Abstract
Background
Diseases that affect the buccal cavity are a public health concern nowadays. Chlorhexidine and nystatin are the most commonly used drugs for the control of buccal affections. In the search for more effective antimicrobials, nanotechnology can be successfully used to improve the physical chemical properties of drugs whilst avoiding the undesirable side effects associated with its use. Herein described are studies using nystatin and chlorhexidine with sodium montmorillonite (MMTNa), and chlorhexidine with β-cyclodextrin and two derivatives methyl-β-cyclodextrin and hydroxypropyl-β-cyclodextrin in the development of antimicrobial nanosystems.
Methods
The nanosystems were prepared by kneading and solubilization followed by freeze-drying technique. The nanosystems were characterized by X-ray powder diffraction (XRPD), differential scanning calorimetry (DSC), and Fourier transform infrared spectroscopy (FTIR). Nanosystem antimicrobial activity against Streptococcus mutans and Candida albicans strains was evaluated with inhibition halo analysis.
Results
The nanocarriers MMTNa and cyclodextrins showed good yields. XRPD, FTIR, and DSC analysis confirmed the proposed nanosystems formation and the suitability of the production methods. The nanosystems that showed best antimicrobial effect were chlorhexidine gluconate (CHX) and cyclodextrin inclusion complexes and CHX:MMTNa 60% cation exchange capacity – 24 hours.
Conclusion
The nanosystem formulations present higher stability for all chlorhexidine inclusion complexes compared with pure chlorhexidine. The nystatin nanosystems have the potential to mask the bitter taste, justifying subsequent in-vivo studies. For these reasons, further studies are being carried out to evaluate their application in professional formulations.
Keywords: sodium montmorillonite, chlorhexidine gluconate, buccal diseases, nanotechnology, cyclodextrins
Introduction
Diseases that affect the buccal cavity are considered a serious public health problem. Among the more prevalent are caries, periodontal disease, and the different types of oral candidiasis. Periodontal diseases and dental caries are usually caused by the presence of biofilm as a result of ineffective mechanical oral hygiene practices. For this reason, chemical agents are important to reduce gingivitis, periodontal disease, and dental caries.1 Candida albicans has already been isolated from the dental biofilm, caries, and the periodontal pockets, which together with Streptococcus mutans has more severe consequences.2 There are also several clinical issues associated with C. albicans which represent serious problems for treatment and prophylaxis.3 Therefore, the development of more effective therapies to treat these diseases is one of the major public dental health challenges. Chlorhexidine gluconate (CHX) is an antiseptic agent widely used to inhibit the formation of gingivitis and periodontitis.4 This cationic biguanidine interacts with the bacterial anionic surface and alters the integrity of the bacterial cell membrane leading to cytoplasm precipitation.5 However, besides the usual stability problems, this drug presents frequent side effects including taste perception alteration and an increase in tooth discoloration.6,7 For these reasons, several cyclodextrin (CD) inclusion complexes have been developed to increase stability and improve antimicrobial activity.4,8 The synergic association of CHX and nystatin (NYS) is a good option for the simultaneous control of C. albicans and S. mutans. Like CHX, NYS has an unpleasant flavor that leads to nausea during oral application, limiting its use and reducing patient compliance despite its undoubted effectiveness in the control of candidiasis and other buccal cavity disorders.9 The formulation of nanostructured NYS and CHX could overcome the low solubility and stability problems of these drugs, producing a controlled release system with new improved antimicrobial activity. Previous reports have shown that a NYS and CHX β-cyclodextrin (βCD) inclusion can improve stability and solubility.4,10 However, better results can be obtained using nanocarriers with sustained release properties, such as sodium montmorillonite (MMTNa), the main constituent of bentonite (60%). This nanocarrier may present some synergistic effect due to the capability of these materials to adsorb and fix bacteria and fungi, immobilizing their toxins,11 and also presents parallel lamellae with internal anionic surfaces and interlamellar cations for equilibrium and charge stabilization. Therefore, inclusion processes can occur with several different cations or small molecules, leading to substance encapsulation in the interlamellar space, increasing system tortuosity and water uptake.12 The use of MMTNa for acetate chlorhexidine inclusion has been previously reported,11 as has the use of βCD for CHX and NYS encapsulation.4,10 However, the use of water-soluble methyl-β-cyclodextrin (MβCD) and hydroxypropyl-β-cyclodextrin (HPβCD) for CHX, and MMTNa for both drugs, are other possibilities to be studied in order to obtain stable formulations with tolerable taste.13
Although there are several techniques to obtain CD complexes, such as co-precipitation, paste complexation, extrusion, spray drying, and kneading,6,13–15 considering these drugs, most of the authors show only the application of solution technique. Taking into account the industrial applicability, it has become necessary to evaluate other options, as kneading, due the feasibility and low cost.
The aim of this study is the development of new nano-structured drug-delivery systems with industrial applicability containing classical drugs for control of buccal pathologies, NYS and CHX, in order to increase the drug stability and improve the taste and antimicrobial action. Prepared and tested were inclusion complexes of NYS and CHX with MMTNa, and CHX with βCD and two derivatives, MβCD and HPβCD.
Materials and methods
Materials
Ethanol 95% (Merck, Darmstadt, Germany), MMTNa (Acros Chemical Co, Pittsburgh, PA), βCD, MβCD, and HPβCD (Wacker GmbH, Munich, Germany), NYS and chlorhexidine (Sigma, St Louis, MO) were pharmaceutical grade. Solutions were prepared with purified water obtained using a Milli-Q® system (Millipore, Bedford, MA).
Preparation of CD complexes
The inclusion complexes CHX:CD were prepared by solubilization-freeze-drying and kneading at molar ratios of 1:1, 1:2, and 1:3, using βCD, MβCD, and HPβCD. Physical mixtures were prepared by mixing together CD and freeze-dried CHX in a mortar at the same molar ratios. Using the kneading method, CD and CHX were mixed in a mortar for 5 minutes. An ethanol:water (70:30; v/v) solution was added, and the system mixed for 30 minutes to obtain a homogeneous paste. The paste was dried under reduced pressure and the granulometry adjusted using a 40 mesh sieve. Using the solution method, the appropriate proportions of CHX and CD were mixed in 20 mL of distilled water using a magnetic stirrer for 72 hours. The samples were frozen in liquid nitrogen and lyophilized. The particle size was also calibrated with a 40 mesh sieve. The inclusion yield was calculated by ultraviolet (UV) spectroscopy.
Preparation of clay-based nanosystems
NYS and CHX:MMTNa nanosystems were prepared with the solution method using different cation exchange capacity (CEC) values: 100%, 80%, and 60% of the total MMTNa. The CEC value considered was 100 meqv of cation to 100 g of MMTNa.12 The inclusion reactions were performed in triplicate for different periods (1, 18, 24, and 48 hours), stirring at room temperature. The reaction mixtures were centrifuged at 4000 rpm for 40 minutes, and the precipitates dried in a vacuum desiccator. The inclusion yield was calculated by UV spectroscopy.
Characterization of nanosystems
These nanosystems were characterized by X-ray powder diffraction (XRPD), Fourier transform infrared spectroscopy (FTIR), and differential scanning calorimetry (DSC). XRPD patterns of nanosystems, physical mixtures, and pure substances were recorded with a Rigaku Miniflex diffractometer BD11197 (Rigaku, Tokyo, Japan) using CuKα radiation with a current of 30 mA, voltage of 40 kV, and a 2θ angle between 2° and 20°. FTIR spectra were collected by an IR Prestige-21 Shimadzu A210045 (Shimadzu, Kyoto, Japan) spectrometer using 2% KBr pellets and wavenumber between 4000 and 400 cm−1. DSC analyses were carried out with DSC 882e Mettler-Toledo equipment (Mettler-Toledo, Greifensee, Switzerland) using hermetically sealed aluminum pans under a nitrogen flow of 28 mL·min−1 and heating rate of 10°C · min−1.
Evaluation of nanosystem antimicrobial activity
The S. mutans (ATCC25175) and C. albicans (ATCC36901) strains were grown at 37°C for 24 hours in the presence of the two drugs being tested and the isolated carriers (CD and MMTNa), with simultaneous comparison with the CHX and MMTNa nanosystems and CHX 0.12% solution formulations. The inoculum containing 106 cells/mL was uniformly seeded on plates containing solid brain heart infusion broth. After drying the surface, 10 μL of each sample was placed on the culture medium surface and incubated for 24 and 48 hours at 37°C. The plates containing S. mutans were maintained in anaerobiosis for the incubation time. The inhibition effect was verified by the presence of inhibition zones around the area on the plate where the solution was deposited and sized for analysis and comparison.
New CHX nanosystem formulations and stability test analysis
Mouthwash formulations were developed with CHX (0.120% w/v) or with CD inclusion complexes. Water-soluble mint essence (0.1% v/v), ethanol (0.050% v/v), polysorbate 20 (0.500% v/v), sorbitol (70% w/w aqueous solution, 15.0% v/v), menthol (0.010% w/v), and water (20%) were mixed until complete dissolution, and the volume was adjusted to 100% with water. The formulations were assayed by high efficiency liquid chromatography (HPLC) as described below. The HPLC system used was a LC-10 A/VP Shimadzu (Shimadzu, Kyoto, Japan) with a chromatography C18 column (250 mm × 4.6 mm, 5 μm particle size), the mobile phase was methanol:water with triethylamine 0.4% (63:37; v/v) with a 0.8 mL · min−1 flow rate. The analysis was performed at room temperature, with a 20 μL injection volume and monitored at a wavelength of 240 nm. The running time was established at 15 minutes.6,16 The stability tests were performed based on International Conference on Harmonization guidelines17 with CHX alone and inclusion complex the CHX:βC, CHX:MβCD, and CHX:HPβCD. All the inclusion complexes were at a molar ratio of 1:1 and obtained by kneading. The formulations were conditioned in a Nova Ética (São Paulo, Brazil) climatic chamber set to a relative humidity of 45°C ± 2°C and 75% ± 5%. The CHX:HPβCD inclusion complex preparation, formulation, and stability evaluation have not yet been described in the literature. The CHX assay was carried out according to the USP 34.6,16
Statistical data analysis
One-way analysis of variance and Wilcoxon matched pairs tests were used to analyze all data obtained in this study using StatSoft® STATISTICA (StatSoft Inc, Tulsa, OK) software.
Results and discussion
Preparation and characterization of CHX and CD inclusion complexes
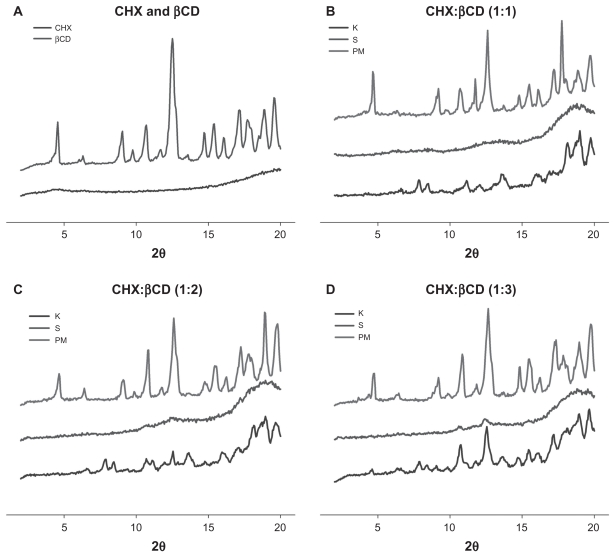
XRPD patterns of lyophilized CHX, βCD, and CHX:βCD complex are presented in Figure 1. As expected, lyophilized CHX shows an amorphous pattern after water uptake, while βCD shows a highly crystalline pattern. The XRPD patterns of the inclusion complexes show greater amorphous characteristics than pure βCD and physical mixtures. This is evidence of inclusion, as this disorder phenomenon has already been described by Cortés and colleagues.18 These amorphous patterns are characterized by the disappearance and decrease of peak intensity – a decrease of 70%–80% with the kneading method and 80%–90% with the solution method. These amorphous patterns were observed at molar ratios of 1:1, 1:2, and 1:3.
Figure 1.
X-ray powder diffraction patterns of (A) CHX and βCD, (B–D) physical mixture and inclusion complexes obtained by solubilization-freeze-drying and kneading at 1:1 (B), 1:2 (C), and 1:3 (D) molar ratios.
Abbreviations: βCD, β-cyclodextrin; CHX, chlorhexidine; K, kneading; PM, physical mixture; S, solubilization-freeze-drying.
The FTIR spectra of the βCD inclusion complex (data not shown) showed the characteristic peaks of CHX at 1700–1500 cm−1, corresponding to aromatic ring C = C stretching which is different from the βCD peaks. The spectra of both the physical mixture and the complex illustrated the typical peaks of each material of which the inclusion complex is composed, with the presence of significant frequency shifts. These results demonstrate the efficacy of the inclusion process with a CHX presence.18
As observed for βCD, MβCD presents a crystalline pattern (XRPD) and inclusion is evidenced by sample amorphization. The peak decrease was observed in samples prepared by both methods and at all molar ratios: 1:1, 1:2, and 1:3. In this case, it was not possible to differentiate between the two inclusion methods by XRPD. This lack of crystallinity indicates inclusion complex formation as shown by Figueiras and colleagues.19 The characteristic peaks of both CHX and MβCD can be seen in the FTIR spectra of the inclusion complexes with significant shifts, confirming the presence of CHX in the inclusion processes.
The characteristic peaks of HPβCD are at 2θ angles of 11.4° and 19.3°. A 60% peak reduction can be observed in the XRPD pattern of the complex produced by kneading at a 1:1 molar ratio. The samples produced by the solution method did not show significant peak reduction, meaning that the method employed is determinative to this complex formation. Wang and colleagues observed the same characteristics producing trans-ferulic acid complexes.20 The characteristic peaks of both CHX and HPβCD were demonstrated by the FTIR spectra of the inclusion complexes with significant shifts, confirming the effect of the presence of CHX in the production processes.
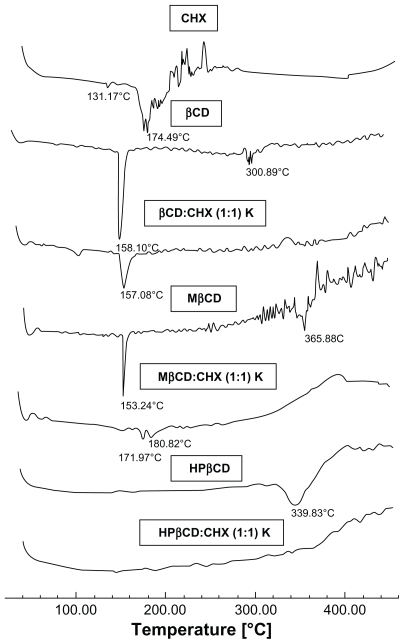
Thermal analysis, which is based on the comparison of the thermal behavior of single components, their physical mixture, and inclusion complexes, is the usual technique for qualitative investigation of CD inclusion complex formation. 21 DSC curves for the pure substances and inclusion compounds are shown in Figure 2. The DSC curve of CHX shows stability from 25°C to 174°C, after which, a sequence of thermal decomposition events were observed. DSC curves of inclusion compounds show that the CHX degradation peak temperature was higher for all inclusion complexes obtained with βCD and its synthetic derivatives (MβCD and HPβCD). This evidences a new supramolecular compound formation characteristic of inclusion complexes with improved stability. The disappearance or flattening of the drug melting point peak are considered conclusive evidence of inclusion compound formation.21 Yallapu and colleagues have observed these same patterns for curcumin complexation, where the melting endothermic peak of the drug completely disappeared in the DSC curves.22
Figure 2.
Differential scanning calorimetry curves of CHX, βCD, MβCD, HPβCD, and inclusion complexes obtained by kneading at a 1:1 molar ratio.
Abbreviations: βCD, β-cyclodextrin; CHX, chlorhexidine; HPβCD, hydroxypropyl- β-cyclodextrin; K, kneading; MβCD, methyl-β-cyclodextrin; Temp, temperature.
In all CD derivatives studied, the kneading method was chosen to prepare inclusion complexes of CHX, βCD, MβCD, and HPβCD at a 1:1 molar ratio, due its low cost and industrial applicability.
Preparation and characterization of clay-based nanosystems
MMTNa was used as a nanocarrier due to its sustained release properties and synergistic antimicrobial activity. The nanosystems produced were characterized by XRPD, DSC, and FTIR. A decrease in the 2θ value observed in the XRPD analysis is an indicative factor of the inclusion process since a reduction in this angle is associated with an increase in basal spacing, which is related to drug inclusion.23 The chlorhexidine nanosystem obtained showed a 2θ value decrease, described in Table 1, when compared with pure MMTNa, indicating the success of the inclusion process. However, there is no significant difference between all the proposed conditions presented in Table 1 (P > 0.05). CHX molecule rearrangement should also be considered since, like an alkyl ammonium chain, CHX may have a particular conformation that expands basal spacing in low concentration between lamellae. Absorption of CHX can occur when a high concentration is used for inclusion. In order to avoid this, a 60% CEC was used (24 hours). Under these conditions, the inclusion complex presented an average lamellar spacing of 16.8 ± 1.3 Å and an inclusion yield of 70.0% ± 0.02%. These results corroborate those observed by Meng and colleagues11 and Yang and colleagues,24 who reported an interlamellar space increase from 15.1 to 19.4 Å and 14.5 to 16.6 Å, respectively, for CHX acetate. These authors have observed that the CHX molecular volume is around 0.5 nm, slightly less than the 0.69 nm calculated by subtracting the MMTNa monolayer size (0.96 nm) from the basal spacing measurement (1.65 nm). This larger molecular volume may be due to a different conformation assumed by CHX in MMTNa lamellae.11
Table 1.
Inclusion process results obtained with MMTNa and CHX
| Intercalation conditions | 2θ (°) | Basal spacing (Å) | Indirect yield (%) | ||
|---|---|---|---|---|---|
| MMTNa | |||||
| MMTNa | 6.55 | 13.81 | – | ||
| CHX:MMTNa | |||||
| CHX:MMTNa 60% | 1 h | C1 | 5.25 | 16.83 | 71 |
| 18 h | C2 | 5.40 | 16.37 | 71 | |
| 24 h | C3 | 5.05 | 17.50 | 70 | |
| 48 h | C4 | 5.20 | 16.99 | 79 | |
| CHX:MMTNa 80% | 1 h | C5 | 5.00 | 17.67 | 50 |
| 18 h | C6 | 5.05 | 17.50 | 58 | |
| 24 h | C7 | 5.10 | 17.33 | 61 | |
| 48 h | C8 | 5.20 | 16.99 | 76 | |
| CHX:MMTNa 100% | 1 h | C9 | 5.00 | 17.67 | 36 |
| 18 h | C10 | 5.00 | 17.67 | 45 | |
| 24 h | C11 | 5.10 | 17.33 | 46 | |
| 48 h | C12 | 4.95 | 17.85 | 50 | |
Abbreviations: CHX, chlorhexidine; h, hour; MMTNa, sodium montmorillonite.
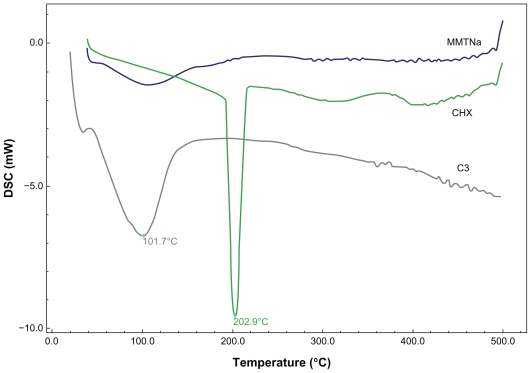
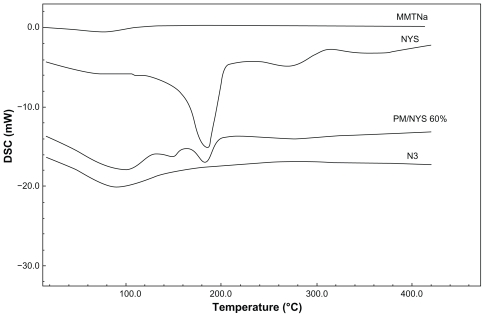
DSC curves for the pure substances and nanosystems are shown in Figure 3. Nanosystem C3 shows an endothermic peak between 200°C and 220°C, which may indicate the release of CHX from lamellar spacing. This result may be evidence that substances intercalated between clay lamellae can be observed through the calorimetric patterns of nanosystems at different temperatures.24
Figure 3.
DSC curves of MMTNa, CHX, and nanosystems with 60% CEC – 24 hours (C3).
Abbreviations: CEC, cation exchange capacity; CHX, chlorhexidine; DSC, differential scanning calorimetry; MMTNa, sodium
FTIR spectra of pure CHX, MMTNa, physical mixtures, and nanosystems present characteristic stretching bands due to water absorption at 3462 cm−1, 3400 cm−1, and 3420 cm−1. The hydroxyl group from the Al-OH bond presents a stretching band at around 3620 cm−1. Vibration bands of silicate between montmorillonite lamellae are shown at 1114 cm−1 and 1047 cm−1 for the Si-OH bond.11,25 Regarding the spectra of CHX, this shows a shift of the stretching bands for the aromatic N-H and C-H bonds at 3396 cm−1 and 3226 cm−1 respectively. It is important to note that the spectra of the physical mixture, which simulates C3, showed differences when compared with the spectra of the inclusion process, indicating their contrasting profiles and confirming the inclusion process.
The NYS:MMTNa inclusion studies showed a 2θ value decrease in relation to pure MMTNa, which is evidence of nanosystem formation under all tested conditions (Table 2). However, the 24-hour inclusion period produced a larger basal spacing with all tested CEC. The nanosystem N3 (60% CEC) was chosen for characterization due to the higher basal spacing compared with 100% CEC and the economic aspect. This nanosystem presented an average lamellae spacing of 16.63 ± 0.33 Å (Figure 4) and an indirect inclusion yield of 54% ± 0.01%.
Table 2.
Inclusion process results obtained with MMTNa and NYS
| Intercalation conditions | 2θ (°) | Basal spacing (Å) | Indirect yield (%) | ||
|---|---|---|---|---|---|
| NYS:MMTNa 60% | 1 h | N1 | 5.90 | 14.98 | 51 |
| 18 h | N2 | 5.65 | 15.64 | 52 | |
| 24 h | N3 | 5.20 | 16.99 | 54 | |
| 48 h | N4 | 5.95 | 14.85 | 46 | |
| NYS:MMTNa 80% | 1 h | N5 | 5.45 | 16.22 | 53 |
| 18 h | N6 | 5.60 | 15.78 | 49 | |
| 24 h | N7 | 5.20 | 16.99 | 55 | |
| 48 h | N8 | 5.75 | 15.37 | 43 | |
| NYS:MMTNa 100% | 1 h | N9 | 5.40 | 16.37 | 47 |
| 18 h | N10 | 5.60 | 15.78 | 46 | |
| 24 h | N11 | 5.55 | 15.92 | 51 | |
| 48 h | N12 | 6.00 | 14.73 | 44 |
Abbreviations: h, hour; MMTNa, sodium montmorillonite; NYS, nystatin.
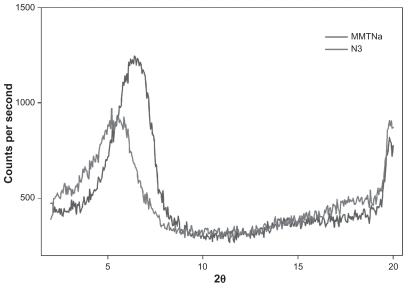
Figure 4.
X-ray powder diffraction patterns of MMTNa and NYS-and-MMTNa nanosystems (NYS:MMTNa) with 60% CEC – 24 hours (N3).
Abbreviations: CEC, cation exchange capacity; MMTNa, sodium montmorillonite; NYS, nystatin.
XRPD profiles for the pure substances and nanosystem N3 at different CEC values are shown in Figure 4. Nanosystem N3 shows a higher 2θ value when compared with MMTNa. This result is evidence of inclusion complex formation in the clay lamellae.
The calorimetric pattern of NYS derivatives are shown in Figure 5. The characteristic thermal event related to NYS, its melting point, occurs between 160°C and 180°C, but this is not registered on the DSC curve of the NYS:MMTNa 60% CEC – 24-hour nanosystem. This pattern is similar to the pure MMTNa with an endothermic peak at 100°C due to water loss. The absence of NYS endothermic melting point is evidence of inclusion, since the presence of the drug in the nanosystem was confirmed by FTIR analysis (data not shown).
Figure 5.
DSC curves of MMTNa, NYS, physical mixture of 60% CEC (NYS:MMTNa PM), and NYS:MMTNa 60% CEC – 24-hour nanosystem (N3).
Abbreviations: CEC, cation exchange capacity; DSC, differential scanning calorimetry; MMTNa, sodium montmorillonite; NYS, nystatin; PM, physical mixture; Temp, temperature.
Evaluation of antimicrobial activity
The inhibition halo technique has the greatest applicability in the evaluation of antimicrobial activity of biologically active montmorillonite–chlorhexidine nanocomposite.24,26 Therefore, the inhibition halo technique was used to evaluate and compare the activity of the nanosystems and pure drugs and the possible synergy of the drugs with clays and CD. The samples tested were CHX:βCD, CHX:MβCD, and CHX:HPβCD. These nanosystems were prepared by kneading, using CHX and CDs at a 1:1 molar ratio. The control solution was 0.12% CHX aqueous solution (w/v). The inhibition halo diameters observed for C. albicans and S. mutans are shown in Table 3. All samples showed very similar activity and were quite similar to the CHX aqueous solution against Gram-positive bacteria and fungi. However, Cortés and colleagues18 have shown that a CHX:βCD inclusion complex demonstrated modified antimicrobial activity when its minimum inhibitory concentration was measured, increasing its efficacy against pathogenic bacteria. These results were not observed in this work. Herein, contrary results were obtained with no statistical difference in the samples tested (P = 0.07653).
Table 3.
Inhibition halo diameters of CHX (control) and inclusion complexes against Streptococcus mutans and Candida albicans
| S. mutans | C. albicans | |||||
|---|---|---|---|---|---|---|
| Inhibition halo diameters (mm) | ||||||
| CHX:βCD | 17 | 17 | 17 | 15 | 15 | 15 |
| CHX:MβCD | 18 | 18 | 18 | 16 | 16 | 16 |
| CHX:HPβCD | 17 | 17 | 17 | 16 | 15 | 15 |
| CHX | 17 | 17 | 17 | 16 | 16 | 15 |
Abbreviations: βCD, β-cyclodextrin; CHX, chlorhexidine; HPβCD, hydroxypropyl-β-cyclodextrin; MβCD, methyl-β-cyclodextrin.
The halo diameters of the MMTNa nanosystems are represented in Table 4 and Figure 6. The MMTNa suspension did show any inhibition activity, which corroborates results shown by other authors.11,24,26,27 These authors not only tested sodium but also calcium montmorillonite. Thus, any synergic effect can be attributed to the absorptive properties of clay.
Table 4.
Inhibition halo diameters of pure substances and developed derivatives against Streptococcus mutans and Candida albicans strains using three different plating techniques
| S. mutans | C. albicans | |||||
|---|---|---|---|---|---|---|
| Plate 1 | Swab 1a | Swab 2b | Plate 1 | Swab 1a | Swab 2b | |
| Inhibition halo diameters (mm) | ||||||
| MMT | 0 | 0 | 0 | 0 | 0 | 0 |
| CHX | 27 | 26 | 27 | 18 | 19 | 26 |
| C3c | 22 | 21 | 21 | 11 | 12 | 20 |
| NYS | 0 | 0 | 0 | 16 | 19 | 27 |
| N3d | 0 | 0 | 0 | 0 | 0 | 0 |
Notes: Swab 1 samples applied in drops;
Swab 2 samples applied to wells;
C3 = CHX:MMTNa 60% CEC – 24 hours;
N3 = NYS:MMTNa 60% CEC – 24 hours.
Abbreviations: CEC, cation exchange capacity; CHX, chlorhexidine; MMT, montmorillonite; MMTNa, sodium montmorillonite; NYS, nystatin.
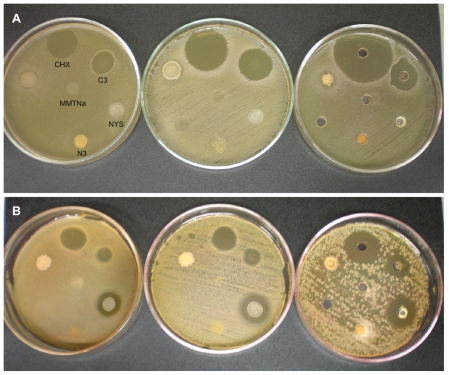
Figure 6.
Inhibition halo analysis using (A) Streptococcus mutans (n = 3) and (B) Candida albicans strains (n = 3) of CHX aqueous solution 0.12%, CHX derivative 60% CEC – 24 hours (C3), NYS, NYS derivative 60% CEC – 24 hours (N3), and MMTNa.
Note: The inoculum in all Petri dishes followed the order displayed on the first dish.
Abbreviations: CHX, chlorhexidine; MMTNa, sodium montmorillonite; NYS, nystatin.
CHX aqueous solution 0.12% and the C3 derivative showed a large growth inhibition zone for both microorganisms. Halos for the CHX solution against S. mutans were between 26 and 27 mm, while the C3 derivative presented halos of around 21–22 mm. Furthermore, halos against C. albicans of 18, 19, and 26 mm were observed using the CHX solution, and 11, 12, and 20 mm with the C3 derivative. The smaller halo diameters presented by the C3 derivative were expected since it is an inclusion product with known prolonged release characteristics.24,27 Yang and colleagues24 studied the antimicrobial activity of chlorhexidine acetate:MMTNa nanosystems and found that CHX was released slowly and the inhibition halo could be seen after 1 year. However, the results of this present study show that the CHX C3 derivative presented acceptable immediate release properties without total drug release, which is ideal for the treatment of oral diseases.
Pure NYS, an antifungal agent, did not show any inhibition activity against S. mutans, as expected, but presented halos ranging from 16, 19, and 27 mm against C. albicans strains (Table 4, Figure 6). These results are similar to those found by Carrillo-Muñoz and colleagues of 20 mm.28 The NYS nanosystem was expected to show the same prolonged release profile as C3, but did not present any initial inhibition, indicating that the release is possibly slower than chlorhexidine due to lower NYS solubility in water and saliva. However, the large fluid volume in vivo could lead to a more effective release, justifying the use of this nanosystem in prolonged release tablets, which could be a therapeutic alternative to more complex pharmaceutical forms.
Development of inclusion complexes in oral solutions and stability test
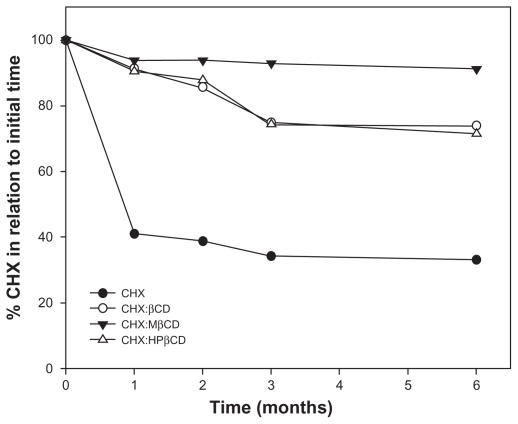
Initially, the CHX stability in a mouthwash formulation was investigated because CHX has several incompatibilities with common excipients, such as some ions and organic compounds. These incompatibility reactions lead to the formation of the degradation product p-chloroaniline and its subsequent precipitation.29,30 No precipitation or incompatibility was observed between CHX and formulation excipients 48 hours after formulation preparation with pure CHX. Mouthwash formulations were produced with pure CHX and the inclusion complexes using 0.12% w/v CHX content. These samples were stored in a climatic chamber at 45°C ± 2°C and 75% ± 5% relative humidity (RH) in order to evaluate the thermal stability. The results of the stability study are presented in Figure 7, and a great increase in the stability of all nanosystems studied can be seen, indicating the potential for the commercial application of these new CD derivatives.
Figure 7.
Stability profile of mouth wash formulation containing CHX not included, and inclusion complexes of βCD, MβCD, and HPβCD stored at 40°C ± 2°C and 75% ± 5% relative humidity for 6 months.
Abbreviations: βCD, β-cyclodextrin; CHX, chlorhexidine; HPβCD, hydroxypropyl-β-cyclodextrin; MβCD, methyl-β-cyclodextrin.
Conclusion
XRPD, FTIR, and DSC analysis were used to confirm the proposed nanosystems formation and the suitability of the production methods. However, the nanosystems that showed best antimicrobial effect were CHX and CD inclusion complexes and CHX:MMTNa 60% CEC – 24 hours. Although NYS nanosystems presented no antimicrobial activity with the technique applied here, this does not preclude their use since this method does not represent in-vivo conditions. Moreover, these nanosystem formulations present the following improvements: higher stability for all chlorhexidine inclusion complexes compared with pure chlorhexidine under 40°C ± 2°C and 75% ± 5% RH and being a sustained release system, the potential for NYS nanosystems to mask the bitter taste, justifying subsequent in-vivo studies. For these reasons, further studies are being carried out to evaluate their application in professional formulations.
Acknowledgments
This work was supported by FAPERJ, CAPES Edital CAPES Nanobiotecnologia 2008 and CNPq.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Skartveit L, Selvig KA, Myklebust S, Tveit AB. Effect of Tif4 Solutions on bacterial-growth in vitro and on tooth surfaces. Acta Odontol Scand. 1990;48(3):169–174. doi: 10.3109/00016359009005872. [DOI] [PubMed] [Google Scholar]
- 2.Ates M, Akdeniz BG, Sen BH. The effect of calcium chelating or binding agents on Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(5):626–630. doi: 10.1016/j.tripleo.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- 4.Escribano M, Herrera D, Morante S, Teughels W, Quirynen M, Sanz M. Efficacy of a low-concentration chlorhexidine mouth rinse in noncompliant periodontitis patients attending a supportive periodontal care programme: a randomized clinical trial. J Clin Periodontol. 2010;37(3):266–275. doi: 10.1111/j.1600-051X.2009.01521.x. [DOI] [PubMed] [Google Scholar]
- 5.Winkel EG, Roldán S, Van Winkelhoff AJ, Herrera D, Sanz M. Clinical effects of a new mouth rinse containing chlorhexidine, cetylpyridinium chloride and zinc-lactate on oral halitosis. J Clin Periodontol. 2003;30(4):300–306. doi: 10.1034/j.1600-051x.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- 6.Soares da Silva LF, do Carmo FA, de Almeida Borges VR, et al. Preparation and evaluation of lidocaine hydrochloride in cyclodextrin inclusion complexes for development of stable gel in association with chlorhexidine gluconate for urogenital use. Int J Nanomedicine. 2011;6:1143–1154. doi: 10.2147/IJN.S20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CS, Arnold RR, Trope M, Teixeira FB. Clinical efficiency of 2% chlorhexidine gel in reducing intracanal bacteria. J Endodont. 2007;33(11):1283–1289. doi: 10.1016/j.joen.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrin I. Drug solubilization and stabilization. J Pharm Sci. 1996;85(10):1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe JE. Treatment of oropharyngeal candidiasis in immunocompetent infants: a randomized multicenter study of miconazole gel vs nystatin suspension. Pediatr Infect Dis J. 1997;16(3):288–293. doi: 10.1097/00006454-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 10.de Soet JJ, Gruythuysen RJ, Bosch JA, van Amerongen WE. The effect of 6-monthly application of 40% chlorhexidine varnish on the microflora and dental caries incidence in a population of children in Surinam. Caries Res. 2002;36(6):449–455. doi: 10.1159/000066536. [DOI] [PubMed] [Google Scholar]
- 11.Meng N, Zhou NL, Zhang SQ, Shen J. Controlled release and antibacterial activity chlorhexidine acetate (ca) intercalated in montmorillonite. Int J Pharm. 2009;382(1–2):45–49. doi: 10.1016/j.ijpharm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Ray SS, Yamada K, Okamoto M, Ogami A, Ueda K. New polylactide/layered silicate nanocomposites. 3. High-performance biodegradable materials. Chem Mater. 2003;15(7):1456–1465. [Google Scholar]
- 13.Martin Del, Valle EM. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–1046. [Google Scholar]
- 14.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: Past, present and future. Nat Rev Drug Discov. 2004;3(12):1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 15.Loftsson T, Duchêne D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329(1–2):1–11. doi: 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 16.United States Pharmacopeia. Monograph: Chlorehexidine Gluconate Oral Rinse. 34th ed. II. Rockville, MD: The United States Pharmacopeial Convention; 2011. p. 2297. [Google Scholar]
- 17.International Conference on Harmonization (ICH) Q1A(R2). Stability testing of new drug substance and products. US FDA Federal Register. 2003. [Accessed October 4, 2011]. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf.
- 18.Cortés ME, Sinisterra RD, Avila-Campos MJ, Tortamano N, Rocha RG. The chlorhexidine: β-cyclodextrin inclusion compound: Preparation, characterization and microbiological evaluation. J Incl Phenom Macrocycl Chem. 2001;40(4):297–302. [Google Scholar]
- 19.Figueiras A, Ribeiro L, Vieira MT, Veiga F. Preparation and physicochemical characterization of omeprazole: methyl-beta-cyclodextrin inclusion complex in solid state. J Incl Phenom Macrocycl Chem. 2007;57(1):173–177. [Google Scholar]
- 20.Wang J, Cao Y, Sun B, Wang C. Characterization of inclusion complex of trans-ferulic acid and hydroxypropyl-β-cyclodextrin. Food Chem. 2011;124(3):1069–1075. [Google Scholar]
- 21.Giordano F, Novak C, Moyano JR. Thermal analysis of cyclodextrins and their inclusion compounds. Thermoch Acta. 2001;380(2):123–151. [Google Scholar]
- 22.Yallapu MM, Jaggi M, Chauhan SC. Beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces. 2010;79(1):113–125. doi: 10.1016/j.colsurfb.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Cheng X, Liu H, Lu W. Synthesis and properties of poly(vinyl alcohol)/synthetic F-montmorillonite nanocomposites. Chin J Chem. 2009;27(8):1611–1616. [Google Scholar]
- 24.Yang D, Yuan P, Zhu JX, He HP. Synthesis and characterization of antibacterial compounds using montmorillonite and chlorhexidine acetate. J Therm Anal Calorim. 2007;89(3):847–852. [Google Scholar]
- 25.Joshi GV, Patel HA, Kevadiya BD, Bajaj HC. Montmorillonite intercalated with vitamin B-1 as drug carrier. Appl Clay Sci. 2009;45(4):248–253. [Google Scholar]
- 26.Meng N, Zhou N, Zhang S, Shen J. Synthesis and antimicrobial activities of polymer/montmorillonite-chlorhexidine acetate nanocomposite films. Appl Clay Sci. 2009;42:667–670. [Google Scholar]
- 27.He H, Yang D, Yuan P, Shen W, Frost RL. A novel organoclay with antibacterial activity prepared from montmorillonite and Chlorhexidini Acetas. J Colloid Interface Sci. 2006;297(1):235–243. doi: 10.1016/j.jcis.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Carrillo-Munoz AJ, Quindós G, Tur C, et al. In-vitro antifungal activity of liposomal nystatin in comparison with nystatin, amphotericin B cholesteryl sulphate, liposomal amphotericin B, amphotericin B lipid complex, amphotericin B desoxycholate, fluconazole and itraconazole. J Antimicrob Chemother. 1999;44(3):397–401. doi: 10.1093/jac/44.3.397. [DOI] [PubMed] [Google Scholar]
- 29.Rasimick BJ, Nekich M, Hladek MM, Musikant BL, Deutsch AS. Interaction between chlorhexidine digluconate and EDTA. J Endod. 2008;34(12):1521–1523. doi: 10.1016/j.joen.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 30.Basrani BR, Manek S, Sodhi RNS, Fillery E, Manzur A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod. 2007;33(8):966–969. doi: 10.1016/j.joen.2007.04.001. [DOI] [PubMed] [Google Scholar]