Abstract
Leishmaniasis is a protozoan vector-borne disease and is one of the biggest health problems of the world. Antileishmanial drugs have disadvantages such as toxicity and the recent development of resistance. One of the best-known mechanisms of the antibacterial effects of silver nanoparticles (Ag-NPs) is the production of reactive oxygen species to which Leishmania parasites are very sensitive. So far no information about the effects of Ag-NPs on Leishmania tropica parasites, the causative agent of leishmaniasis, exists in the literature. The aim of this study was to investigate the effects of Ag-NPs on biological parameters of L. tropica such as morphology, metabolic activity, proliferation, infectivity, and survival in host cells, in vitro. Consequently, parasite morphology and infectivity were impaired in comparison with the control. Also, enhanced effects of Ag-NPs were demonstrated on the morphology and infectivity of parasites under ultraviolet (UV) light. Ag-NPs demonstrated significant antileishmanial effects by inhibiting the proliferation and metabolic activity of promastigotes by 1.5- to threefold, respectively, in the dark, and 2- to 6.5-fold, respectively, under UV light. Of note, Ag-NPs inhibited the survival of amastigotes in host cells, and this effect was more significant in the presence of UV light. Thus, for the first time the antileishmanial effects of Ag-NPs on L. tropica parasites were demonstrated along with the enhanced antimicrobial activity of Ag-NPs under UV light. Determination of the antileishmanial effects of Ag-NPs is very important for the further development of new compounds containing nanoparticles in leishmaniasis treatment.
Keywords: nanotechnology, leishmaniasis, Leishmania, parasite
Introduction
Leishmaniasis is one of the serious health problems of the world. It is currently endemic in 98 countries worldwide. Overall annual prevalence of the disease is approximately 12 million people and the size of the population at risk is approximately 350 million. It is estimated that approximately 1.5 million new cases of cutaneous leishmaniasis and 500,000 cases of visceral leishmaniasis occur worldwide each year.1,2 Furthermore, incidents of leishmaniasis are reported to be on the rise because of an increase in the number of vectors of the disease due to global warming.3,4
Among several drugs used in the treatment of leishmaniasis, pentavalent anti-monials, considered a gold standard in the treatment, are known to be very toxic to humans.5–9 Resistance to antileishmanial drugs has also been observed in Leishmania parasites.10 Furthermore, as the drugs used in treatment are so expensive, their use is mostly limited in undeveloped and developing countries. Ineffectiveness of drugs against several species of Leishmania is another disadvantage.11 For these reasons, new approaches in the treatment of leishmaniasis are urgently needed.12,13
Currently, the interest in nanotechnological approaches in microbiology is growing rapidly, as in all fields of the science.14 The main motive is the expectation that nanoparticles will be able to be used in the treatment of various diseases in the future.15–17 In recent studies it was determined that through their unique properties and large surface areas, metal oxide nanoparticles possess effective antimicrobial activities.18 Particularly, owing to their great chemical reactivity, nanoparticles are capable of producing reactive oxygen species (ROS), which have the ability to kill infectious agents.
The antibacterial and antiviral behaviors of silver, silver ions, and silver-containing compounds have long been investigated.19–21 The catalytic oxidation caused by metallic silver and reactions with dissolved single-valent silver ions are thought to contribute to its bactericidal effects within bacterial cells. However, some bacteria have recently shown resistance to the action of silver compounds, similar to resistance to traditional antibiotics.22 On the other hand, promising results were recently reported with respect to the antimicrobial effect of nanoparticles by themselves or within composites.23,24 Several studies have reported that silver nanoparticles (Ag-NPs) showed high efficacy in inactivating bacteria and viruses.25–28
As mentioned, the basis for the antimicrobial effect of nanoparticles is their ability to produce ROS.29 Leishmania parasites are also known to be sensitive to ROS.30 Macrophages, being host cells for Leishmania parasites, can produce high amounts of ROS in order to kill microbial agents.31 However, this production is prevented by Leishmania through the inhibition of the enzymatic mechanism responsible for producing ROS, and Leishmania can survive inside macrophages without being exposed to any damage.32 Therefore, it may be posited that in order to inhibit Leishmania parasites with a ROS-based treatment, these oxygen derivatives must be produced in a physical way such as through Ag-NPs, instead of in an enzymatic way that can be blocked by parasites. The authors hypothesize that Ag-NPs, with their unique properties including the ability to produce high amounts of ROS independently of the host cells, can be used as an effective agent in the treatment of leishmaniasis.
In a previous study, Butkus et al33 explored the effects of silver ions, obtained from silver nitrate (AgNO3, Merck, Darmstadt, Germany), and ultraviolet (UV) light on the bacterial virus MS2 and demonstrated that while silver ions and UV together exert a synergistic effect in inactivating viruses, UV light by itself did not have such an effect. The present authors consider that the antimicrobial effects of Ag-NPs, which are capable of releasing silver ions, may be enhanced by irradiation with UV light. However, no examples of an antimicrobial effect of Ag-NPs under UV light can be found in the literature.
Therefore, the aim of this study was to investigate in vitro antileishmanial effects of Ag-NPs on morphology and various cellular biological activities including growth, metabolic activity, proliferation, infectivity, and infection index of Leishmania tropica parasites both in the presence and in the absence of UV light. Results showed that Ag-NPs demonstrate an antileishmanial effect alone by inhibiting all biological activities of L. tropica parasites and that this effect was increased further under UV light.
Material and methods
Preparation and characterization of Ag-NPs
To produce Ag nanopowder, initially sodium hydroxide solution (1 mol/L, 300 mL, [Merck, Darmstadt, Germany] and AgNO3 solution (0.5 mol/L, 500 mL) were mixed with the help of magnetic stirring. As a result, a brown precipitate with pH 10 was observed. Following this, 5 mL of sodium dodecyl sulfate solution (Merck, Darmstadt, Germany) 1 mol/L was added and mixed until a homogeneous solution was obtained. Consequently, 3 mL of hydrazine hydrate (Merck, Hohenbrunn, Germany) was added drop-wise. After the washing step with distilled water, the solution was dried at 80°C for 48 hours and finally was calcined at 350°C for 2 hours.
The solution of Ag-NPs (1 mg/mL) was prepared in distilled water. The solution was mixed by vortexing for 1–2 minutes and was sonicated for 30 minutes in order to disperse aggregates. After sonication, the solution was autoclaved and was again mixed by vortexing before each use.
The collected Ag-NPs were characterized by transmission electron microscopy and their size and morphology were assessed. For characterization, nanoparticle powders were diluted in pure acetone and the prepared suspension was ultrasonicated. The suspension was put drop-wise on carbon- coated copper grids and dried. Transmission electron microscopy characterization of the Ag-NPs was performed using a JEOL-1010 microscope (Jeol Korea Ltd, Korea).
To measure the hydrodynamic size of the nanoparticles in dynamic light scattering (DLS), 100 μL of the particle solution was diluted with 1.5 mL of water and placed into the cuvette of a Zetasizer Nano instrument (Malvern Instruments Ltd, Philadelphia, PA). Experiments were conducted in triplicate to obtain an average number-size distribution. The same apparatus was used to measure zeta potentials.
Cell culture
L. tropica promastigotes (MHOM/TR/99/EP39) were cultured in RPMI-1640 medium with L-glutamine (Sigma, St Louis, MO) supplemented with 10% fetal calf serum (FCS) (Sigma, St Louis, MO). Parasites were passaged weekly by transferring 3 × 105 promastigotes from previous culture to 5 mL of RPMI-1640 plus 10% FCS medium. The cultivation of J774 macrophage cells (kindly obtained from Professor Ayhan Bilir, Istanbul University, School of Medicine, Histology and Embryology Department) was maintained in RPMI-1640 medium with L-glutamine plus 10% FCS. Macrophage cells (5 × 105 cells/mL) were added to a flask containing 5 mL of RPMI-1640 plus 10% FCS medium and the flask was incubated at 37°C. Cells were passaged weekly.
Giemsa staining
Parasite cultures (10–20 μL) that had been exposed to Ag- NPs in test tubes in the dark and under UV light were spread on microscope slides. These slides were then fixed in methanol for 5 minutes. After fixation, Giemsa stain (Sigma, St Louis, MO) was applied to the slides for 45 minutes and they were then examined with an inverted microscope (magnification 20×) (Olympus CK40; Olympus, Japan).
5-diphenyl-tetrazolium bromide assay
The effects of different concentrations of Ag-NPs (25, 50, 100, 150, and 200 μg/mL) on the viability of L. tropica parasites in the dark and under UV light were determined by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay. For each determination, two microplates (TPP, Zurich, Switzerland) were used. After the addition of different concentrations of Ag-NPs into the wells, one of the microplates was exposed to UV light (36 W PL-L; Philips, Somerset, NJ) while the other was held in the dark for 1 hour. The microplates were then incubated at 27°C for 48 hours. After incubation, 10 μL of MTT solution (10 mg/mL) was added to all of the wells and the microplates were then incubated at 27°C for 4 hours. Generation of formazan crystals was observed microscopically while cell viability values were determined quantitatively using a microplate reader (Fluoroskan Ascent, Thermo Labsystems, Helsinki, Finland) at 570 nm.
Determination of effects of Ag-NPs on proliferation of parasites
Determinations were carried out in culture of L. tropica promastigotes. Every assay tube contained 3 mL of medium with 1 × 106 parasites/mL of L. tropica promastigotes. Assays were divided into two groups: the first group was exposed to UV light, whereas the second group was held in the dark. Control Leishmania culture was not exposed to Ag-NPs. Exposure to UV light lasted for 1 hour, and following that, each test tube was incubated at 27°C. Parasites were counted by hemocytometer (Marienfeld, Germany) at 24, 48, and 72 hours of incubation.
Determination of effects of Ag-NPs on infectivity of parasites
To investigate the infectivity of L. tropica promastigotes exposed to Ag-NPs (100 μg/mL), J774 cells (5 × 104) were seeded into a six-well plate (TPP, Zurich, Switzerland) that had coverslips within each well. At the same time, Leishmania parasites (1 × 106 parasites/mL) were seeded into six experimental tubes. Three of the tubes were exposed to Ag-NPs (100 μg/mL): the first tube was kept under the dark condition; the second was kept under visible light; and the last one was exposed to UV light. The remaining three tubes were used as controls. After 24 hours of incubation, 5 × 105 promastigotes from the experiment were added to each well of the six-well plate with a ratio of 10 promastigotes to each macrophage. The plate was then incubated at 37°C for 3–4 hours. After incubation, each well of the plate was rinsed with 1–2 mL phosphate-buffered saline (PBS) (Sigma, St Louis, MO) in order to remove non-phagocytized promastigotes. The plate was then incubated again at 37°C overnight and 24 hours later each sample from the experimental plates was stained with Giemsa. Infectivity was determined using an inverted microscope (magnification 100×).
Cytotoxicity evaluation
For the cytotoxicity assay, a suspension of 2.5 × 105 J774 macrophages in RPMI-1640 medium, supplemented with 10% FCS and 1% penicillin (10000 UI/mL)/streptomycin (10 mg/mL) were added to each well of 96-well plates. The plates were incubated at 37°C for 24 hours for adhesion of macrophages to the bottom of the well. The nonadherent cells were removed by washing with the RPMI-1640 medium. Then different concentrations of Ag-NPs (in the range from 1 to 500 μg/mL) were added to wells and one of the plates was held in dark, while the other was exposed to UV light. After 30 minutes, the plates were incubated at 37°C for 48 hours. Then 10 μL of MTT was added to each well at concentration of 10 mg/mL, followed by incubation for 4 hours. After that, 100 μL of dimethyl sulfoxide (AppliChem, Darmstadt, Germany) was added to each well and the contents of each well were homogenized for 15 minutes with subsequent determination of the absorbance at 570 nm.
Antileishmanial activity against amastigotes
Ag-NPs that were found to eliminate L. tropica promastigotes in the initial microscope-based studies were further tested to compare their leishmanicidal activities with amastigotes. J774 macrophage cells 2, 5 × 105 cells/mL were seeded in 24-well plates and allowed to adhere for 4 hours at 37°C and 5% CO2. Nonadherent cells were washed with PBS, and the macrophages were incubated overnight in RPMI-1640 media. Adherent macrophages were infected with L. tropica promastigotes at a parasite/macrophage ratio of 10:1 and incubated at 37°C in 5% CO2 for 4 hours. Free promastigotes were removed by washing the wells with PBS and nanoparticles in different dilutions (in the range of 1 to 200 μg/mL) were then added to the wells. After 24 hours, chamber slides were fixed with methanol, stained with 10% Giemsa, and examined using oil-immersion light microscopy. The number of infected macrophages and the average number of parasites per macrophage was determined in 200 cells. The results were expressed as the infection index, which is the percentage of infected macrophages multiplied by the average number of amastigotes per macrophage.
Data processing and statistics
All experiments were repeated at least three times in triplicate wells. The results were expressed as the mean plus or minus the standard deviation. The Levene test was used to determine the homogeneity of the variances. Parametric tests (Student’s t-test, analysis of variance) were used to evaluate the results for significant differences. Data were analyzed using SPSS software (v 16.0 for Windows; SPSS Inc, Chicago, IL) and P < 0.05 values were considered statistically significant.
Results
Characterization of Ag-NPs
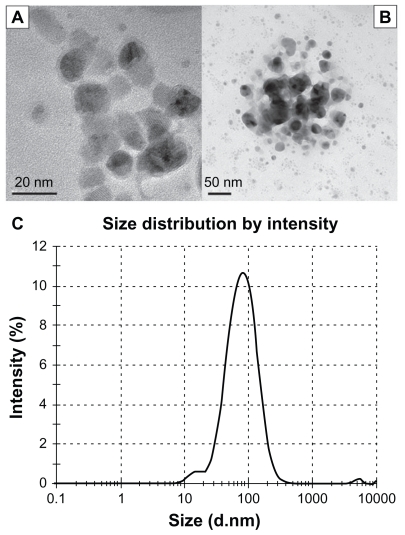
The particle size analysis and distribution of the nanoparticles was performed by transmission electron microscopy and DLS analysis. Transmission electron microscope (TEM) images and results of the DLS analysis are presented in Figure 1. TEM images of the aqueous dispersion show the particle size to be in the range of 10–40 nm (Figure 1A and B). It is also clearly seen that Ag-NPs were round-shaped with a smooth surface morphology and tended to form clumps. DLS analysis of an aqueous dispersion revealed the formation of nanoparticles with an average hydrodynamic diameter of approximately 65 nm (Figure 1C).
Figure 1.
Characterization of silver nanoparticles: (A) transmission electron microscope (TEM) image at the scale of 20 nm, (B) TEM image at the scale of 50 nm, and (C) size distributions with dynamic light scattering analysis.
Effects of Ag-NPs on morphology, metabolic activity, proliferation, and infectivity of L. tropica promastigotes
Morphology
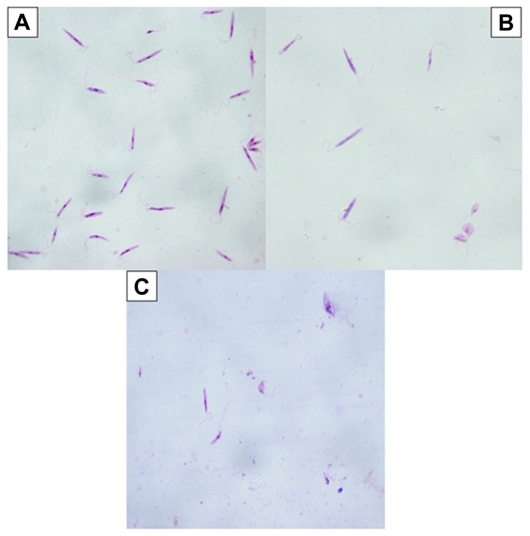
The promastigotes in the control group not exposed to Ag- Nps or UV light were seen to retain their shape and organelles (nucleus, kinetoplast, flagella) when viewed after Giemsa staining (Figure 2A). However, when exposed to Ag-NPs in the dark, L. tropica promastigotes lost their shape and their internal organelles were not distinguishable (Figure 2B). Similarly, when exposed to Ag-NPs in UV light, promastigotes had an atypical appearance, owing to disruption of their membranes (Figure 2C). Furthermore, as seen in Figure 2B and C, the number of cells decreased significantly, whether exposed to Ag-NPs in the dark or to Ag-NPs in UV light, when compared with the control groups.
Figure 2.
Microscopic view of parasites (A) in the control group (not exposed to silver nanoparticles [Ag-NPs]), (B) in the group exposed to Ag-NPs in the dark, and (C) in the group exposed to Ag-NPs under ultraviolet light (Giemsa staining, magnification 100×).
Metabolic activity (viability)
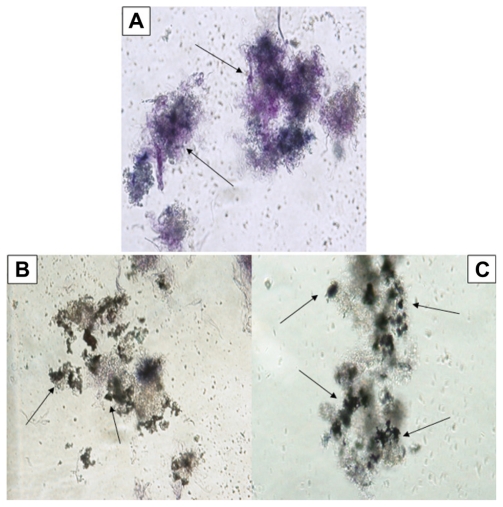
The effect of Ag-NPs on metabolic activity of L. tropica promastigotes at different concentrations (25, 50, 100, 150, and 200 μg/mL) was determined microscopically and quantitatively by the MTT method in the dark and under UV light. Figure 3A shows the occurrence of formazan crystals (which are the signature of metabolic activity) in the control group held in the dark. Formazan crystal formation in promastigotes was very intense under these conditions. However, exposure to Ag-NPs in the dark resulted in the formation of fewer formazan crystals than in the control group. A low rate of formazan crystal formation was especially noticeable on exposure to nanoparticle concentrations of 150 μg/mL (Figure 3B) and 200 μg/mL (Figure 3C).
Figure 3.
Microscopic views of the effects of silver nanoparticles (Ag-NPs) on metabolic activity of Leishmania tropica promastigotes in the dark: (A) parasites in the control group (not exposed to Ag-NPs), (B) the cytopathic effects of Ag-NPs at 150 μg/mL, and (C) the cytopathic effects of Ag-NPs at 200 μg/mL. Arrows in (A) indicate the formation of formazan crystals and in (B) and (C) indicate the clusters of Ag-NPs (magnification 40×).
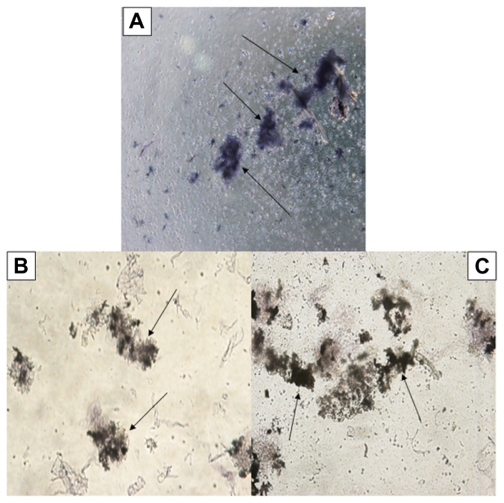
Similar to exposure in the dark, metabolic activity of the L. tropica promastigotes decreased after exposure to nanoparticles under UV light. Even though high amounts of formazan crystals were formed when parasites were exposed to UV light alone (Figure 4A), in experimental groups when parasites were exposed to high concentrations of Ag-NPs (150 μg/mL and 200 μg/mL) in addition to UV light, the rate of formation of formazan crystals was very low (Figure 4B and C).
Figure 4.
Microscopic views of the effects of silver nanoparticles (Ag-NPs) on metabolic activity of Leishmania parasites under ultraviolet light: (A) parasites in the control group (not exposed to Ag-NPs), (B) the cytopathic effects of Ag-NPs at 150 μg/mL, and (C) the cytopathic effects of Ag-NPs at 200 μg/mL. Arrows in (A) indicate the formation of formazan crystals and in (B) and (C) indicate the clusters of Ag-NPs (magnification 40×).
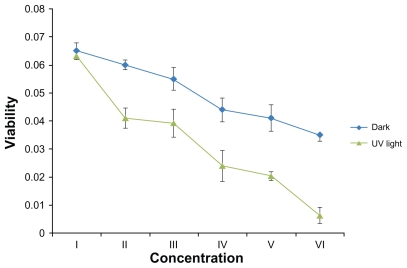
The quantitative effects of Ag-NPs on the metabolic activity of L. tropica promastigotes were also studied. The effects of different concentrations of Ag-NPs in dark conditions are shown in Figure 5: with rising concentrations of Ag-NPs, the metabolic activity of L. tropica parasites decreased. In comparison with the control group, it was obvious that each concentration of Ag-NPs negatively affected the metabolic activity of L. tropica promastigotes. As a result, Ag-NPs showed the biggest suppressive effect on parasites at a concentration of 200 μg/mL, and at the same concentration the metabolic activity values of L. tropica promastigotes decreased nearly twofold, in contrast to the control group (P < 0.05).
Figure 5.
The effects of different concentrations of silver nanoparticles on metabolic activity of Leishmania tropica promastigotes both in the absence and in the presence of ultraviolet (UV) light (I, control group; II, 25 μg/mL; III, 50 μg/mL; IV, 100 μg/mL; V, 150 μg/mL; VI, 200 μg/mL).
Results of the study on the effects of Ag-NPs on the metabolic activity of L. tropica promastigotes under UV light showed that the cell viability values also decreased gradually with the increase in Ag-NP concentrations (Figure 5). The biggest effect on the metabolic activity of promastigotes under UV light was seen in the highest concentration of Ag- NPs (200 μg/mL). These results demonstrated a different effect of Ag-NPs on cell viability of L. tropica promastigotes under the dark condition and under application of UV light. Compared with the control group, the metabolic activity values of the parasites exposed to high concentrations of Ag-NPs decreased approximately twofold in the dark and 6.5-fold under UV light (both P values <0.05).
Proliferation
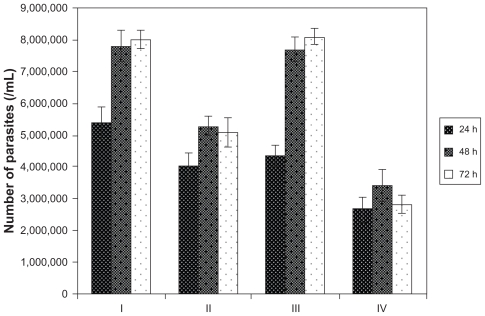
The effects of Ag-NPs on the proliferation of L. tropica promastigotes were investigated in the dark and under UV light. Since the authors observed that metabolic activity values of parasites decreased conversely with the increase in the concentration of Ag-NPs, in this experiment the authors used a concentration of 200 μg/mL of Ag-NPs, which demonstrated the most significant effect on metabolic activity. To make determinations, promastigotes were counted in control and experimental groups at 24, 48, and 72 hours of incubation. In Figure 6, a significant decrease in the number of parasites is seen in each group, in comparison with the control groups, after exposure to Ag-NPs. This effect was observed during all analyzed incubation periods. As Figure 6 clearly shows, the effects of Ag-NPs on the proliferation of promastigotes under UV light were more pronounced than they were in the dark (P < 0.05). In the experimental group exposed to both Ag-NPs and UV light, the number of parasites decreased nearly threefold compared with the control group held under UV light (P < 0.05). These results are an indicator of the effects of Ag-NPs on the growth of Leishmania parasites in the dark and especially under UV light.
Figure 6.
Effects of silver nanoparticles (Ag-NPs) on the proliferation of Leishmania tropica promastigotes in the dark and under ultraviolet (UV) light in different incubation periods (I, parasites in control group in the dark; II, parasites exposed to Ag-NPs in the dark; III, parasites in control group exposed to UV light; IV, parasites exposed to Ag-NPs under UV light). Ag-NPs had significant effects on Leishmania parasites in the experiment groups compared with the control groups. The biggest effect was observed in the experiment group exposed to Ag-NPs and UV light.
Infectivity
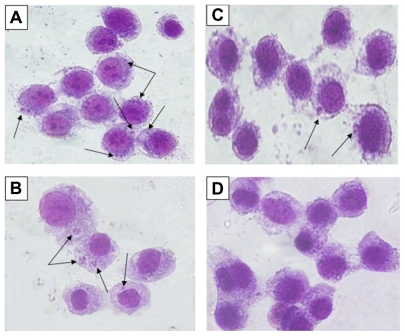
The effects of Ag-NPs on infectivity of L. tropica parasites were also investigated, since infectivity is one of the important biological parameters of Leishmania parasites. Figure 7A shows that the promastigotes not exposed to nanoparticles or UV light had infected macrophages actively, and, consequently, promastigotes had transformed into amastigote forms. Similarly, promastigotes infected the macrophages in the control group exposed to UV light (Figure 7B). However, Figure 7C shows that L. tropica promastigotes exposed to Ag-NPs in the dark infected the macrophages less efficiently than they did in the control groups, while the promastigotes exposed to Ag-NPs with UV light (Figure 7D) did not infect the macrophages. These results indicate that infectivity ratios of L. tropica promastigotes decrease in association with their exposure to non-UV-exposed Ag-NPs, but they are totally inhibited when they were faced with UV-exposed Ag-NPs.
Figure 7.
Microscopic views of macrophages infected or not infected by parasites after being stained with Giemsa. Leishmania tropica promastigotes (A) in the control group held in the dark and (B) in the control group exposed to ultraviolet (UV) light infected the macrophages. (C) L. tropica promastigotes exposed to silver nanoparticles (Ag-NPs) in the dark also infected macrophages, but their infectivity was very low compared with the control groups. (D) L. tropica promastigotes exposed to Ag-NPs under UV light did no infect the macrophages. (Arrows indicate macrophage-infected parasites.)
Effects of Ag-NPs on L. tropica amastigotes
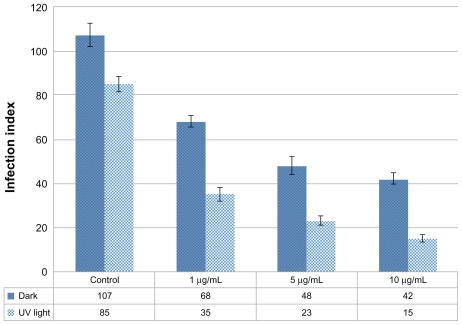
Primarily, toxicity assay for Ag-NPs was conducted on macrophage cells in order to determine nontoxic concentrations of Ag-NPs. It was found that low concentrations such as 1, 5, and 10 μg/mL had no toxic effects on macrophages in the dark or under UV light (data not shown). Therefore, the authors tested the antileishmanial effects of these nontoxic concentrations on L. tropica amastigotes that infected the macrophages, both in the presence and in the absence of UV light. According to infection index values, in dark conditions the inhibitory effects of Ag-NPs on amastigotes showed a concentration-dependent increase compared with the control groups (Figure 8). The infection index of amastigotes in the control group held in the dark was 107, while it was 68, 48, and 42 for amastigotes exposed to 1, 5, and 10 μg/mL of Ag-NPs, respectively. Results were similar in the presence of UV light, and the infection index inversely correlated with Ag-NP concentrations. Also, it was demonstrated that Ag-NPs had more inhibitory effect on amastigotes under UV light when compared with dark conditions (Figure 8). Infection index values for amastigotes were evaluated as 35, 23, and 15 when amastigotes were exposed to 1, 5, and 10 μg/mL of Ag-NPs, respectively, while it was 85 for the control group exposed to UV light.
Figure 8.
Effects of different concentrations of silver nanoparticles on the infection index of Leishmania tropica amastigotes both in the absence and in the presence of ultraviolet (UV) light.
Discussion
The present study is the first to investigate the antileishmanial effects of UV-exposed and non-UV-exposed Ag-NPs on various cellular biological parameters of L. tropica parasites. It was determined that both UV-exposed and non-UV-exposed Ag-NPs demonstrated antileishmanial effects by inhibiting growth, metabolic activity, and infectivity of promastigotes by preventing survival of amastigotes inside host cells. It was also determined that this antileishmanial effect increased further under UV light. Despite there being several studies about their antibacterial, antiviral, and antifungal activities, no study has been reported regarding the antileishmanial effect of Ag-Nps on L. tropica parasites. Moreover, although there are limited numbers of studies demonstrating the synergistic antibacterial and antiviral effects of silver ions and UV light together, this study is the first to show increased antimicrobial effects of Ag-NPs under UV light.
Some researchers have investigated the antibacterial effects of Ag-NPs. It has been shown that Ag-NPs have different mechanisms for killing bacteria cells. Ag-NPs have the ability to bind strongly to sulfur- and phosphor-containing compounds34 and, because of this property, they can damage the bacterial cell membrane by impairing sulfur-containing proteins. Ag-NPs can penetrate the bacteria and can damage sulfur-containing enzymes and phosphor-containing DNA. Ag-NPs are also known to accumulate heavily within mitochondria and are reported to impair mitochondrial function via oxidative stress.35,36 It is well known that adenosine triphosphate (ATP) synthesis ceases when mitochondrial enzymes are damaged. Therefore, it is thought that their antibacterial effects are due to the ability to inhibit ATP synthesis. Another antimicrobial mechanism of Ag-NPs is the releasing of silver ions.37,38 These ions contribute to cell death by producing high amounts of ROS.39,40 After penetrating the bacterial cytoplasm, silver ions also form complexes with cysteine groups of enzymes. These complexes prevent the enzymatic functions of proteins. According to this mechanism, silver ions exhibit antibacterial effects.41–45
The main reason for using Ag-NPs in this study was their capacity to produce ROS, which Leishmania parasites are known to be susceptible to. Also, the authors hypothesized that their wide surface areas, small sizes, and their ability to bind sulfur- and phosphor-containing groups may lead to an increase in their antileishmanial effects. In parallel with this opinion, it was observed that Ag-NPs decreased the metabolic activity and proliferation values of parasites compared with the control groups. However, more extensive effects were seen in Leishmania parasites exposed to Ag-NPs and UV light together.
Butkus et al33 explored for the first time the synergistic effects of silver ions and UV light together on a RNA virus, and demonstrated that RNA viruses were not affected by UV light only but by silver ions and UV light giving rise to inactivation of viruses together. In light of this study, Kim et al46 explored the synergistic effects of silver ions and UV light on E. coli. It was demonstrated that UV irradiation substantially increased the effect of silver ions for inactivation of bacteria cells. It was suggested that this synergism was bound to the impact of UV light on silver-cysteine complexes. It was posited that silver-cysteine complexes irradiated by UV light would generate monosulfide radicals. These radicals can react with the other functional components and this enhances their impact on bacterial cell damage.46 On the other hand, while titanium dioxide nanoparticles gain photocatalytic features and have enhanced antimicrobial effects under UV light, reports on studies examining activation of Ag-NPs by exposure to UV light are lacking.47,48 However, in the present study, the authors first determined that the effects on parasites of Ag-NPs with UV light were more than the effects of Ag-NPs by themselves. These effects were demonstrated on various biological parameters relating to the parasite. Since Ag-NPs are known to have the ability to release silver ions, the authors consider the reason for Ag-NPs showing greater effects under UV light may be the demonstration of synergistic effects of silver ions released from Ag-NPs with UV light. Released silver ions may make complexes with cysteine groups of enzymes and proteins within parasites, and UV irradiation may also lead to monosulfur radical generation from the complex. This may lead to extra damage within parasites, on top of that shown by silver ions alone within bacteria or viruses.
The results also show that live parasites lose their infection abilities following exposure to nanoparticles. This appears more significantly in parasites exposed to both Ag- NPs and UV light. The authors consider the reason for this is the interaction of Ag-NPs with the surface of parasites. It may be posited that Ag-NPs impair the structure of lipophosphoglycan and glycoprotein 63 molecules that are found on the surface of parasites and which are responsible for the infection. The authors also propose that these molecules may be more seriously affected from ROS generated more intensively because of interactions between Ag-NPs and UV light, as described, and this may lead to inhibition of parasite infection. This result indicates that Ag-NPs, alone or with UV light, can protect the host cells from Leishmania infection and make the parasites harmless, even though the parasites are still alive. Therefore, the authors consider a new approach, one that includes the application of Ag-NPs on vectors that carry infective Leishmania promastigotes, may be offered in the near future, and that this may prevent the disease from spreading.
Results also demonstrated that Ag-NPs had inhibitory effects on amastigote forms of Leishmania parasites even in low concentrations that had no toxic effects on macrophages. Interestingly, Ag-NP concentrations that had no antileishmanial effects on promastigotes significantly inhibited the survival of amastigotes inside host macrophage cells. This indicates that amastigotes are more sensitive to Ag-NPs than promastigotes. The authors consider that it can be related to the action of macrophages following nanoparticle exposure. Macrophages may produce more lytic enzymes or nitric oxide to inhibit Leishmania parasites, depending on their interactions with nanoparticles. Also, it may be posited that amastigotes exposed to nanoparticles may not negatively affect the ROS production by macrophages, and that these host cells may contribute to inhibition of parasites by releasing more ROS in addition to the efficacy mechanism of nanoparticles. The results clearly show there is no need to apply high concentrations of Ag-NPs for inhibiting amastigotes, and this may be very promising for the treatment of leishmaniasis.
Conclusion
This study was the first time that Ag-NPs were determined to possess antileishmanial effects on L. tropica parasites through examining their effects on various cellular parameters of promastigote and amastigote forms. Results demonstrate that use of Ag-Nps may represent a future alternative to current antileishmanial drugs. Since leishmaniasis is spreading rapidly worldwide and because antileishmanial drugs have several disadvantages, the authors posit that treatment based on Ag-NPs may have a very important role in overcoming leishmaniasis. For this reason, it is suggested that new studies on this issue are urgently required. Additionally, the authors believe that determination of enhanced antiparasitic effects of Ag-NPs under UV light may be very useful for further antimicrobial applications, as this combination provides more effective elimination of infectious agents such as Leishmania parasites.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization. Leishmaniasis. [Accessed January 16, 2010]. Available from: http://www.who.int/leishmaniasis/en/
- 2.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27(5):305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Peterson AT, Shaw J. Lutzomyia vectors for cutaneous leishmaniasis in Southern Brazil: ecological niche models, predicted geographic distributions, and climate change effects. Int J Parasitol. 2003;33(9):919–931. doi: 10.1016/s0020-7519(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 4.Hemmer CJ, Frimmel S, Kinzelbach R, Gürtler L, Reisinger EC. Global warming: trailblazer for tropical infections in Germany? Dtsch Med Wochenschr. 2007;132(48):2583–2589. doi: 10.1055/s-2007-993101. German. [DOI] [PubMed] [Google Scholar]
- 5.Berman JD. Chemotherapy for leishmaniasis: biochemical mechanisms, clinical efficacy, and future strategies. Rev Infect Dis. 1988;10(3):560–586. doi: 10.1093/clinids/10.3.560. [DOI] [PubMed] [Google Scholar]
- 6.Croft SL. Recent development in the chemotherapy of leishmaniasis. Trends Pharmacol Sci. 1988;9(10):376–381. doi: 10.1016/0165-6147(88)90258-1. [DOI] [PubMed] [Google Scholar]
- 7.Herwaldt BL, Berman JD. Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am J Trop Med Hyg. 1992;46(3):296–306. doi: 10.4269/ajtmh.1992.46.296. [DOI] [PubMed] [Google Scholar]
- 8.Aronson NE, Wortmann GW, Johnson SC, et al. Safety and efficacy of intravenous sodium stibogluconate in the treatment of leishmaniasis: recent US military experience. Clin Infect Dis. 1998;27(6):1457–1464. doi: 10.1086/515027. [DOI] [PubMed] [Google Scholar]
- 9.Seaton RA, Morrison J, Man I, Watson J, Nathwani D. Out-patient parenteral antimicrobial therapy: a viable option for the management of cutaneous leishmaniasis. QJM. 1999;92(11):659–667. doi: 10.1093/qjmed/92.11.659. [DOI] [PubMed] [Google Scholar]
- 10.Natera S, Machuca C, Padrón-Nieves M, Romero A, Diaz E, Ponte-Sucre A. Leishmania spp: proficiency of drug-resistant parasites. Int J Antimicrob Agents. 2007;29(6):637–642. doi: 10.1016/j.ijantimicag.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Croft SL, Coombs GH. Leishmaniasis: current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19(11):502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Ouellette M, Drummelsmith J, Papadopoulou B. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist Updat. 2004;7(4–5):257–266. doi: 10.1016/j.drup.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Sundar S, Rai M, Chakravarty J, et al. New treatment approach in Indian visceral leishmaniasis: single-dose liposomal amphotericin B followed by short-course oral miltefosine. Clin Infect Dis. 2008;47(8):1000–1006. doi: 10.1086/591972. [DOI] [PubMed] [Google Scholar]
- 14.Curtis A, Wilkinson C. Nantotechniques and approaches in biotechnology. Trends Biotechnol. 2001;19(3):97–101. doi: 10.1016/s0167-7799(00)01536-5. [DOI] [PubMed] [Google Scholar]
- 15.Angeli E, Buzio R, Firpo G, et al. Nanotechnology applications in medicine. Tumori. 2008;94(2):206–215. doi: 10.1177/030089160809400213. [DOI] [PubMed] [Google Scholar]
- 16.Swai H, Semete B, Kalombo L, Chelule P, Kisich K, Sievers B. Nanomedicine for respiratory diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(3):255–263. doi: 10.1002/wnan.33. [DOI] [PubMed] [Google Scholar]
- 17.Debbage P. Targeted drugs and nanomedicine: present and future. Curr Pharm Des. 2009;15(2):153–172. doi: 10.2174/138161209787002870. [DOI] [PubMed] [Google Scholar]
- 18.Elechiguerra JL, Burt JL, Morones JR, et al. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnology. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tokumaru T, Shimizu Y, Fox CL., Jr Antiviral activities of silver sulfadiazine and ocular infection. Res Commun Chem Pathol Pharmacol. 1974;8(1):151–158. [PubMed] [Google Scholar]
- 20.Oka H, Tomioka T, Tomita K, Nishino A, Ueda S. Inactivation of enveloped viruses by a silver-thiosulfate complex. Met Based Drugs. 1994;1(5–6):511. doi: 10.1155/MBD.1994.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oloffs A, Grosse-Siestrup C, Bisson S, Rinck M, Rudolph R, Gross U. Biocompatibility of silver-coated polyurethane catheters and silver-coated Dacron material. Biomaterials. 1994;15(10):753–758. doi: 10.1016/0142-9612(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 22.Chopra I. The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J Antimicrob Chemother. 2007;59(4):587–590. doi: 10.1093/jac/dkm006. [DOI] [PubMed] [Google Scholar]
- 23.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Li J, Wu C, Wu Q, Li J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology. 2005;16:1912–1917. [Google Scholar]
- 25.Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18:1–9. doi: 10.1088/0957-4484/18/22/225103. [DOI] [PubMed] [Google Scholar]
- 26.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73(6):1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu L, Sun RW, Chen R, et al. Silver nanoparticles inhibit hepatitis B virus replication. Antivir Ther. 2008;13(2):253–262. [PubMed] [Google Scholar]
- 28.Lara HH, Ayala-Nunez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnology. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi O, Hu Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol. 2008;42(12):4583–4588. doi: 10.1021/es703238h. [DOI] [PubMed] [Google Scholar]
- 30.Murray HW. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J Exp Med. 1981;153(5):1302–1315. doi: 10.1084/jem.153.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lodge R, Descoteaux A. Phagocytosis of Leishmania donovani amastigotes is Rac1 dependent and occurs in the absence of NADPH oxidase activation. Eur J Immunol. 2006;36(10):2735–2744. doi: 10.1002/eji.200636089. [DOI] [PubMed] [Google Scholar]
- 32.Mehta A, Shaha C. Mechanism of metalloid-induced death in Leishmania spp: role of iron, reactive oxygen species, Ca2+, and glutathione. Free Radic Biol Med. 2006;40(10):1857–1868. doi: 10.1016/j.freeradbiomed.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Butkus MA, Labare MP, Starke JA, Moon K, Talbot M. Use of aqueous silver to enhance inactivation of coliphage MS-2 by UV disinfection. Appl Environ Microbiol. 2004;70(5):2848–2853. doi: 10.1128/AEM.70.5.2848-2853.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng J, Wu X, Wang M, Ran D, Xu W, Yang J. Study on the interaction between silver nanoparticles and nucleic acids in the presence of cetyltrimethylammonium bromide and its analytical application. Talanta. 2008;74(4):526–532. doi: 10.1016/j.talanta.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Xia T, Kovochich M, Brant J, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6(8):1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 36.AshaRani PV, Low Kah, Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3(2):279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 37.Song HY, Ko KK, Oh IH, Lee BT. Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur Cell Mater. 2006;11(Suppl 1):58. [Google Scholar]
- 38.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52(4):662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Sambhy V, MacBride MM, Peterson BR, Sen A. Silver bromide nanoparticle/polymer composites: dual action tunable antimicrobial materials. J Am Chem Soc. 2006;128(30):9798–9808. doi: 10.1021/ja061442z. [DOI] [PubMed] [Google Scholar]
- 40.Ratte HT. Bioaccumulation and toxicity of silver compounds: a review. Environ Toxicol Chem. 1999;18(1):89–108. [Google Scholar]
- 41.Bragg PD, Rainnie DJ. The effect of silver ions on the respiratory chain of Escherichia coli. Can J Microbiol. 1974;20(6):883–889. doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- 42.Liau SY, Read DC, Pugh WJ, Furr JR, Russell AD. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett Appl Microbiol. 1997;25(4):279–283. doi: 10.1046/j.1472-765x.1997.00219.x. [DOI] [PubMed] [Google Scholar]
- 43.Matsumura Y, Yoshikata K, Kunisaki S, Tsuchido T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl Environ Microbiol. 2003;69(7):4278–4281. doi: 10.1128/AEM.69.7.4278-4281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell AD, Hugo WB. Antimicrobial activity and action of silver. Prog Med Chem. 1994;31:351–370. doi: 10.1016/s0079-6468(08)70024-9. [DOI] [PubMed] [Google Scholar]
- 45.Thurman RB, Gerba CP, Bitton G. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. CRC Crit Rev Environ Contr. 1989;18(4):295–315. [Google Scholar]
- 46.Kim JY, Lee C, Cho M, Yoon J. Enhanced inactivation of E. coli and MS-2 phage by silver ions combined with UV-A and visible light irradiation. Water Res. 2008;42(1–2):356–362. doi: 10.1016/j.watres.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Tsuang YH, Sun JS, Huang YC, Lu CH, Chang WH, Wang CC. Studies of photokilling of bacteria using titanium dioxide nanoparticles. Artif Organs. 2008;32(2):167–174. doi: 10.1111/j.1525-1594.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu P, Duan W, Wang Q, Li X. The damage of outer membrane of Escherichia coli in the presence of TiO2 combined with UV light. Colloids Surf B Biointerfaces. 2010;78(2):171–176. doi: 10.1016/j.colsurfb.2010.02.024. [DOI] [PubMed] [Google Scholar]