Abstract
Background
In the present study, we investigated whether DJ-1 could serve as a biomarker for assessing the biocompatibility of multiwalled carbon nanotubes (MWCNTs), using the highly purified carbon nanotube, HTT2800.
Methods
Using Western blot analysis, we determined DJ-1 protein levels in two different types of cells (one capable and the other incapable of HTT2800 endocytosis). Using quantitative real-time polymerase chain reaction, we also investigated the ability of purified nanotubes to alter DJ-1 mRNA levels.
Results
We demonstrated that the DJ-1 protein concentration was reduced, regardless of the cytotoxic activity of intracellular HTT2800. Furthermore, HTT2800 decreased the DJ-1 mRNA levels in a dose-dependent manner. This decrease in DJ-1 mRNA levels was not observed in the case of Sumi black or cup-stacked carbon nanotubes.
Conclusion
These data indicate that modification of DJ-1 expression is caused by the cell response to MWCNTs. We conclude that DJ-1 is a promising candidate biomarker for the development of biocompatible MWCNTs.
Keywords: multiwalled carbon nanotubes, DJ-1 protein, Western blot, quantitative real-time polymerase chain reaction
Introduction
Carbon nanomaterial technology, including the development of sp2-based carbon nanotubes, has advanced greatly during the last 25 years. The nanoscale size and extraordinary physicochemical properties of multiwalled carbon nanotubes (MWCNTs) have proven very useful in a wide variety of applications, including nanocomposites, sensors, energy storage, energy-conversion systems, field-emission displays, radiation sources, medical applications, and other nanodevices.1,2 MWCNTs can be mass-produced by chemical vapor deposition methods, especially the floating reactant method.3 Simultaneously, occupational and public exposure to manufactured nanomaterials has increased significantly. Carbon materials boast high biocompatibility. However, because of their unique nanoscale size and high aspect ratio (>100), MWCNTs, like asbestos, may present important human health hazards.4–7
The human body can be exposed to MWCNTs through five possible routes, ie, inhalation of airborne carbon nanotubes, dermal penetration by skin contact, injection of engineered MWCNTs, implantation of composite MWCNTs, and ingestion with drinking water or food additives. Once in the body, MWCNTs are thought to be biopersistent. 8 Safety evaluations of MWCNTs have been performed using in vitro and in vivo models.9–12 However, there is no consensus as to whether or not MWCNTs affect the human body. Thus, it is important to identify biomarkers that clarify whether MWCNTs are taken into the system, regardless of their ultimate effect. Such biomarkers will also prove useful for the development of biocompatible MWCNTs.
DJ-1 protein, encoded by the PARK7 gene, belongs to the peptidase C56 family. It functions as a redox-sensitive chaperone (a sensor for oxidative stress), ultimately protecting neurons against oxidative stress and cell death.13–15 Moreover, it has proven to be a biomarker for Parkinson’s disease and some types of cancer.16–18 Using a proteomic approach, we recently revealed that expression of this protein decreased when U937 cells were exposed to MWCNTs for 4 days. However, we did not detect biological responses, such as pathological or histological changes, by conventional methods.19,20
In the present study, we investigated whether DJ-1 could serve as a biomarker for biocompatible MWCNTs. We used the highly purified carbon nanotube, HTT2800, and two different types of cells (characterized by their contrasting biological responses to HTT2800).
Methods
Carbon nanomaterials
We used the highly purified MWCNT, known as HTT2800, described in detail previously.3 In brief, HTT2800 is characterized by a high aspect ratio, a diameter of 100–150 nm, and length of 10–20 μm. The autoclave sterilization conditions were 121°C for 15 minutes. HTT2800 was dispersed with 0.1% gelatin (Nacalai Tesque, Kyoto, Japan) in phosphate-buffered saline and sonicated for 30 minutes. We used other carbon materials, ie, Sumi black (Unique Tattoos, Subiaco, Australia) 30–50 nm in particle size, and cup-stacked carbon nanotubes (GSI Creos, Tokyo, Japan) 100 nm in diameter and 0.5–20 μm in length. These were autoclaved and dispersed in the same manner used for HTT2800.
Cell culture
The monoblastic human cell line, THP-1, and the human malignant pleural mesothelioma cell line, MESO-121, were purchased from Riken (Ibaraki, Japan). The cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, at 37°C in a 5% CO2 humidified incubator, and passaged twice a week. For each study, the cells were seeded at a density of 4 × 104 cells/mL (THP-1) and 2 × 104 cells/mL (MESO-1). The MESO-1 cells were allowed to adhere for 24 hours.
Alamar blue assay
To determine the proliferation of cells exposed to different concentrations of HTT2800, we performed an Alamar blue assay (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The cells were incubated for 4 days at 37°C in the culture medium, with various concentrations of HTT2800, in 96-well culture plates. The control cells were cultured in a dispersant medium containing 0.001% gelatin (DM). Viable cells metabolized the dye. This resulted in an increase in fluorescence by excitation/emission at 550 nm/600 nm, recorded by a fluorescence multiplate reader (PowerScan 4, DS Pharma Biomedical, Osaka, Japan). Cell proliferation was calculated as follows: proliferation rate = 100× experimental value/each dispersant medium value. Test media were assayed six times.
Observation of cells exposed to HTT2800
THP-1 and MESO-1 cells were incubated for 4 days with 100 μg/mL and 10 μg/mL of HTT2800, respectively, and stained with 1 μg/mL of bisbenzimide H33342 fluorochrome trihydrochloride (H33342, Nacalai Tesque) for 30 minutes at 37°C in a 5% CO2 incubator. The stained cells were observed through an Axio Observer Z1 fluorescence microscope (Zeiss, Jena, Germany) with excitation at 360 nm. The cells were visualized by light or fluorescence microscopy using a ×40 objective.
Western blot analysis
The cells were incubated with or without HTT2800 for 4 days in culture dishes (Ø6 cm). After incubation, the cells were washed three times with 250 mM saccharose in 10 mM Tris-HCl (pH 7.0) and solubilized in lysis solution containing 7 M urea, 2 M thiourea, 4% CHAPS, 5% β-mercaptoethanol, and 0.5% IPG buffer (pH 3–10, Bio-Rad, Irvine, CA). Cell lysates were centrifuged at 14,000× g for 5 minutes, and the protein in the supernatant was quantified using Coomassie Plus Assay Reagent (Thermo Scientific, Rockford, IL). The total protein solution was diluted 1:1 with Laemmli sample buffer (Bio-Rad) and boiled for 5 minutes. The proteins were then separated on 12.5% sodium dodecyl sulfate polyacrylamide electrophoresis gel (e-PAGEL, ATTO, Tokyo, Japan) and transferred onto a polyvinylidene fluoride membrane (Bio-Rad). The membrane was blocked with × 1 non-protein blocking agent (ATTO) in phosphate-buffered saline with 0.1% Tween 20, for 30 minutes at room temperature, and probed with the primary rabbit antihuman DJ-1 antibody (sc-32874, Santa Cruz Biotechnology, Santa Cruz, CA) for one hour at room temperature. After washing, the membrane was incubated with the secondary antirabbit IgG, horseradish peroxidase-linked species-specific whole antibody from a donkey (GE Healthcare, Little Chalfont, UK) for one hour at room temperature, and then developed with ECL-plus chemiluminescent detection reagent (GE Healthcare, Piscataway, NJ). The membrane was stripped with Restore Plus Western blot stripping buffer (Thermo Scientific), and detection was repeated using the primary monoclonal anti-β-actin antibody (Sigma-Aldrich, St Louis, MO) and the anti-mouse IgG, horseradish peroxidase-linked species-specific whole antibody from sheep (GE Healthcare). The obtained bands were quantified by Quantity One software (Version 4, Bio-Rad). The expression of DJ-1 protein was represented as a percentage of dispersant medium, after correcting the value by the quantified β-actin.
Immunocytochemistry
The cells were incubated with or without HTT2800 for 4 days on culture chamber slide. After incubation, the cells were washed once with phosphate-buffered saline, blocked with 10% normal goat serum in phosphate-buffered saline for 30 minutes, and probed with the primary rabbit antihuman DJ-1 antibody overnight at 4°C. After washing, the slides were incubated with the secondary antibody (Alexa Fluor® 594 goat antirabbit IgG, Invitrogen) for one hour at room temperature, and then mounted with 90% glycerol containing 1 μg/mL H33342. The cells were visualized using an LSM510 NLO laser-scanning confocal microscope (Zeiss) with a ×63 objective.
Isolation of total RNA and quantitative real-time polymerase chain reaction
MESO-1 cells were washed three times with ice-cold phosphate-buffered saline. The cells were harvested in 500 μL of TRIzol reagent (Invitrogen) and total RNA was extracted. One microgram of each total RNA preparation was then reverse transcribed using ReverTra Ace (Toyobo, Osaka, Japan) and 2.5 μM random primers. Quantification of mRNA levels was performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) and the First Start Universal SYBR Green PCR Master Mixture (Roche Applied Science, Mannheim, Germany) with the following primer pair sets: DJ-1, QuantiTect® Primer Assay (Hs_PARK7_1_SG, Qiagen, Dusseldorf, Germany); and GAPDH 5′-CTGCACCACCAACTGCTTAG-3′ (forward) and 5′-GGGCCATCCACAGTCTTCT-3′ (reverse). The cycling parameters for these assays were: 50°C for 2 minutes, 95°C for 2 minutes, 40 cycles of 95°C for 30 seconds, and finally, 60°C for one minute. Quantitative results were obtained from the Ct values, which were the inverse ratios relative to the starting polymerase chain reaction product. The relative quantification was obtained by calculating 2−ΔCt, where ΔCt = Cttarget gene − CtGAPDH. Quantification of the cDNA levels for each gene was performed using three replicates of each cDNA sample obtained.
Statistical analysis
Data are presented as the mean ± standard error of the mean. The Student’s t-test was applied, and P < 0.05 was considered to be statistically significant.
Results and discussion
To identify potential biomarkers for MWCNTs in vitro, we used two different cell lines, ie, THP-1 and MESO-1. We recently reported that DJ-1 protein expression decreased when U937 cells were exposed to MWCNTs for 4 days; however, carbon black levels were not altered in response to DJ-1.20 Similar to U937 cells, THP-1 human acute monocytic leukemia cells have the ability to differentiate into macrophages. 22 The objective of the present study was to confirm whether the expression of DJ-1 in U937 cells is universal. We recently also reported that exposure of BEAS-2B cells (derived from human bronchial epithelium) to HTT2800 proved cytotoxic,23 causing arrest of proliferation. One of the most significant differences between U937 and BEAS-2B cells is their surface adhesiveness. Therefore, in the present study, we used adherent MESO-1 cells (derived from human malignant pleural mesothelioma) to evaluate DJ-1 protein expression upon exposure to MWCNTs. Pulmonary mesothelial cells are thought to constitute the crucial cell type for asbestos toxicity.
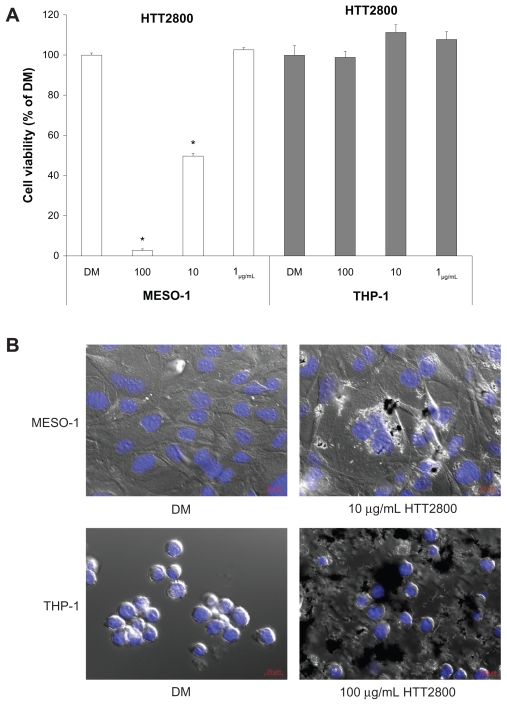
The proliferation of THP-1 and MESO-1 cells exposed to HTT2800 for 4 days was clearly different (Figure 1A). While the propagation of MESO-1 cells was suppressed by HTT2800 in a concentration-dependent manner (with a few dead cells observed in the culture dish), THP-1 cells were not affected by the nanomaterial. Figure 1B depicts the cell images after 4 days of culture with or without HTT2800. In the case of MESO-1 cells, endocytosis of MWCNTs was clearly observed. These results were in accordance with a previous report, in which a similar effect was observed in the BEAS-2 cell line.23 By contrast, in the case of THP-1 cells, MWCNTs adhered around the cells, but were not ingested. This response was similar to that previously observed in U937 cells.19
Figure 1.
Influence of HTT2800 on cell viability. MESO-1 and THP-1 cells were exposed to varying concentrations of HTT2800 for 4 days. (A) Cell viability assessed by Alamar blue assay (B) Merged image of differential interference contrast and fluorescence in MESO-1 and THP-1 cells exposed to HTT2800 (nuclei were stained with H33342; bars in red indicate 20 μm).
Notes: Data are compared with dispersant medium, n = 6, mean ± standard error of the mean, *P < 0.001.
Abbreviation: DM, dispersant medium containing 0.001% gelatin.
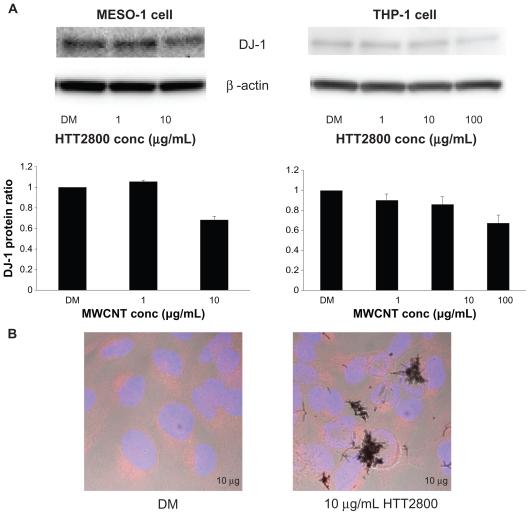
Figure 2A shows the expression levels of DJ-1 protein, determined by means of Western blot analysis. In comparison with the vehicle control, the quantity of DJ-1 detected in THP-1 cells exposed to 100 μg/mL of HTT2800 was reduced to 67%. This finding is in accordance with the results obtained with U937 cells exposed to MWCNTs for 4 days, analyzed using the proteomic approach.19 The decrease rate observed in the present study was lower than that previously documented,24 because our Western blot quantitative analysis was performed by summing oxidized and reduced DJ-1, whereas Duan et al separated the protein using two-dimensional electrophoresis. Correspondingly, in MESO-1 cells exposed to 10 μg/mL of HTT2800, DJ-1 expression was reduced to 68% compared with the vehicle control.
Figure 2.
DJ-1 protein expression after HTT2800 exposure. MESO-1 and THP-1 cells were exposed to varying concentrations of HTT2800 for 4 days. (A) Expression of DJ-1 protein was normalized to β-actin and represented as the ratio compared with dispersant medium (n = 4, mean ± standard error of the mean). (B) Cellular localization of DJ-1 protein in MESO-1 cells after exposure to 10 μg/mL of HTT2800 for 96 hours (DJ-1 was stained with Alexa Fluor® 594 and nuclei were stained with H33342).
Note: Bars in white indicate 10 μm.
Abbreviation: DM, dispersant medium containing 0.001% gelatin.
It has been reported that oxidative stress induces intracellular redistribution of DJ-1.25 Therefore, we performed immunofluorescence staining of DJ-1. However, we observed no changes in intracellular localization of the protein (Figure 2B).
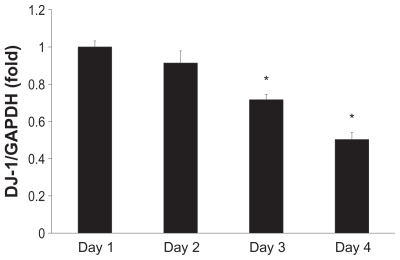
We further investigated the expression of DJ-1 mRNA in MESO-1 cells by quantitative real-time polymerase chain reaction. MESO-1 cells are adherent and separation of the MWCNTs is easy. Thus, in comparison with cell lines characterized by nonadherent cells, MESO-1 cells are more advantageous when developing an evaluation method for highly biocompatible MWCNTs in vitro. The decrease in DJ-1 mRNA expression was time-dependent and statistically significant after day 2 (Figure 3). Our quantitative real-time polymerase chain reaction results indicate approximately 50% reduction in expression, which is larger than that observed in the case of Western blotting. However, this finding is in accordance with the decrease in DJ-1 expression previously demonstrated using the proteomic approach.19
Figure 3.
Time-course analysis of DJ-1 mRNA expression. MESO-1 cells were exposed to 10 μg/mL of HTT2800. RNA was isolated every day for 4 days; mRNA levels of DJ-1 were determined by quantitative real-time polymerase chain reaction and normalized to glyceraldehyde 3-phosphate dehydrogenase.
Notes: n = 3, mean ± standard error of the mean, *P < 0.01.
Duan et al reported that total and reduced DJ-1 expression in human pneumocytes was decreased by hydrogen peroxide. 24 Moreover, we recently demonstrated that purified MWCNTs tended to increase the production of intracellular hydrogen peroxide in human bronchial epithelial cells, one hour and 24 hours after exposure. This was accompanied by the accumulation of other intracellular reactive oxygen species after 24 hours (submitted for publication). MWCNT endocytosis does not occur as early as one hour after exposure. Nevertheless, augmentation of the intracellular hydrogen peroxide level at this time point may indicate the requirement for cells to come into contact with MWCNTs. In the present study, THP-1 cells only contacted MWCNTs, and did not endocytose them. Thus, the decrease in DJ-1 expression may have been caused by an intracellular hydrogen peroxide boost, generated by the contact between THP-1 cells and MWCNTs.
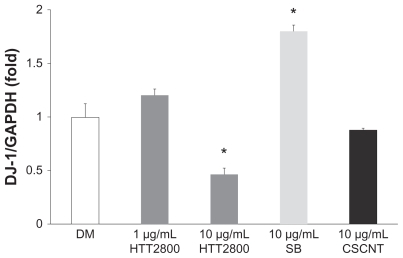
We evaluated the influence of other types of carbon nanomaterials on DJ-1 mRNA levels in MESO-1 cells (Figure 4). Cup-stacked carbon nanotubes did not change the expression level. By contrast, Sumi black increased it by 1.8-fold. At a concentration of 10 μg/mL, neither of the carbon nanomaterials showed cytotoxicity, but both were endocytosed (data not shown). Moreover, the pattern of cytokine secretion in THP-1 cells exposed to Sumi black differed from that observed in THP-1 cells exposed to MWCNTs (submitted for publication). Our results indicate that different physical properties of the carbon nanomaterials used were recognized by the cell membrane, resulting in distinctive responses. Our previous finding that HTT2800 converted the differentiation pathway of C2C12 myoblasts into that of adipoblast-like cells26 further supports this hypothesis.
Figure 4.
DJ-1 mRNA expression after exposure to HTT2800, Sumi black, and cup-stacked carbon nanotubes. MES O-1 cells were exposed to diverse carbon nanomaterials for 4 days; mRNA levels of DJ-1 were determined by quantitative real-time polymerase chain reaction and normalized to G glyceraldehyde 3-phosphate dehydrogenase.
Notes: n = 3, mean ± standard error of the mean, *P < 0.01.
Abbreviations: DM, dispersant medium containing 0.001% gelatin; SB, Sumi black; CSCNT, cup-stacked carbon nanotubes.
DJ-1 exists abundantly in the human body, with extremely diverse functions. However, it remains to be determined whether it has a tissue-specific or organ-specific role. We propose that, by measuring the expression of DJ-1 in blood cells, it may be possible to determine the extent of MWCNT exposure. In the present study, we observed a reduction in DJ-1 protein expression, regardless of the cytotoxic activity of intracellular HTT2800. Moreover, exposure to HTT2800, but not to Sumi black or cup-stacked carbon nanotubes, caused a reduction in DJ-1 mRNA levels. Thus, we conclude that DJ-1 is a novel biomarker for the development of biocompatible MWCNTs.
Acknowledgments
We thank the staff of the Division of Instrumental Analysis at the Research Center for Human and Environmental Sciences of Shinshu University for their help. This research was supported by the Program for Fostering Regional Innovation in Nagano, and a grant-in-aid (No. 19002007) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Saito N, Usui Y, Aoki K, et al. Carbon nanotubes: biomaterial applications. Chem Soc Rev. 2009;38(7):1897–1903. doi: 10.1039/b804822n. [DOI] [PubMed] [Google Scholar]
- 2.Endo M, Strano M, Ajayan P. Potential applications of carbon nanotubes. Carbon Nanotubes. 2008;111:13–61. [Google Scholar]
- 3.Endo M. Grow carbon fibers in the vapor phase. Chemtech. 1988;18(9):568–576. [Google Scholar]
- 4.Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaurand MC, Renier A, Daubriac J. Mesothelioma: Do asbestos and carbon nanotubes pose the same health risk. Part Fibre Toxicol. 2009;6:16. doi: 10.1186/1743-8977-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pacurari M, Castranova V, Vallyathan V. Single- and multi-wall carbon nanotubes versus asbestos: are the carbon nanotubes a new health risk to humans? J Toxicol Environ Health A. 2010;73(5):378–395. doi: 10.1080/15287390903486527. [DOI] [PubMed] [Google Scholar]
- 7.Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 8.Muller J, Huaux F, Moreau N, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207(3):221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Porter DW, Hubbs AF, Mercer RR, et al. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269(2–3):136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Hirano S, Fujitani Y, Furuyama A, Kanno S. Uptake and cytotoxic effects of multi-walled carbon nanotubes in human bronchial epithelial cells. Toxicol Appl Pharmacol. 2010;249(1):8–15. doi: 10.1016/j.taap.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Magrez A, Kasas S, Salicio V, Pasquier N, Schwaller B, Forrò L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6(6):1121–1125. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 12.Muller J, Delos M, Panin N, Rabolli V, Huaux F, Lison D. Absence of carcinogenic response to multiwall carbon nanotubes in a 2-year bioassay in the peritoneal cavity of the rat. Toxicol Sci. 2009;110(2):442–448. doi: 10.1093/toxsci/kfp100. [DOI] [PubMed] [Google Scholar]
- 13.Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5(2):213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2(11):e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res. 2009;87(1):123–129. doi: 10.1002/jnr.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Naour F, Misek DE, Krause MC, et al. Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001;7(11):3328–3335. [PubMed] [Google Scholar]
- 17.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer-and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103(41):15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 19.Haniu H, Matsuda Y, Takeuchi K, Kim YA, Hayashi T, Endo M. Proteomics-based safety evaluation of multi-walled carbon nanotubes. Toxicol Appl Pharmacol. 2010;242(3):256–262. doi: 10.1016/j.taap.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Haniu H, Matsuda Y, Usui Y, et al. Toxicoproteomic evaluation of carbon nanomaterials in vitro. J Proteomics. 2011 Mar 23; doi: 10.1016/j.jprot.2011.03.004. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 21.Usami N, Fukui T, Kondo M, et al. Establishment and characterization of four malignant pleural mesothelioma cell lines from Japanese patients. Cancer Sci. 2006;97(5):387–394. doi: 10.1111/j.1349-7006.2006.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drexler HG, Otsuka K, Gaedicke G, Minowada J. Changes in isoenzyme profiles during induction of differentiation in human myelomonocytic leukemia cell lines. Cancer Res. 1986;46(12 Pt 1):6078–6082. [PubMed] [Google Scholar]
- 23.Tsukahara T, Haniu H. Cellular cytotoxic response induced by highly purified multi-wall carbon nanotube in human lung cells. Mol Cell Biochem. 2011;352(1–2):57–63. doi: 10.1007/s11010-011-0739-z. [DOI] [PubMed] [Google Scholar]
- 24.Duan X, Kelsen SG, Merali S. Proteomic analysis of oxidative stress-responsive proteins in human pneumocytes: insight into the regulation of DJ-1 expression. J Proteome Res. 2008;7(11):4955–4961. doi: 10.1021/pr800295j. [DOI] [PubMed] [Google Scholar]
- 25.Lev N, Ickowicz D, Melamed E, Offen D. Oxidative insults induce DJ-1 upregulation and redistribution: implications for neuroprotection. Neurotoxicology. 2008;29(3):397–405. doi: 10.1016/j.neuro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Tsukahara T, Haniu H. Nanoparticle-mediated intracellular lipid accumulation during C2C12 cell differentiation. Biochem Biophys Res Commun. 2011;406:558–563. doi: 10.1016/j.bbrc.2011.02.090. [DOI] [PubMed] [Google Scholar]