Abstract
Background
Nanomaterials have unique advantages in controlling stem cell function due to their biomimetic characteristics and special biological and mechanical properties. Controlling adhesion and differentiation of stem cells is critical for tissue regeneration.
Methods
This in vitro study investigated the effects of nano-hydroxyapatite, nano-hydroxyapatite-polylactide- co-glycolide (PLGA) composites, and a bone morphogenetic protein (BMP-7)- derived short peptide (DIF-7c) on osteogenic differentiation of human mesenchymal stem cells (MSC). The peptide was chemically functionalized onto nano-hydroxyapatite, incorporated into a nanophase hydroxyapatite-PLGA composite or PLGA control, or directly injected into culture media.
Results
Unlike the PLGA control, the nano-hydroxyapatite-PLGA composites promoted adhesion of human MSC. Importantly, nano-hydroxyapatite and nano-hydroxyapatite-PLGA composites promoted osteogenic differentiation of human MSCs, comparable with direct injection of the DIF-7c peptide into culture media.
Conclusion
Nano-hydroxyapatite and nano-hydroxyapatite-PLGA composites provide a promising alternative in directing the adhesion and differentiation of human MSC. These nanocomposites should be studied further to clarify their effects on MSC functions and bone remodeling in vivo, eventually translating to clinical applications.
Keywords: human mesenchymal stem cells, osteogenesis, stem cell differentiation, bone morphogenetic protein, peptide delivery, nanocomposites
Introduction
Nanomaterials for bone regeneration
Nanomaterials have demonstrated promising capabilities in stimulating cell function and enhancing tissue regeneration due to their biomimetic features and unique physicochemical, mechanical, and biological properties. Nanomaterials can be composed of metals, ceramics, polymers, and composites, with at least one dimension less than 100 nm. Natural bone is a nanocomposite, which contains nanoscale building blocks comprising mainly collagen fibrils and mineral hydroxyapatite plates. Biodegradable and bioactive nanocomposites are attractive for orthopedic and maxillofacial applications due to their advantageous properties for better bone tissue regeneration. Nanocomposites not only provide better surface and physicochemical properties for osteoblast attachment and long-term function (collagen and calcium synthesis), but also have better mechanical properties (elastic modulus and strength) for certain load-bearing conditions.1–4
Nanophase hydroxyapatite, a derivative of calcium phosphate, is chemically and structurally similar to natural bone minerals and is biodegradable. Additionally, increased new bone formation has been observed as early as 2 weeks on nano-hydroxyapatite-coated tantalum scaffolds compared with micron-sized hydroxyapatite-coated scaffolds and uncoated scaffolds when implanted into rat calvarial bone.5 Polylactideco-glycolide (PLGA) has been approved by the US Food and Drug Administration for certain human clinical applications and has been extensively studied for drug delivery.6–8 As a copolymer of polylactic acid and polyglycolic acid, PLGA is a biodegradable polyester, the variable molecular weight and composition of which can be rationally designed to control the release kinetics of encapsulated therapeutic agents. The incorporation of osteoconductive nano-hydroxyapatite into PLGA can improve the mechanical properties and moderate the degradation rate of the scaffold.9–11 Specifically, nano-hydroxyapatite slows the degradation of PLGA. Further, nano-hydroxyapatite-PLGA scaffolds support greater rabbit mesenchymal stem cell (MSC) growth and alkaline phosphatase activity than PLGA scaffolds.12 The addition of nano-hydroxyapatite particles into the PLGA scaffold confers better biological, mechanical, and degradation properties.
Bone morphogenetic proteins and functional peptides for bone regeneration
Bone morphogenetic proteins (BMPs), including rhBMP-2 and BMP-7, are the most potent growth factors for enhancing bone formation.13–16 BMPs have several hundred amino acids, approximately 2–3 nm in size, depending on their conformation. The complex secondary structure of BMPs renders them prone to degradation, and they tend to lose their bioactivity quickly in aqueous physiological conditions. A short functional peptide derived from the active regions of the BMP has the potential to increase the efficacy of BMP delivery. Short peptides can attach to drug carriers more efficiently due to their small size while still maintaining functionality and bioactivity. Chen and Webster investigated three short peptides derived from the bioactive regions of BMP-7.17 These three peptides were composed of 10 amino acids and were designated as peptide A (SNVILKKYRN), B (KPCCAPTQLN), and C (AISVLYFDDS). The results showed that peptide B increased osteoblast proliferation, while peptides A and C promoted osteoblast differentiation (eg, mineralization).17 In this study, peptide C was chosen as the model peptide because of its ability to promote osteoblast differentiation and was slightly modified for ease of chemical functionalization. The BMP-7-derived short peptide was either directly injected into cell culture media or delivered by nano-hydroxyapatite, PLGA, or nanocomposites to study the effects on human MSC function.
Human MSC for bone regeneration
Human MSCs are desirable cells for bone tissue regeneration due to their capability of self-renewal and potential for osteogenic differentiation.18,19 Specific bioactive growth factors, nutrients, and environmental cues can direct human MSC differentiation into the mesodermal lineage, including osteoblasts, chondrocytes, and adipocytes.20 BMP-2 and BMP-7 can induce osteoblast-like genes and matrix mineralization in primary human MSC culture.21 Importantly, the biological and mechanical properties of scaffold materials play important roles in the attachment and differentiation of human MSC.
The objective of this study is to investigate the effects of nano-hydroxyapatite, PLGA, and nano-hydroxyapatite- PLGA composites on bone marrow-derived human MSC functions in comparison with a short functional peptide of BMP-7.
Materials and methods
Synthesis of nanocrystalline hydroxyapatite
Nanophase hydroxyapatite was synthesized by wet chemistry precipitation followed by hydrothermal treatment to gain control over desirable grain sizes and crystallinities. Briefly, hydroxyapatite was precipitated by mixing solutions of calcium nitrate and diammonium hydrogen phosphate in an alkaline pH region.22 Specifically, a 1 M calcium nitrate solution and a 0.6 M ammonium phosphate solution were prepared by dissolving their respective solid state powders in deionized water separately. The ammonium phosphate solution produced was mixed with deionized water, which was adjusted to pH 10 by ammonium hydroxide. The premade 1 M calcium nitrate solution was then added into the mixture of ammonium phosphate and ammonium hydroxide at a rate of 3.6 mL/minute. Precipitation occurred as soon as the calcium nitrate was added. Chemically, the hydroxyapatite precipitation occurred through the reaction:
| (1) |
Precipitation was continued for 10 minutes at room temperature with constant stirring. The supernatant was collected, centrifuged (Eppendorf centrifuge, model 5810 R) to reduce 75% of the solution volume, and placed into a 125 mL Teflon liner (Parr Instruments, Moline, IL). The Teflon liner was sealed tightly in a Parr 4748 acid digestion bomb (Parr Instruments) and treated hydrothermally at 200°C for 20 hours to obtain nanocrystalline hydroxyapatite. Hydrothermal treatment has a great advantage for preparing a stoichiometric, ultrafine hydroxyapatite powder with a homogeneous shape and size distribution due to higher than atmospheric applied pressures.23,24 After hydrothermal treatment, the nano-hydroxyapatite particles were rinsed with deionized water and dried in an oven at 80°C for 12 hours.
The size and morphology of the nano-hydroxyapatite particles have been previously characterized using field emission scanning electron microscopy. Specifically, nano-hydroxyapatite had an average particle size of 36 nm as determined by image analysis of scanning electron micrographs.22,25 The calcium to phosphate ratio of nano-hydroxyapatite was 1.67 according to energy dispersive x-ray analysis.25 X-ray diffraction confirmed that the nano-hydroxyapatite was crystalline. 26 A full characterization of nano-hydroxyapatite has been published previously.22,25,26
Design and synthesis of model peptide derived from BMP-7
Peptide C (AISVLYFDDS) derived from BMP-7 was modified at its N-terminal with a cysteine-containing spacer to ease chemical conjugation onto the nano-hydroxyapatite particles using aminosilane chemistry, followed by a maleimide cross-linker molecule. In this study, the peptide with a 12 amino-acid sequence of CKAISVLYFDDS was used as the model peptide and called DIF-7c. The peptide DIF-7c was obtained as carboxyl terminal acids to more than 98.2% purity according to the high-pressure liquid chromatography profile provided by the manufacturer (GenScript Corporation, Piscataway, NJ). The molecular weight of the peptide DIF-7c was 1360.56 g/mol.
Immobilization of peptide using aminosilane chemistry
The challenges of peptide delivery lie in efficient loading and controlled release. For peptide loading, nano-hydroxyapatite was functionalized by aminosilane chemistry under dry conditions to avoid surface contamination and, thus, ensure stability of the peptide.26,27 Briefly, nano-hydroxyapatite was first silanized in 3-aminopropyltriethoxysilane in anhydrous hexane. Second, for substituting a hetero-bifunctional cross-linker for the terminal amine, the silanized nano-hydroxyapatite was coupled with N-succinimidyl-3-maleimido propionate (also called 3-maleimidopropionic acid N-hydroxysuccinimide ester, Sigma, St Louis, MO) in anhydrous N,N-dimethylformamide. Third, the peptide DIF-7c was immobilized onto nano-hydroxyapatite in anhydrous dimethylformamide through a reaction between the outer maleimide group with the thiol group of cysteine present in the terminal of DIF-7c. The nano-hydroxyapatite and the peptide DIF-7c conjugates bonded by aminosilane chemistry were called hydroxyapatite-Ps.
Fabrication of nanocomposites and control scaffolds
PLGA pellets were purchased from Polysciences (Warrington, PA) and fabricated into a solid scaffold using the solvent casting method. PLGA pellets had a 50/50 wt% polylactic/glycolide ratio, a molecular weight of 100,000–120,000 g/mol, an intrinsic viscosity of 66–80 cm3/g, a polydispersity of 1.8, a density of 1.8 g/cm3, and a glass transition temperature of 45°C–50°C.2 For the hydroxyapatite- PLGA nanocomposites, nanocrystalline hydroxyapatite (average particle size 36 nm) was added to the PLGA solution to give a 30:70 ceramic to polymer weight ratio. The composite mixture was sonicated for 10 minutes using a Misonix 3000 sonicator (Misonix Inc, New York) with its microtip immersed in the mixture. After sonication, the composite suspension was cast into a Teflon mold, evaporated in air at room temperature for 24 hours, and dried in a vacuum chamber at room temperature for 48 hours. For the peptide-loaded nanocomposites (hydroxyapatite-Ps-PLGA), hydroxyapatite-Ps was dispersed in PLGA using a similar method as for hydroxyapatite-PLGA nanocomposites.
To fabricate the PLGA scaffold with peptide (PLGA-P) as a control, the peptide DIF-7c was dispersed directly in PLGA matrix using the solvent casting method. The amount of peptide dispersed in PLGA was calculated to be the same as the amount used for hydroxyapatite-Ps preparation.
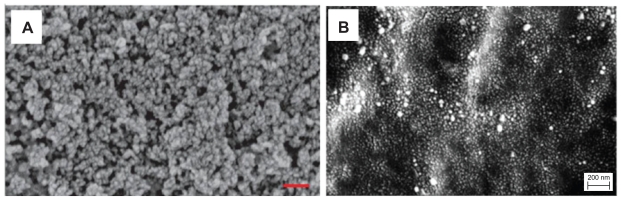
All the nanocomposite and PLGA scaffolds were cut into 1 cm × 1 cm squares for use in the material characterization and in vitro studies. The hydroxyapatite-PLGA and hydroxyapatite-Ps-PLGA nanocomposites had been previously characterized using a field emission scanning electron microscope, as shown in Figure 1.2,25
Figure 1.
Scanning electron micrographs of (A) HA-PLGA (well-dispersed nano-hydroxyapatite in PLGA composite), (B) nano-HA-Ps-PLGA (HA-PLGA nanocomposite with chemically immobilized DIF-7c peptide). Original magnification 100,000×.
Notes: Magnification bars are 200 nm. A is adapted from Liu and Webster25 and B is adapted from Liu and Webster2 with permission of the respective publishers.
Abbreviations: HA, hydroxyapatite; PLGA, polylactide-co-glycolide; DIF-7c, bone morphogenetic protein (BMP-7)-derived short peptide; P, peptide; Ps, peptide loaded by aminosilane chemistry.
In vitro functions of human MSC
Stem cell adhesion and morphology
Human MSCs (Poietics® PT2501, Lonza, Walkersville, MD) were thawed and cultured in MSC growth medium (Lonza) under standard cell culture conditions, ie, a sterile, 37°C, humidified, 5% CO2/95% air environment. MSC growth medium is composed of MSC basal medium (Lonza) plus MSC growth supplements, L-glutamine, and GA-1000 (aqueous solution of gentamicin sulfate and amphotericin B), all from Lonza. The human MSCs were harvested from the posterior iliac crest of the pelvic bone of healthy volunteers according to the Lonza data sheet. Stem cells at the second passage were used for all the cell culture experiments.
All scaffolds of nanocomposites, peptide-loaded nanocomposites, and controls were placed in 12-well tissue culture plates (Corning Inc, Lowell, MA), sterilized under ultraviolet radiation, and then rinsed three times with sterilized phosphate-buffered saline (containing 8 g NaCl, 0.2 g KCl, 1.5 g Na2HPO4, and 0.2 g KH2PO4 in 1000 mL of deionized water adjusted to a pH of 7.4; all chemicals from Sigma). For the bioactive glass control, Thermanox® glass coverslips (Fisher Scientific, Barrington IL) surface-treated for cell culture were utilized. Human MSCs (second passage) were seeded at a density of 5000 cells/cm2 onto the scaffolds of interest and were then incubated in the MSC growth media under standard cell culture conditions for 6 days. After that time period, nonadherent cells were removed by rinsing with phosphate-buffered saline, and adherent cells were then fixed with formaldehyde (Fisher Scientific) and stained with DAPI and Alex Fluor® 488 (Invitrogen, Carlsbad, CA). The cell nuclei and cytoskeleton were visualized under a fluorescence microscope (Leica DM5500B upright) and recorded by an attached digital camera. Cells that adhered onto the surface of specimens were counted manually and confirmed using Image J software. Cell adhesion density was calculated as the average number of cells per centimeter squared. All samples were tested in triplicate.
Stem cell osteogenic differentiation
The human MSCs from Lonza were cultured until the second passage and seeded at a density of 5000 cells/cm2 in MSC growth media into 12-well culture plates with the scaffolds of interest and the controls as listed in Table 1. For the first 6 days, cells were cultured in MSC growth media. After that, the cells were cultured in MSC osteogenic induction media to induce osteogenesis. The total length of cell culture was 40 days and the media were changed every 3 days. Osteogenic induction media is composed of human MSC osteogenic basal media with dexamethasone, L-glutamine, ascorbate, penicillin/streptomycin, mesenchymal cell growth supplement, and β-glycerophosphate (Lonza). At the end of the prescribed time period, the human MSCs were lysed using three freeze-thaw cycles in distilled water. Alkaline phosphatase activity and calcium deposition in the supernatant were determined according to standard protocols as described below. All experiments were run in triplicate and repeated at three separate times.
Table 1.
Osteogenic differentiation of human mesenchymal stem cells was studied on the scaffolds and controls as listed below
| Label | Abbreviation | Description | |
|---|---|---|---|
| Nanocomposite | 1 | HA-Ps-PLGA | Nano-HA-PLGA composites loaded with peptide using aminosilane chemistry |
| 2 | HA-PLGA | Nano-HA-PLGA without peptide | |
| Nanophase HA | 3 | HA-Ps | Nano-HA loaded with peptide using aminosilane chemistry |
| 4 | HA | Nano-HA without peptide | |
| Polymer control | 5 | PLGA-P | PLGA scaffold loaded with peptide |
| 6 | PLGA | PLGA scaffold without peptide | |
| Peptide control | 7 | DIF-7c | The DIF-7c peptide was directly injected into the cell culture media |
| Reference | 8 | Glass | Thermanox® surface treated borosilicate glass coverslip |
| 9 | PSTC | Polystyrene tissue culture plate |
Abbreviations: HA, hydroxyapatite; PLGA, polylactide-co-glycolide; DIF-7c, bone morphogenetic protein (BMP-7)-derived short peptide; P, peptide; Ps, peptide loaded by aminosilane chemistry.
Alkaline phosphatase is an enzyme, the production of which signifies increased osteogenic differentiation of human MSC.28 An alkaline phosphatase assay kit ( QuantiChromTM, DALP-250, BioAssay Systems, Hayward, CA) was used to determine alkaline phosphatase activity in the cell lysates prepared as described above. Briefly, aliquots of 50 μL cell lysates were mixed with a 150 μL working solution of magnesium acetate and p-nitrophenyl phosphate in assay buffer. Then, the reaction was read at time = 0 and time = 4 minutes using a spectrophotometer (Spectra MAX 190, Molecular Devices, San Jose, CA) at 405 nm. Alkaline phosphatase activity of the samples (IU/L) was calculated using the equation established in the assay data sheet.
The ultimate indicator of human MSC differentiation is calcium deposition, which was quantified using a commercial calcium assay kit (QuantiChromTM, DICA-500, BioAssay Systems). Briefly, calcium-containing supernatant from human MSC culture was mixed with the working reagent, incubated for 3 minutes, and the optical density was read at 612 nm using a spectrophotometer (Spectra MAX 190, Molecular Devices). Total calcium was calculated from standard curves of absorbance versus known concentrations of calcium standards run in parallel with the experimental samples. Calcium concentration values were normalized by substrate area and expressed as μg/cm2.
Statistical analysis
Experiments were conducted in triplicate and repeated at least three times. Numerical data were statistically analyzed using the standard analysis of variance technique, and statistical significance was accepted at P < 0.05.
Results
Stem cell adhesion
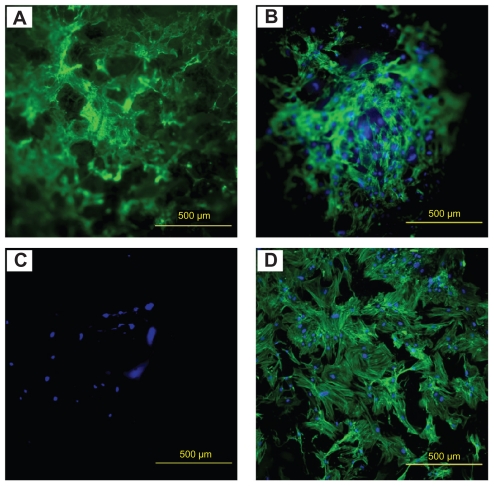
The fluorescence images suggest that human MSCs attached onto all substrates, as shown in Figure 2. On the hydroxyapatite-Ps-PLGA and hydroxyapatite-PLGA nanocomposite scaffolds, human MSCs not only attached to but also distributed three-dimensionally throughout the scaffolds, as shown in Figure 2A and B. In comparison with the nanocomposites, much fewer human MSCs adhered onto the PLGA control, as shown in Figure 2C. Cell adhesion on PLGA-P was not found (image not shown). The human MSCs started to spread into the three-dimensional matrix when the nanocomposites and PLGA scaffolds degraded. On the borosilicate glass reference, the human MSCs attached and spread out on the two-dimensional surface and showed well pronounced actin stress fibers in Figure 2D.
Figure 2.
Fluorescence images of human MSC adhesion on (A) HA-Ps-PLGA, (B) HA-PLGA, (C) PLGA, and (D) glass after 6 days of culture.
Notes: Scale bar = 500 μm. Green stains, F-actin cytoskeleton. Blue stains, nucleus of human MSC.
Abbreviations: HA, hydroxyapatite; PLGA, polylactide-co-glycolide; P, peptide; MSC, mesenchymal stem cells; Ps, peptide loaded by aminosilane chemistry.
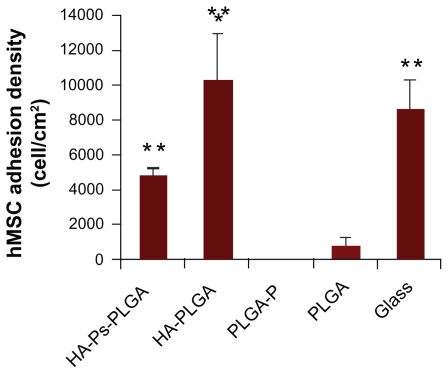
The adhesion density of human MSC after 6 days of standard cell culture was quantified and is summarized in Figure 3. Comparing the nanocomposites, human MSC adhesion density was greater on the hydroxyapatite-PLGA scaffold than on the hydroxyapatite-Ps-PLGA scaffold. The human MSC adhesion density was much lower on the PLGA and PLGA-P scaffolds compared with the nanocomposites. This indicates that incorporation of nanophase hydroxyapatite into the PLGA scaffold could enhance human MSC adhesion. There was no human MSC adhesion detected on the PLGA-P scaffold. The human MSCs adhered to the surface of the glass control at a similar density to that of hydroxyapatite-PLGA. The larger amount of human MSCs adhered on the glass control was expected because the glass surface was plasma-treated for better cell adhesion.
Figure 3.
Human MSC (hMSC) adhesion density was calculated as cells per centimeter squared on the scaffolds of interest and controls.
Notes: Data are presented as the mean ± standard error of the mean (n = 3). *P < 0.05 compared with HA-Ps-PLGA and **P < 0.05 compared with PLGA-P and PLGA.
Abbreviations: HA, hydroxyapatite; PLGA, polylactide-co-glycolide; DIF-7c, bone morphogenetic protein (BMP-7)-derived short peptide; P, peptide; Ps, peptide loaded by aminosilane chemistry.
The increased cell adhesion density after 6 days compared with an initial seeding density of 5000 cells/cm2 also indicated human MSC proliferation. On the hydroxyapatite-Ps-PLGA and hydroxyapatite-PLGA nanocomposites, the average cell density was 4708 cells/cm2 and 10,193 cells/cm2, respectively. Incorporation of the peptide into the nanocomposites did not increase cell adhesion or proliferation. On the PLGA-P and PLGA scaffolds, the cell adhesion density was significantly lower as compared with that of the nanocomposites. There were no detectable cells on the PLGA-P scaffold. On the bioactive glass control, the average density of human MSC increased to 8508 cells/cm2 after 6 days of culture, which was about 1.7 times that of the initial seeding density of human MSC.
Stem cell osteogenic differentiation
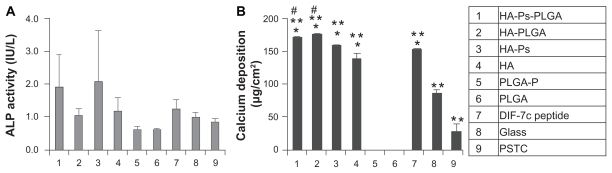
Alkaline phosphatase activity of the human MSC on the scaffolds of interest after 40 days of culture was quantified, as shown in Figure 4A. When comparing hydroxyapatite-Ps-PLGA with hydroxyapatite-PLGA, although the average alkaline phosphatase activity of the human MSC on the hydroxyapatite-Ps-PLGA was greater than on hydroxyapatite-PLGA, statistical significance was not detected between the nanocomposites with or without the DIF-7c peptide. Similarly, there was no statistically significant increase in the alkaline phosphatase activity of human MSC cultured with hydroxyapatite-Ps as compared with hydroxyapatite without the DIF-7c peptide. The incorporation of nano-hydroxyapatite or hydroxyapatite-Ps in PLGA slightly increased the average alkaline phosphatase activity of human MSC when compared with PLGA alone, but this was not statistically significant. There was no statistically significant difference in the alkaline phosphatase activity of human MSC when hydroxyapatite-Ps was dispersed in the PLGA or when it was used alone. The alkaline phosphatase activity of human MSC was very similar on the PLGA-P and PLGA scaffolds, and there were no statistically significant differences. When the DIF-7c peptide was directly injected into culture media, the average alkaline phosphatase activity of human MSC was slightly greater than the glass and polystyrene tissue culture plate (PSTC) references, but the difference was not statistically significant. To determine the human MSC osteogenic differentiation further, calcium deposition was quantified in conjunction with alkaline phosphatase activity.
Figure 4.
Osteogenic differentiation of human MSCs was characterized by (A) ALP activity and (B) calcium deposition. The human MSCs were cultured with the materials as listed in the column.
Notes: Data are presented as the mean ± standard error of the mean (n = 3). No statistical difference was found for ALP activity. For calcium deposition, *P < 0.05 compared with glass and PSTC (8 and 9), **P < 0.05 compared with PLGA-P and PLGA (5 and 6), and #P < 0.05 compared with DIF-7c peptide (7).
Abbreviations: ALP, alkaline phosphatase; HA, hydroxyapatite; PLGA, polylactide-co-glycolide; P, peptide; MSC, mesenchymal stem cells; DIF-7c, bone morphogenetic protein (BMP-7)-derived short peptide; Ps, peptide loaded by aminosilane chemistry.
Calcium deposition by human MSC is an important indicator of osteogenic differentiation. Calcium deposition per area of the scaffolds after 40 days of culture is shown in Figure 4B. No statistically significant difference in calcium deposition was detected between hydroxyapatite-Ps-PLGA and hydroxyapatite-PLGA. Similarly, the calcium deposition results did not show any statistically significant difference between hydroxyapatite-Ps and hydroxyapatite. Although the average calcium deposition per area was greater on the nanocomposites than on the nano-hydroxyapatite, it was not statistically significant. For the PLGA-P and PLGA scaffolds, there was no detectable calcium deposition. When the DIF-7c peptide was directly injected into the cell culture media, the calcium deposition per area was similar to that of nano-hydroxyapatite. This indicates that the effects of nano-hydroxyapatite on osteogenic differentiation of human MSC were similar to that of the peptide. The nano-hydroxyapatite-containing materials, including hydroxyapatite-Ps-PLGA, hydroxyapatite-PLGA, hydroxyapatite-Ps, and hydroxyapatite, have demonstrated very similar or slightly greater calcium deposition when compared with the peptide delivered by direct injection, and they showed much greater calcium deposition than PLGA and PLGA-P. Calcium deposition on the nanocomposites with or without peptide, on the nano-hydroxyapatite with or without peptide, and under direct injection of DIF-7c peptide was much greater than on the glass and PSTC references. The human MSCs deposited more calcium on the glass than on the PSTC plate.
Discussion
Nanomaterials have unique advantages for tissue engineering and drug delivery applications due to their biomimetic characteristics and unique biological and mechanical properties. Due to their smaller size, the BMP-7 derived short functional peptide, DIF-7c, can maintain bioactivity for a longer period in comparison with the large BMP-7 protein. The results of this study demonstrated that nanophase hydroxyapatite and nanocomposites (nano-hydroxyapatite in PLGA composites) can induce osteogenic differentiation of human MSC, similar to DIF-7c peptide.
Human MSC adhesion
Nanocomposites improved human MSC adhesion as compared with polymer scaffolds. Specifically, there was a higher human MSC attachment on both hydroxyapatite-Ps-PLGA and hydroxyapatite-PLGA scaffolds than on PLGA. PLGA showed less human MSC adhesion due to its inherent material properties and its acidic degradation products.29 When PLGA degrades, the degradation products, lactic acid and glycolic acid, increase the local pH, which has adverse effects on human MSC adhesion and proliferation.30 It has been reported that nanophase hydroxyapatite promoted osteoblast adhesion and long-term function.31,32 Moreover, nano-hydroxyapatite can positively mediate and slow down the degradation of PLGA scaffolds because the presence of nano-hydroxyapatite in the PLGA matrix interferes with water and oligomer diffusion in and out of the scaffolds. Therefore, as expected, human MSC adhesion increased when nano-hydroxyapatite was dispersed in PLGA.
However, incorporation of the DIF-7c peptide into the scaffolds did not increase human MSC adhesion. For example, the cell adhesion density on hydroxyapatite-Ps-PLGA was lower than on hydroxyapatite-PLGA. Two factors, ie, surface morphology and binding affinity, were speculated to affect human MSC adhesion. Scanning electron micrographs of the hydroxyapatite-PLGA and hydroxyapatite-Ps-PLGA showed that they have different surface morphologies (Figure 1).2,25 Specifically, the hydroxyapatite-PLGA contained more noticeable nanofeatures, whereas the hydroxyapatite-Ps-PLGA had a relatively smoother surface. These nanofeatures can enhance human MSC adhesion, and this might explain why greater human MSC adhesion was observed on hydroxyapatite-PLGA than on hydroxyapatite-Ps-PLGA. BMPs have knuckle epitopes that are thought to bind to specific BMP receptors in the cell membrane.33 The knuckle epitope of BMP-7 contains 20 amino acids (TVPKPCCAPTQLNAISVLYF); however, the DIF-7c peptide only contains seven end amino acids of the knuckle epitope (CKAISVLYFDDS).33 The DIF-7c peptide contained an incomplete knuckle epitope, which might have decreased the binding affinity. Similarly, previous research showed that there was no significant increase in osteoblast adhesion in the presence of the BMP-7 short peptide C (AISVLYFDDS).17 Both surface morphology and binding affinity may have contributed to the significant decrease of human MSC adhesion density on hydroxyapatite-Ps-PLGA as compared with the hydroxyapatite-PLGA nanocomposite.
Osteogenic differentiation of human MSC
Osteoinductive biomaterials could stimulate stem cells to differentiate towards the osteogenic pathway. Both alkaline phosphatase activity and calcium deposition were important factors to determine the osteogenic differentiation of human MSC. Calcium deposition by human MSC increased in the presence of nano-hydroxyapatite and nano-hydroxyapatite-PLGA composites and when the DIF-7c peptide was directly injected into the media. Additionally, no significant difference in calcium deposition was found between nano-hydroxyapatite with or without DIF-7c peptide and between the nanocomposites with or without DIF-7c peptide. This indicates that the effects of nano-hydroxyapatite on the osteogenic differentiation of human MSC are well pronounced and nanohydroxyapatite can enhance osteogenesis even in the absence of the peptide. Previous studies on nano-hydroxyapatite showed similar results. For example, enhanced calcium deposition by osteoblasts was observed on nano-hydroxyapatite in comparison with micron-hydroxyapatite.22,31
The results of this study also indicate that incorporation of nano-hydroxyapatite into nanocomposite scaffolds could promote both adhesion and osteogenic differentiation of human MSC. DIF-7c peptide can promote osteogenic calcium deposition when directly injected. Therefore, the applications of nano-hydroxyapatite and nano-hydroxyapatite in polymer composites are promising alternatives in directing the functions of human MSC.
Conclusion
Nanocomposites not only improved adhesion of human MSC but also promoted their osteogenic differentiation. Incorporation of nano-hydroxyapatite within bioabsorbable PLGA scaffolds played an important role in promoting human MSC adhesion and differentiation. Direct injection of DIF-7c peptide into the culture media resulted in a similar calcium deposition level as compared to the nano-hydroxyapatite and nanocomposites, which was greater than the glass and PSTC references. However, DIF-7c peptide functionalization to nano-hydroxyapatite and into nanocomposites did not show any significant effect in promoting adhesion and human MSC osteogenic differentiation when compared with nano-hydroxyapatite and nanocomposites without peptide. When both the nano-hydroxyapatite and the DIF-7c peptide are present in human MSC culture, the enhancing effects of nano-hydroxyapatite on the osteogenic differentiation of human MSC were more clearly pronounced than the DIF-7c peptide. It is believed that the effects of the nano-hydroxyapatite may overshadow any effect that the peptide may have on differentiation. The nanocomposites demonstrated promising in vitro results in controlling osteogenic differentiation of human MSC without the help of the peptide. As nano-hydroxyapatite-PLGA composites could promote new bone growth without using the expensive BMPs or BMP-derived peptides, their effects on human MSC and bone tissue regeneration should be further studied in vivo for clinical translation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Meng Y, Liu M, Wang SA, et al. Cellular reactions of osteoblast-like cells to a novel nanocomposite membrane for guided bone regeneration. Appl Surf Sci. 2008;255(2):267–269. [Google Scholar]
- 2.Liu H, Webster TJ. Ceramic/polymer nanocomposites with tunable drug delivery capability at specific disease sites. J Biomed Mater Res A. 2010;93(3):1180–1192. doi: 10.1002/jbm.a.32614. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Slamovich EB, Webster TJ. Increased osteoblast functions among nanophase titania/poly(lactide-co-glycolide) composites of the highest nanometer surface roughness. J Biomed Mater Res A. 2006;78(4):798–807. doi: 10.1002/jbm.a.30734. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Webster TJ. Nanomedicine for implants: A review of studies and necessary experimental tools. Biomaterials. 2006;28(2):354–369. doi: 10.1016/j.biomaterials.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 5.Balasundaram G, Webster TJ. Nanotechnology and biomaterials for orthopedic medical applications. Nanomedicine. 2006;1(2):169–176. doi: 10.2217/17435889.1.2.169. [DOI] [PubMed] [Google Scholar]
- 6.Jiang T, Petersen RR, Call G, Ofek G, Gao JZ, Yao JQ. Development of chondroitin sulfate encapsulated PLGA microsphere delivery systems with controllable multiple burst releases for treating osteoarthritis. J Biomed Mater Res Part B Appl Biomater. 2011;97B(2):355–363. doi: 10.1002/jbm.b.31822. [DOI] [PubMed] [Google Scholar]
- 7.Shi XT, Wang YJ, Varshney RR, Ren L, Gong YH, Wang DA. Microsphere-based drug releasing scaffolds for inducing osteogenesis of human mesenchymal stem cells in vitro. Eur J Pharm Sci. 2010;39(1–3):59–67. doi: 10.1016/j.ejps.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Mori M, Isobe M, Yamazaki Y, Ishihara K, Nakabayashi N. Restoration of segmental bone defects in rabbit radius by biodegradable capsules containing recombinant human bone morphogenetic protein-2. J Biomed Mater Res. 2000;50(2):191–198. doi: 10.1002/(sici)1097-4636(200005)50:2<191::aid-jbm14>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Schiller C, Epple M. Carbonated calcium phosphates are suitable pH-stabilising fillers for biodegradable polyesters. Biomaterials. 2003;24(12):2037–2043. doi: 10.1016/s0142-9612(02)00634-8. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Slamovich EB, Webster TJ. Less harmful acidic degradation of poly(lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int J Nanomedicine. 2006;1(4):541–545. doi: 10.2147/nano.2006.1.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Webster TJ. Enhanced biological and mechanical properties of nanophase ceramic in polymer composites with nano-scale dispersion compared to micron-scale agglomeration: from 2D surface to 3D printed structure. Materials Science and Engineering: C. 2011;31(2):77–89. [Google Scholar]
- 12.Huang YX, Ren J, Chen C, Ren TB, Zhou XY. Preparation and properties of poly(lactide-co-glycolide) (PLGA)/nano-hydroxyapatite (NHA) scaffolds by thermally induced phase separation and rabbit MSCs culture on scaffolds. J Biomater Appl. 2008;22(5):409–432. doi: 10.1177/0885328207077632. [DOI] [PubMed] [Google Scholar]
- 13.Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 14.Wang EA, Rosen V, D’Alessandro JS, et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87(6):2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White AP, Vaccaro AR, Hall JA, Whang PG, Friel BC, McKee MD. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31(6):735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31(6):721–727. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Webster TJ. Increased osteoblast functions in the presence of BMP-7 short peptides for nanostructured biomaterial applications. J Biomed Mater Res. 2009;91A(1):296–304. doi: 10.1002/jbm.a.32246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Arvidson K, Abdallah BM, Applegate LA, et al. Bone regeneration and stem cells. J Cell Mol Med. 2011;15(4):718–746. doi: 10.1111/j.1582-4934.2010.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem-cells in bone-development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56(3):283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavery K, Hawley S, Swain P, Rooney R, Falb D, Alaoui-Ismaili MH. New insights into BMP-7 mediated osteoblastic differentiation of primary human mesenchymal stem cells. Bone. 2009;45(1):27–41. doi: 10.1016/j.bone.2009.03.656. [DOI] [PubMed] [Google Scholar]
- 22.Sato M, Sambito MA, Aslani A, Kalkhoran NM, Slamovich EB, Webster TJ. Increased osteoblast functions on undoped and yttrium-doped nanocrystalline hydroxyapatite coatings on titanium. Biomaterials. 2006;27(11):2358–2369. doi: 10.1016/j.biomaterials.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 23.Ioku K, Yoshimura M. Stoichiometric apatite fine single crystals by hydrothermal synthesis. Phosphorus Res Bull. 1991;1:15–20. [Google Scholar]
- 24.Somiya S, Ioku K, Yoshimura M. Hydrothermal synthesis and characterization of fine apatite crystals. Mater Sci Forum. 1988;34:371–378. [Google Scholar]
- 25.Liu H, Webster TJ. Mechanical properties of dispersed ceramic nanoparticles in polymer composites for orthopedic applications. Int J Nanomedicine. 2010;5:299–313. doi: 10.2147/ijn.s9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balasundaram G, Sato M, Webster TJ. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials. 2006;27(14):2798–2805. doi: 10.1016/j.biomaterials.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Hong HG, Jiang M, Sligar SG, Bohn PW. Cysteine-specific surface tethering of genetically-engineered cytochromes for fabrication of metalloprotein nanostructures. Langmuir. 1994;10(1):153–158. [Google Scholar]
- 28.Siffert RS. The role of alkaline phosphatase in osteogenesis. J Exp Med. 1951;93(5):415–429. doi: 10.1084/jem.93.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chastain SR, Kundu AK, Dhar S, Calvert JW, Putnam AJ. Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. J Biomed Mater Res A. 2006;78A(1):73–85. doi: 10.1002/jbm.a.30686. [DOI] [PubMed] [Google Scholar]
- 30.Yang SF, Leong KF, Du ZH, Chua CK. The design of scaffolds for use in tissue engineering. Part 1. Traditional factors. Tissue Eng. 2001;7(6):679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 31.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803–1810. doi: 10.1016/s0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 32.Webster TJ, Siegel RW, Bizios R. Osteoblast adhesion on nanophase ceramics. Biomaterials. 1999;20(13):1221–1227. doi: 10.1016/s0142-9612(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 33.Senta H, Bergeron E, Drevelle O, Park H, Faucheux N. Combination of synthetic peptides derived from bone morphogenetic proteins and biomaterials for medical applications. Can J Chem Eng. 2011;89(2):227–239. [Google Scholar]