of forebrains from GD18 LPS-exposed foetuses compared with saline. Scale bars = 50 μm. (D) Representative immunoblots showing that the protein levels of the 180 kDa isoform of reelin were significantly decreased in whole tissue lysates from the cerebral cortex of GD18 LPS-exposed foetuses. Values are shown as a percentage of the value for saline-exposed animals. Histograms are the means±S.E.M. for five foetuses from three dams injected with saline and five foetuses from three LPS-injected dams. *P<0.05 versus saline-exposed foetuses, Student's t test.
Among the factors involved in the regulation of radial glia development, maintenance of the radial glia scaffold as well as proper orientation of the radial glia processes is the glycoprotein reelin (Hartfuss et al., 2003; Forster et al., 2010). In the mammalian brain, reelin has different roles depending upon the developmental stage. Prenatally, it is secreted by the Cajal-Retzius cells in the MgZ and plays major roles in the regulation of neuronal migration and cortical layer formation; whereas, in the adult brain, reelin is secreted by GABAergic interneurons and participates in synaptic plasticity and memory formation (Fatemi, 2005; Forster et al., 2010). In addition to its proposed role as a susceptibility gene for neuropsychiatric disorders, such as schizophrenia and autism, its levels were reported to be decreased in adult mice following exposure to antenatal insults such as maternal viral infection (Fatemi, 2005). Reelin expression was analysed in foetuses from LPS- and saline-injected dams at GD18 by both immunohistochemistry and Western-blot analysis. Reelin expression was present in the Cajal-Retzius cells of the MgZ in both animal groups, even though its immunoreactivity was lower in the LPS-exposed foetuses compared with controls (Figure 4C). These results were further confirmed by Western-blot analysis. Reelin exists in various isoforms, which are generated by cleavage of the 400 kDa isoform, considered to be the full form and the others proteolytic products (Ignatova et al., 2004; Jossin et al., 2004, 2007). Three main isoforms (400, 330 and 180 kDa) were revealed by Western blotting in the cerebral cortex of LPS-exposed foetuses and controls. A significant decrease (30%) in the protein levels of the 180 kDa form was found in the LPS-exposed foetuses (Figure 4D) as compared with controls, whilst the other bands displayed no significant changes, suggesting that foetal inflammation may interfere with cleavage of reelin.
The expression of neural progenitor cell markers is altered by inflammation
Brain development from GD17 to GD20 is characterized by the presence of actively proliferating precursor cell populations, which will give rise to late-born neurons and cells of the glial lineage. Maturation of neural precursors is tightly regulated by both cell specific, intrinsic and extrinsic factors (Berger-Sweeney and Hohmann, 1997; Cameron et al., 1998; Pomeroy and Kim, 2000; Nguyen et al., 2001; Sauvageot and Stiles, 2002). Because of the documented role of radial glial cells as neural progenitors (Campbell and Gotz, 2002; Pinto and Gotz, 2007), and the decrease in GLAST levels found in LPS-exposed foetuses (Figures 4A and 4B), we reasoned that activation of the foetal inflammatory response might also affect progenitor cell development. Hence, the expression of markers for neural progenitors was analysed.
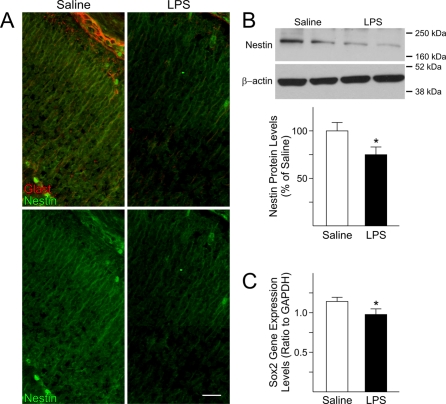
The effects of foetal brain inflammation on neural progenitor cells were investigated by examining the distribution and protein levels of nestin, an intermediate neurofilament typically found in neural progenitors. As shown in Figure 5(A), nestin immunoreactivity was decreased in the neocortex of LPS-exposed foetuses compared with controls. In agreement with this finding, decreased protein levels (25%) for nestin were found in forebrains of LPS-exposed animals (Figure 5B). Finally, expression of Sox2, a transcription factor expressed in neural precursors, was measured by qRT–PCR and found to be significantly decreased (15%) in the forebrain of LPS-exposed animals compared with foetuses from saline-injected dams (Figure 5C).
Figure 5. Expression of neural progenitor cells markers in the foetal forebrain is disturbed after maternal injections of LPS.
(A) Immunoreactivity for nestin, a marker for neural progenitors, was decreased at GD18 in the foetal cerebral cortex of LPS-exposed foetuses compared with foetuses from saline-injected dams. Upper panels: double immunostaining for nestin in green and GLAST in red; lower panels: single immunostaining for nestin. Scale bar = 50 μm. (B) Representative immunoblots showing that nestin protein levels were significantly decreased in whole tissue lysates from the cerebral cortex of GD18 LPS-exposed foetuses. Values derived from densitometric analysis were corrected for the background, normalized to β-actin, and are shown as a percentage of the value for saline-exposed animals. Histograms are the means±S.E.M. for ten foetuses from five dams injected with saline and ten foetuses from five LPS-injected dams. *P<0.05 versus saline-exposed foetuses, Student's t test. (C) The gene expression levels of the transcription factor Sox2 were decreased in the foetal cerebral cortex after maternal injections of LPS. Sox2 expression was measured by qRT–PCR in the cerebral cortex of GD18 foetuses from saline and LPS-injected dams. The levels were normalized to GAPDH. Histograms represent the means±S.E.M. for 15 foetuses from seven saline-injected dams and 15 foetuses from seven LPS-injected dams. *P<0.05 versus saline-exposed foetuses, Student's t test.
The expression levels of the immediate early gene Arc are influenced by maternal injections of LPS
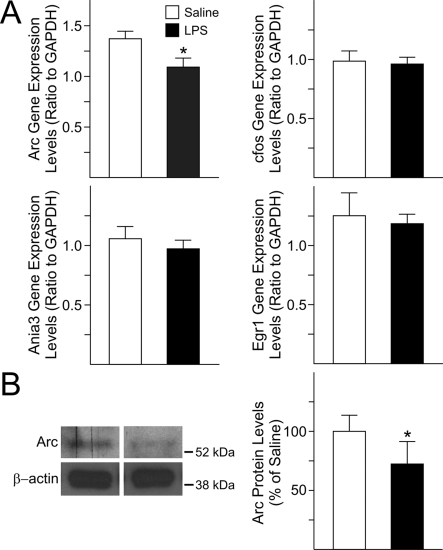
IEGs (immediate early genes) are among the most highly expressed genes during CNS development and their activation in response to neuronal activity is fundamental for controlling downstream genes involved in specification and maturation of neural progenitors in the neocortex as well as in other brain areas (Herdegen and Leah, 1998; Kaufmann and Worley, 1999). We sought to determine whether the anomalous neuronal patterning seen in GD18 foetal forebrains after maternal injections of LPS at GD15 and GD16 was accompanied by abnormal expression levels of IEGs. The activity-regulated IEGs Ania-3, cfos, and Egr-1 showed a trend towards decrease, although such changes were not significant. Conversely, Arc displayed a significant decrease in gene expression levels (20%) in the neocortex of LPS-exposed foetuses as compared with controls (Figure 6A). Furthermore, a significant decrease in the total protein levels of Arc was found (27%) in forebrains of LPS-exposed foetuses when compared with control (Figure 6B).
Figure 6. Gene and protein expression levels of IEG in the foetal forebrain following maternal immune system activation with LPS.
(A) Only the gene expression levels of Arc were significantly decreased in the cerebral cortex of GD18 foetuses from LPS-treated dams compared with control. Changes in gene expression levels of Arc, cfos, Ania-3 and Egr-1 were measured by qRT–PCR in the cerebral cortex of foetuses from saline and LPS-injected dams. The levels were normalized to GAPDH. Histograms represent the means±S.E.M. for 16 foetuses from five saline-injected dams and 14 from five LPS-injected dams. *P<0.05 versus saline-exposed foetuses, Student's t test. (B) The total protein levels of Arc were decreased in whole tissue lysates from GD18 foetal cerebral cortex after maternal injections of LPS. Values derived from densitometric analysis were corrected for the background, normalized to β-actin, and are shown as a percentage of the value for saline-exposed animals. Histograms are the means±S.E.M. for ten foetuses from five dams injected with saline and 11 from five LPS-injected dams. *P<0.05 versus saline-exposed foetuses, Student's t test.
Postnatal effects of maternal injections of LPS at GD15 and GD16
In order to investigate if the changes in brain structures and neural cell development identified at GD18 were still present postnatally and to assess whether foetal inflammation elicited long-lasting effects, the brains of offspring from saline- and LPS-injected dams were analysed at P1. H&E staining of P1 brains revealed lack of differences in the size of the CP between pups born to dams injected with LPS or saline at GD15 and GD16 (Figures 7A, 7B and 7G). However, the total thickness of the neocortex was significantly enlarged (17%; Figure 7G) when compared with control pups and the cells appeared to be more closely aggregated than in control (Figures 7C and 7D). The mean value of the hippocampal areas, measured at the transition between CA1 and CA2 areas, were increased in the LPS-exposed pups, although this difference was not significant (Figures 7E–7G). Furthermore, the expression pattern of βIII-tubulin (Supplementary Figures S1A and S1B available at http://www.asnneuro.org/an/003/an003e068add.htm) and doublecortin (Supplementary Figures S1C and S1D) was still altered in P1 cerebral cortex of pups born to LPS-injected dams compared with control pups.
Figure 7. Changes in the offspring forebrain after prenatal foetal and maternal immune activation with LPS.
Dams received two consecutive injections of LPS (200 μg/kg, intraperitoneally) at GD15 and GD16 and the offspring were killed at postnatal day (P)1. (A, B) H&E staining revealed lack of significant differences in the thickness of the CP in P1 rats born to dams injected with saline or LPS; conversely, a significant enlargement of the cerebral cortex was found in P1 rats born to LPS-injected dams. (C, D) Higher magnification images showing increased cell density in the CP of P1 rats prenatally exposed to the effects of maternal injections of LPS. (E, F) No significant differences were found in the thickness of the hippocampus measured at the transition between the CA1 and CA2 areas. White bars indicate where the measurements were performed. LV, lateral ventricle. (G) Quantifications of the differences in the thickness of the cerebral cortex, CP and hippocampus. Histograms represent the means±S.E.M. for three P1 control (saline) and three P1 rats prenatally exposed to LPS. *P<0.05 versus saline-exposed P1 rats, Student's t test.
DISCUSSION
Among many proposed environmental factors, epidemiological data support an association between maternal infections and neuropsychiatric disorders (Mednick et al., 1988; Brown, 2006; Ellman and Susser, 2009). Here, we present evidence that maternal infection, mimicked by injections of LPS, and subsequent foetal inflammation during mid–late gestation in rats (equivalent to mid-second trimester in human) considerably perturbed brain development, shortly after the inflammatory response was triggered in the foetus, by interfering with signalling pathways involved in neuronal cell distribution pattern. Enhanced microglia activation, reactive astroglia and increased expression of pro-inflammatory cytokines were detected in the foetal brain after maternal exposure to LPS, suggesting that the observed effects might, at least in part, be generated by these immune mediators. Previous studies have reported increased levels of pro-inflammatory cytokines in the maternal serum as well as at the maternal/foetal interface (amniotic fluid, placenta) after stimulation of the maternal immune system with different agents [LPS, poly(I:C), etc.] (Boksa, 2010). Altogether these findings advocate for a role of these immune mediators in the morphological and neurobehavioural changes in offspring exposed to prenatal inflammation.
Studies in humans as well as in accepted animal models of maternal infection have described a series of abnormalities in adult brain cytoarchitecture, including decreased dendritic arborization and aberrant neuronal migration (Fatemi and Folsom, 2009; Deutsch et al., 2010), pointing at a neurodevelopmental origin of severe psychiatric disorders and implying that certain defects are present before the onset of the disorder. Alterations in normal cortical development caused by small disturbances of neurogenesis and neuronal migration may elicit maldevelopment of these areas, affecting the formation of neuronal networks and resulting in the neuropathological defects described in the post-mortem brain of persons affected by schizophrenia and other neurodevelopmental disorders. Perhaps some pathways active in the adult brain are already dysregulated during foetal life by maternal conditions leading to the formation of malfunctioning neuronal circuits in the adolescent and adult brain. In the present study, an enlarged CP and abnormal expression of markers for immature neurons were observed in the neocortex of LPS-exposed foetuses at GD18, i.e. 2 days after LPS exposure, in comparison with the brains of age-matched control foetuses. In addition, a number of important molecules, including reelin, GLAST and Arc, were decreased in foetal brains following LPS exposure. Finally, at P1, the cerebral cortex of LPS-exposed animals was significantly larger than in age-matched control offspring and the cells appeared to be more compact, resulting in a reduction of the space these cells have available to extend processes compared with control.
Our results suggest an effect on the cleavage of reelin triggered by inflammation and the consequent cascade of events. In the developing mammalian brain, reelin has a pivotal role in cortical layer formation by regulating neuronal migration (Fatemi, 2005; Forster et al., 2010). In adult brain, this glycoprotein participates in synaptic plasticity and memory formation and is considered a susceptibility gene for neuropsychiatric disorders, such as schizophrenia and autism (Fatemi, 2005; Forster et al., 2010). Proteolytic cleavage of reelin produces five fragments, among them the central fragment was shown to mimic reelin functions in vitro. The function(s) of the full-length and shorter fragments of reelin has hitherto not been completely elucidated, albeit it was reported that inhibition of reelin processing in vivo prevents signalling and hampers development in cortical embryonic slices (Jossin et al., 2004, 2007). Cleavage appears to be required for reelin to be released in the intercellular space and to bind to its receptors on receptive cells. In reeler Orleans (relnorl/orl) mice, lack of reelin signalling is due to abnormal protein processing and expression of a truncated, non-releasable reelin fragment (de Bergeyck et al., 1997; Derer et al., 2001). In contrast with the C-terminus fragments, both the N-terminus and the central fragments have been detected in the CP and are considered important for reelin function. Moreover, Jossin et al. (2007) showed that, while the larger fragments as well as the full length could be only detected in the proximity of the MgZ, antibodies, raised against the N-terminal region or the central fragment, detected reelin among the cells of the CP. Hence, reelin processing seems important for his diffusion, which might be influenced by the fragments' size (ranging from 100 to 330 kDa), i.e. the smaller N-terminal and central fragments may diffuse farther into the CP and influence late born neurons which are at a greater distance from the MgZ. These differences would create a gradient of reelin throughout the developing cortex (Zhao and Frotscher, 2010). The decreased levels of the 180 kDa isoform reported in this study suggest an impairment of reelin processing, which could, at least in part, explain the increased presence of doublecortin and βIII-tubulin positive cells in the IZ in the LPS-exposed foetuses compared with controls.
During brain development, proper synaptic activity must activate a cascade of genes involved in transforming immature neuronal connections into functional circuits. IEGs are highly expressed during CNS development and have important roles in the adult brain. Their expression is developmentally regulated and influenced by exogenous signals such as neurotransmitters and second messenger signalling pathways. IEG activation in response to neural activity is fundamental for controlling expression of downstream genes and their products, which are involved in specification and maturation of neural progenitors in the cerebral cortex as well as in other brain areas (Herdegen and Leah, 1998; Kaufmann and Worley, 1999). Interference with the early expression and activity of IEGs, such as Arc, likely plays a role in the brain maldevelopment found in individuals with schizophrenia. As a consequence, synaptic transmission and plasticity are affected in young adulthood, when refinement of synaptic connections requires higher activity potentially leading to a loss of synaptic plasticity (Fatemi and Folsom, 2009).
These findings portray an intricate process by which foetal inflammation perturbs neuronal patterning and cortical development contributing to cognitive and/or psychotic manifestations later in adulthood. Such a process acts upon a number of different pathways, a number of which then have additional roles in mediating some of the experience-dependent plasticity in the adult brain. Based on these results, we surmise that the formation of neuronal networks in offspring from LPS-injected dams is altered, and such abnormalities may represent a major underlying pathophysiology of psychiatric disorders with a neurodevelopmental origin.
The LPS model used in this study does not fully recapitulate the events triggered by bacterial pathogens and their toxins in the foetal brain, and reproduces only part of the inflammation-mediated effects. Nonetheless, our study has set the stage to unravel the sequelae of events that underlie the neurobehavioural deficits reported in animals exposed to an antenatal insult.
Online data
ACKNOWLEDGEMENTS
We thank Donna Crandall for her help with the preparation of the Figures, Catalina Abad for insightful discussions and help with the ELISA assays, Elvira Khialeeva and Diane Anthony for their help with the H&E staining, Justin Lam and Linda L. Kalamkeryan for technical help. We are grateful to Christopher S. Colwell and David E. Krantz for critically reading the manuscript before submission.
Footnotes
N.S.M. was supported by an NINDS-Rita L. Kirschstein NRSA Fellowship. This work was supported by a Pilot and Feasibility grant from the UCLA Center for Neurobiology of Stress and a Stein-Oppenheimer Award to J.d.V and C.A.G., a Semel Young Investigator Award to C.A.G., and grants from the National Institutes of Health [P01-HD06576 and HD04612] to J.d.V.
REFERENCES
- Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Neocortical Development: Raven Press. 1991 [Google Scholar]
- Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70:570–579. doi: 10.1002/jnr.10423. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Hallenbeck JM, Gallo V. Determining the fetal inflammatory response in an experimental model of intrauterine inflammation in rats. Pediatr Res. 2004;56:541–546. doi: 10.1203/01.PDR.0000139407.89883.6B. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Hohmann CF. Behavioral consequences of abnormal cortical development: insights into developmental disabilities. Behav Brain Res. 1997;86:121–142. doi: 10.1016/s0166-4328(96)02251-6. [DOI] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- Campbell K, Gotz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–238. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- Cui K, Ashdown H, Luheshi GN, Boksa P. Effects of prenatal immune activation on hippocampal neurogenesis in the rat. Schizophr Res. 2009;113:288–297. doi: 10.1016/j.schres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- de Bergeyck V, Nakajima K, Lambert de Rouvroit C, Naerhuyzen B, Goffinet AM, Miyata T, Ogawa M, Mikoshiba K. A truncated Reelin protein is produced but not secreted in the ‘Orleans’ reeler mutation (Reln[rl-Orl]). Brain Res Mol Brain Res. 1997;50:85–90. doi: 10.1016/s0169-328x(97)00166-6. [DOI] [PubMed] [Google Scholar]
- Derer P, Derer M, Goffinet A. Axonal secretion of Reelin by Cajal-Retzius cells: evidence from comparison of normal and Reln(Orl) mutant mice. J Comp Neurol. 2001;440:136–143. doi: 10.1002/cne.1375. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Burket JA, Katz E. Does subtle disturbance of neuronal migration contribute to schizophrenia and other neurodevelopmental disorders? Potential genetic mechanisms with possible treatment implications. Eur Neuropsychopharmacol. 2010;20:281–287. doi: 10.1016/j.euroneuro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Susser ES. The promise of epidemiologic studies: neuroimmune mechanisms in the etiologies of brain disorders. Neuron. 2009;64:25–27. doi: 10.1016/j.neuron.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. Reelin glycoprotein: structure, biology and roles in health and disease. Mol Psychiatry. 2005;10:251–257. doi: 10.1038/sj.mp.4001613. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster E, Bock HH, Herz J, Chai X, Frotscher M, Zhao S. Emerging topics in Reelin function. Eur J Neurosci. 2010;31:1511–1518. doi: 10.1111/j.1460-9568.2010.07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Gallo V. Inhibition of cyclin E-cyclin-dependent kinase 2 complex formation and activity is associated with cell cycle arrest and withdrawal in oligodendrocyte progenitor cells. J Neurosci. 2001;21:1274–1282. doi: 10.1523/JNEUROSCI.21-04-01274.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Lelievre V, Beltran-Parrazal L, Sforza DM, Malvar J, Smith DJ, Charles AC, Ferchmin PA, de Vellis J. Gene expression is differentially regulated by neurotransmitters in embryonic neuronal cortical culture. J Neurochem. 2006;97(Suppl 1):35–43. doi: 10.1111/j.1471-4159.2006.03713.x. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell'Angelica EC. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry. 2010;15(115):204–115. doi: 10.1038/mp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Tsai LH, Wynshaw-Boris A. Life is a journey: a genetic look at neocortical development. Nat Rev Genet. 2002;3:342–355. doi: 10.1038/nrg799. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol. 2005;18:117–123. doi: 10.1097/01.wco.0000162851.44897.8f. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Forster E, Bock HH, Hack MA, Leprince P, Luque JM, Herz J, Frotscher M, Gotz M. Reelin signaling directly affects radial glia morphology and biochemical maturation. Development. 2003;130:4597–4609. doi: 10.1242/dev.00654. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Ignatova N, Sindic CJ, Goffinet AM. Characterization of the various forms of the Reelin protein in the cerebrospinal fluid of normal subjects and in neurological diseases. Neurobiol Dis. 2004;15:326–330. doi: 10.1016/j.nbd.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Jonakait GM. The effects of maternal inflammation on neuronal development: possible mechanisms. Int J Dev Neurosci. 2007;25:415–425. doi: 10.1016/j.ijdevneu.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Jossin Y, Ignatova N, Hiesberger T, Herz J, Lambert de Rouvroit C, Goffinet AM. The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J Neurosci. 2004;24:514–521. doi: 10.1523/JNEUROSCI.3408-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Gui L, Goffinet AM. Processing of Reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons. J Neurosci. 2007;27:4243–4252. doi: 10.1523/JNEUROSCI.0023-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarranz Y, Abad C, Martinez C, Arranz A, Gutierrez-Canas I, Rosignoli F, Gomariz RP, Leceta J. Protective effect of vasoactive intestinal peptide on bone destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1034–R1045. doi: 10.1186/ar1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF. Neural activity and immediate early gene expression in the cerebral cortex. Ment Retard Dev Disabil Res Rev. 1999;5:41–50. [Google Scholar]
- Mattan NS, Ghiani CA, Lloyd M, Matalon R, Bok D, Casaccia P, de Vellis J. Aspartoacylase deficiency affects early postnatal development of oligodendrocytes and myelination. Neurobiol Dis. 2010;40:432–443. doi: 10.1016/j.nbd.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- Meyer U, Engler A, Weber L, Schedlowski M, Feldon J. Preliminary evidence for a modulation of fetal dopaminergic development by maternal immune activation during pregnancy. Neuroscience. 2008a;154:701–709. doi: 10.1016/j.neuroscience.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008b;33:441–456. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009a;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009b;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Parnavelas JG. Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci. 2002;3:423–432. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- Nawa H, Takei N. Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neurosci Res. 2006;56:2–13. doi: 10.1016/j.neures.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Pinto L, Gotz M. Radial glial cell heterogeneity – the source of diverse progeny in the CNS. Prog Neurobiol. 2007;83:2–23. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Pomeroy SL, Kim JY. Biology and pathobiology of neuronal development. Ment Retard Dev Disabil Res Rev. 2000;6:41–46. doi: 10.1002/(SICI)1098-2779(2000)6:1<41::AID-MRDD6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Rees S, Harding R. Brain development during fetal life: influences of the intra-uterine environment. Neurosci Lett. 2004;361:111–114. doi: 10.1016/j.neulet.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. Int J Dev Neurosci. 2008;26:3–11. doi: 10.1016/j.ijdevneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Sauvageot CM, Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol. 2002;12:244–249. doi: 10.1016/s0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci. 2010;64:217–230. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- Zhao B, Schwartz JP. Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. J Neurosci Res. 1998;52:7–16. doi: 10.1002/(SICI)1097-4547(19980401)52:1<7::AID-JNR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Zhao S, Frotscher M. Go or stop? Divergent roles of Reelin in radial neuronal migration. Neuroscientist. 2010;16:421–434. doi: 10.1177/1073858410367521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.