Abstract
In addition to being an attractive source for cell replacement therapy, human induced pluripotent stem cells (iPSCs) also have great potential for disease modeling and drug development. During the recent several years, cell reprogramming technologies have evolved to generate virus-free and integration-free human iPSCs from easily accessible sources such as patient skin fibroblasts and peripheral blood samples. Hematopoietic cells from umbilical cord blood banks and Epstein Barr virus (EBV) immortalized B lymphocyte repositories represent alternative sources for human genetic materials of diverse backgrounds. Ability to reprogram these banked blood cells to pluripotency and differentiate them into a variety of specialized and functional cell types provides valuable tools for studying underlying mechanisms of a broad range of diseases including rare inherited disorders. Here we describe the recent advances in generating disease specific human iPSCs from these different types of hematopoietic cells and their potential applications in disease modeling and regenerative medicine.
Key words: induced pluripotent stem cells (iPSCs), blood, B lymphocytes, hematopoietic differentiation, hepatic differentiation, disease modeling, drug testing
Introduction
Like embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) can be expanded in culture for a prolonged time while maintaining their pluripotency (i.e., the ability to differentiate into cell types representing progenies of all three embryonic germ layers). Unlike ESCs, which are derived from the inner cell mass of blastocyst stage embryos, iPSCs can be generated from adult somatic cells, thus having the advantage of being patient-specific. This property of iPSCs provide histocompatibility, and with our ability to generate a variety of specialized cell types from them, iPSCs become an attractive source for cell replacement therapy. More specifically the iPSC-derived donor cells would share the same genetic identity with the recipient patient hence reducing the risk of immune-rejection or the graft-vs.-host disease. Each patient's genetic information, including disease causing mutation(s), can be preserved in the reprogrammed iPSCs and further passed onto the re-differentiated cell types, making it possible to model diseases in either tissue culture dishes or xeno-graft animal models. The conventional reprogramming methods utilize retroviruses to infect fibroblast cells with four reprogramming associated transcription factors, Oct4, Sox2, Klf4 and c-Myc. The reprogramming technologies have also been developed for additional somatic cell types such as blood cells, keratinocytes and hepatocytes.1–14 There have been particular interests in reprogramming blood cells, because blood samples can be easily obtained from patients with a relatively less invasive procedure. What makes reprogramming of blood cells even more attractive is the availability of a large number of samples that have been already stored in banks for cord blood or EBV-immortalized lymphocytes. Here we describe our recent progress in generating disease-specific iPSCs from these blood cell types and the direct differentiation of these iPSCs into specialized and functional cell types.2,3 We also discuss the potentials and limitations of the diversely sourced human iPSCs in regenerative medicine and disease modeling.4–6
Patient-Specific iPSCs Derived from Blood Cells
Human iPSCs have been generated from various types of blood cells including peripheral blood, bone morrow aspirates and umbilical cord blood.1,2,7–14 Initial reports of blood cell reprogramming utilized the traditional retroviruses to infect human CD34+ hematopoietic cells that are enriched for hematopoietic progenitor cells. Human iPSCs containing the hematopoietic cell-restricted somatic mutations associated with myeloproliferative neoplasms (MPNs) were generated by this method.2 Upon directed differentiation back to hematopoietic cells, these MPN-patient specific iPSCs demonstrated the abnormal erythropoiesis similar to that observed in primary hematopoietic progenitor cells from the patient.2 These observations have proved the principle that diseases with an acquired somatic mutation(s) can be modeled through the iPSC generation and differentiation technologies.
The reprogramming technology has been recently improved with several new methods for creating safer, virus-free iPSCs.3,7,17–19 Study has shown that blood cells possess a unique epigenetic signature that is closer to ESC/iPSCs, compared with that of fibroblast to ESC/iPSCs, making them more amenable to reprogramming.7 It has been shown that with a short time of priming, human primary blood cells can be efficiently reprogrammed into integration-free iPSCs using episomal plasmids without the need for enriching hematopoietic progenitors first.7,14
Reprogramming of Human B Lymphocytes into iPSCs
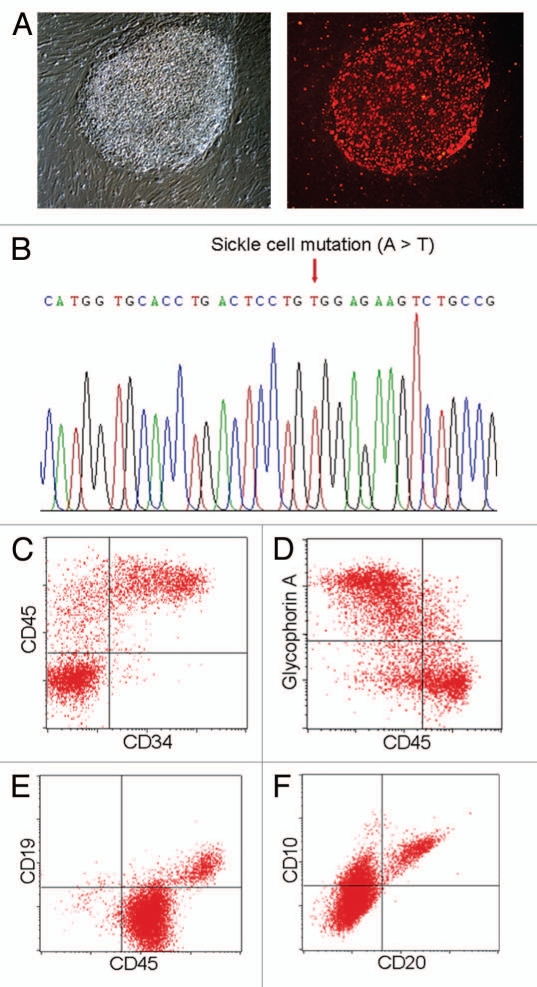
For disease modeling purposes, the EBV-immortalized B cells are of particular interest as a potential source for iPSC generation (Table 1). In past decades, blood cells from a diverse population of patients have been immortalized through infection with EBV.15,16 In addition to storing patient DNA samples and frozen tissues, this immortalization procedure provided a way to preserve and expand cells that contain important genetic information of the patients. Reprogramming of these EBV-immortalized B cells, however, presented a challenge to the traditional reprogramming protocols.3 Similar to mouse B cells, human primary B cells have been difficult to reprogram. Currently there is still no evidence for generating iPSC clones from human B cells, though various studies have reported successful reprogramming of human blood cells including CD34+ cells and T lymphocytes.2,7–14 The underlying mechanisms to the biological phenomenon of why B cells are more refractory to reprogramming are not fully understood. There are also technical challenges that may limit human B cell line reprogramming: (1) The transduction efficiencies of human B lymphocytes (both primary B cells and EBV-immortalized B cells) with traditional retroviruses are extremely low (less than 1% as compared with ∼90% for fibroblasts); (2) The presence of EBV genome in the cells and the expression of EBV-related genes may interfere the reprogramming process; (3) Unlike most primary cell types, the immortalized B cells can extensively proliferate, presenting another challenge to select the reprogrammed clones. To overcome these hurdles, we have utilized an episomal vector system coupled with the nucleofection technology to deliver the reprogramming factors more efficiently into human B cells.3 During the reprogramming process, we also supplemented the culture medium with small molecules such as sodium butyrate, a HDAC inhibitor that has been shown to not only significantly enhance reprogramming efficiency of other types of human cells20 but also to significantly suppress the proliferation of EBV-immortalized B cells.3 Through these improvements, we have successfully generated human iPSCs from EBV-B cells immortalized from multiple patients including those with sickle cell anemia and α 1-antitrypsin deficiency.3 These iPSCs display typical morphology of human ESCs and iPSCs along with expression of markers specific for pluripotent stem cells (Fig. 1A).3 Sequencing analyses confirmed that the EBV-iPSCs carry the same genetic mutations that are specific to the patients (Fig. 1B). The EBV genome that is present in the parental EBV-immortalized B cells was also evidently lost from the cultured iPSCs during the reprogramming and/or iPSC expansion processes.3 This is likely caused by the epigenetic landscape changes during the reprogramming, which may induce silencing of the EBV genes including EBNA-1 which is essential for the replication of EBV genome. Silencing and the consequent loss of EBV genome occurs stochastically during routine culturing of the immortalized B cells, and it is the selection pressure for cell growth that maintains the EBV genome in the proliferating B cells. However, during the process of generating iPSCs, the successfully reprogrammed iPSCs gain indefinite proliferation ability therefore the selection pressure for the presence of EBV genome may no longer exist, which might be an alternative explanation for this phenomenon. These two potential mechanisms are not mutually exclusive and both are likely to contribute to the generation of EBV-free iPSCs.
Table 1.
Comparison of different sources of hematopoietic cells for generating human iPSCs
| Sources for reprogramming | Advantages | Disadvantages | Potential application of iPSCs | |
| Disease modeling | Cell therapy | |||
| Postnatal blood (peripheral blood and bone marrow) |
|
Limited by the availability of patients. | Yes. Patient blood cells are essential for modeling acquired/chronic blood disorders. | Yes. The most accessible sources for patient-specific iPSCs. |
| Umbilical cord blood |
|
Relatively limited cell number per stored sample. | Yes. Can be used for modeling inherited diseases. | Yes. May provide a safer source for cell replacement therapy. |
| EBV-immortalized lymphocytes |
|
Prolonged culture may increase the probability of accumulating additional mutations and/or karyotypic abnormalities. | Yes. Valuable for disease modeling, especially for rare diseases. Samples of deceased patients and their family members are often available. | May not be suitable due to a potentially compromised genomic integrity. |
Figure 1.
Generation and differentiation of patient specific iPSCs from EBV-B cell lines. (A) Human iPSCs derived from a sickle cell anemia patient EBV-immortalized B cells display typical ESC/iPSC morphology (left partI) and ESC-specific cell surface antigen TRA-1–60 (red right partI). (B) Sequencing of the patient iPSCs confirmed the disease-specific point mutation was preserved in the patient iPSC lines. (C) The patient EBV-B-cell derived iPSCs are also capable of directed hematopoietic differentiation. After 14-d hematopoietic differentiation in serum free media using a spin-EB protocol, ∼20% cells express hematopoietic progenitor markers, CD34 and CD45. (D) The iPSC derived hematopoietic progenitor cells can be further differentiated into glycophorin A+ CD45low committed erythroblasts after 5-d culture in the presence of SCF, IL-3 and EPO. (E) The iPSCs also exhibit lymphocyte differentiation capacity in vitro. After 21 d co-culture on OP9 stromal cells in the presence of FL and IL-7, ∼10% CD19+ cells were detected in the culture. These cells also expressed other pre-B-cell markers such as CD20 and CD10 (F).
The successful generation of human iPSCs from the immortalized B cells also raises the question of why human primary B cells could not be reprogrammed at similar efficiencies using the same protocol. On the basis of our experience, primary B cells (obtained from either cord blood or peripheral blood) are far more difficult to transfect compared with fibroblasts or EBV-immortalized B cells.3 In addition, a certain intrinsic epigenetic feature of human primary B cells could make them more refractory to reprogramming using the current methods, as suggested by a mouse study.21 There are still many unknowns about direct cellular reprogramming although studies have started to uncover the mechanisms underlying this process.22–25 A better understanding of the reprogramming mechanisms and future development of new reagents or technologies that can enhance the efficiency of reprogramming factor delivery are required in order to achieve efficient reprogramming of primary B cells.
Directed Differentiation of Human iPSCs Generated from Blood Cells
To utilize patient specific iPSC derived functional cell types for disease modeling and drug screening, effective differentiation technologies of human iPSCs into cells of interest such as hematopoietic, hepatic or neuronal lineage cells are critical. Recently, we have improved the human iPSC differentiation protocols and demonstrated the feasibility of using directed differentiation of patient iPSCs to model both acquired diseases (i.e., polycythemia vera, liver cirrhosis and liver cancer) and inherited diseases (i.e., α1-antitrypsin deficiency and sickle cell anemia);2,3,6 it was feasible to differentiate these patient iPSCs into either hematopoietic progenitors or hepatic cells, and to recapitulate key features of some of these diseases (i.e., polycythemia vera and α 1-antitrypsin deficiency) in a culture dish.2,3 Patient-specific iPSCs derived from EBV-immortalized B cells also showed a similar differentiation capability to those iPSCs from other tissue origin.3,5 In addition to becoming multi-stage hepatic cells under endoderm/hepatic differentiation conditions, these cells also demonstrated the ability to generate CD34+CD45+ hematopoietic stem/progenitor cells following a serum-free embryoid body (EB)-mediated differentiation protocol (Fig. 1C).2 Upon further differentiation, the iPSC-derived hematopoietic progenitor cells, like their primary counterparts, gave rise to more specialized cell types that constitute the lymphohematopoietic system (Fig. 1D–F). When supplemented with a cytokine erythropoietin, the sickle cell anemia iPSCs-derived progenitor cells underwent erythroid differentiation and generated glycophorin A+ CD45low erythroblast (Fig. 1D), demonstrating their potential to model red blood cell diseases.
For future clinical applications of treating patients with immunologic deficiencies or for research applications of creating animal models with humanized immune system, it is essential to direct human iPSCs to lymphoid cells such T, B or NK cells. The lymphoid differentiation potential of the EBV-iPSCs was also accessed using a co-culture differentiation system with OP9 stromal cells. In the presence of added cytokines, such as Flt3-ligand (FL) and interleukine-7 (IL-7), the hematopoietic progenitors derived from iPSCs can differentiate to CD45+CD19+ B cells after 21 d co-culture (Fig. 1E). These cells also express other pre-B cell surface markers such as CD20 and CD10 (Fig. 1F). Together with a recent report in reference 26, these results suggest that human iPSCs derived from diverse sources have the capacity to undergo lymphoid differentiation and have the potential to reconstitute host immune system upon transplantation.
Applications of Blood Origin Human iPSCs from Diverse Sources
With many cell types having been reprogrammed, it is important to evaluate the potentials and limitations of differently sourced human iPSCs in order to take full advantage of this technology.4–6,27–29 Our recent data suggest that immortalized B cells from patients with blood or liver diseases can be reprogrammed into disease-specific iPSCs and further induced to differentiate into cells of interest including early and late developmental stages of both hematopoietic and hepatic lineages (Fig. 1).3 Compared with peripheral blood and cord blood, the EBV-B cells have a unique advantage as a source for generating disease-specific iPSCs for modeling diseases: a large quantity of EBV-B cell lines that reflect a variety of different diseases, including rare inherited disorders, and patients samples with diverse genetic backgrounds (and their family members, even the deceased ones) have been stored in various repositories. It would be highly useful to reprogram these patient derived EBV-B cells for creating various parts of iPSC-based in vitro disease models. Such models will accelerate the pathogenesis study for many rare diseases with unknown mechanisms, and help facilitate the development of novel drug therapy, with a reduced need for recruiting new patients and obtaining fresh tissue samples. Despite the great potential of the EBV-B cell derived human iPSCs in disease modeling and drug development, these iPSCs may not be an ideal choice for cell regeneration therapy (Table 1). This is due to the concern associated with the prolonged culture of these immortalized B cell lines, which may result in a higher number of genetic mutations compared with primary blood samples. The rearranged immunoglobulin loci of these lymphocytes might pose an additional safety concern as it has been shown in mouse, where mice derived from a reprogrammed T cell had a higher incidence of lymphomagenesis.30
Among the different sources of blood, umbilical cord blood might represent the best source for cell replacement therapy (Table 1). These cells are more likely to maintain higher genomic integrity due to the reduced risk of age-related somatic mutations and genomic rearrangements. Although the cell number is usually limited per individual, the unique epigenetic features of the cord blood cells make them one of the easiest cell types to be reprogrammed using virus-free and integration-free protocols.7 Not only can the iPSCs be used clinically for the same individual who had the cord blood banked at birth, the large number of the available samples stored in cord blood banks would also make it more feasible for other patient-in-need to find HLA-matched existing stem cell lines. If no such cell line is available, patient-specific iPSCs can always be generated using patient's own peripheral blood (Table 1). Although new technologies have made the reprogramming of peripheral blood more efficient and the iPSCs safer, caution still needs to be taken when using patient peripheral blood-derived iPSCs for cell therapy purposes. Individuals who suffer from chronic hematopoietic disorders often carry somatic mutations in their blood cells; therefore these iPSCs may not be an ideal choice for therapy and alternative sources should be considered.
The challenge for creating a relatively large part of disease-specific iPSCs from peripheral blood samples to study complex multifactorial diseases is often the limited availability of consent patients with diverse genetic backgrounds. The new technologies to generate patient-specific iPSCs from the banked cells, such as cord blood and EBV-immortalized B cells, will provide alternative and complementary ways to extend the potential of iPSCs in both regenerative medicine and disease modeling.
Acknowledgments
This work was supported by NIH grant 109577 and 5T32HL007525-27 and by MSCRF grants 108563 and 106408.
Abbreviations
- EBV
epstein-barr virus
- ESCs
embryonic stem cells
- iPSCs
induced pluripotent stem cells
- MPNs
myeloproliferative neoplasms
References
- 1.Chun YS, Chaudhari P, Jang YY. Applications of patient-specific induced pluripotent stem cells; focused on disease modeling, drug screening and therapeutic potentials for liver disease. Int J Biol Sci. 2010;6:796–805. doi: 10.7150/ijbs.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Z, Zhan H, Mali P, Dowey S, Williams DM, Jang YY, et al. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114:5473–5480. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi SM, Liu H, Chaudhari P, Kim Y, Cheng L, Feng J, et al. Reprogramming of EBV-immortalized B-lymphocyte cell lines into induced pluripotent stem cells. Blood Press. 2011 doi: 10.1182/blood-2011-03-340620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Ye Z, Kim Y, Sharkis S, Jang YY. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology. 2010;51:1810–1819. doi: 10.1002/hep.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Kim Y, Sharkis S, Marchionni L, Jang YY. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med. 2011;3:82ra39. doi: 10.1126/scitranslmed.3002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SM, Kim Y, Liu H, Chaudhari P, Ye Z, Jang YY. Liver engraftment potential of hepatic cells derived from patient specific induced pluripotent stem cells. Cell Cycle. 2011;10:2423–2427. doi: 10.4161/cc.10.15.16869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou BK, Mali P, Huang X, Ye Z, Dowey SN, Resar LM, et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loh YH, Agarwal S, Park I, Urbach A, Huo H, Heffner GC, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodríguez-Pizà I, Vassena R, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009;5:353–357. doi: 10.1016/j.stem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown ME, Rondon E, Rajesh D, Mack A, Lewis R, Feng X, et al. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLoS ONE. 2010;5:11373. doi: 10.1371/journal.pone.0011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7:15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, et al. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7:20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Hu K, Yu J, Suknuntha K, Tian S, Montgomery K, Choi KD, et al. Efficient Generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood. 2011;117:109–119. doi: 10.1182/blood-2010-07-298331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen F, Zou JZ, di Renzo L, Winberg G, Hu LF, Klein E, et al. A subpopulation of normal B cells latently infected with Epstein-Barr virus resembles Burkitt lymphoma cells in expressing EBNA-1 but not EBNA-2 or LMP1. J Virol. 1995;69:3752–3758. doi: 10.1128/jvi.69.6.3752-3758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altmann M, Pich D, Ruiss R, Wang J, Sugden B, Hammerschmidt W. Transcriptional activation by EBV nuclear antigen 1 is essential for the expression of EBV's transforming genes. Proc Natl Acad Sci USA. 2006;103:14188–14193. doi: 10.1073/pnas.0605985103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia F, Wilson KD, Sun IJ, Gupta DM, Huang M, Li Z, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montserrat N, Garreta Bahima E, Gonzalez F, Gutiérrez J, Eguizábal C, Ramos V, et al. Simple generation of human induced Pluripotent stem cells using Poly({beta}-Amino Esters) as non-viral gene delivery system. J Biol Chem. 2011;286:12417–12428. doi: 10.1074/jbc.M110.168013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mali P, Chou BK, Yen J, Ye Z, Zou J, Dowey S, et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells. 2010;28:713–720. doi: 10.1002/stem.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menendez S, Camus S, Izpisua Belmonte JC. p53: guardian of reprogramming. Cell Cycle. 2010;9:3887–3891. doi: 10.4161/cc.9.19.13301. [DOI] [PubMed] [Google Scholar]
- 23.Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagarkova MA, Shutova MV, Bogomazova AN, Vassina EM, Glazov EA, Zhang P, et al. Induction of pluripotency in human endothelial cells resets epigenetic profile on genome scale. Cell Cycle. 2010;9:937–946. doi: 10.4161/cc.9.5.10869. [DOI] [PubMed] [Google Scholar]
- 25.Bhutani N, Brady JJ, Damian M, Sacco A, Corbel SY, Blau HM. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature. 2010;463:1042–1047. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpenter L, Malladi R, Yang CT, French A, Pilkington KJ, Forsey RW, et al. Human induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood. 2011;117:4008–4011. doi: 10.1182/blood-2010-08-299941. [DOI] [PubMed] [Google Scholar]
- 27.Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 28.Ye Z, Cheng L. Potential of human induced pluripotent stem cells derived from blood and other postnatal cell types. Regen Med. 2010;5:521–530. doi: 10.2217/rme.10.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun N, Longaker MT, Wu JC. Human iPS cell-based therapy: considerations before clinical applications. Cell Cycle. 2010;9:880–885. doi: 10.4161/cc.9.5.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serwold T, Hochedlinger K, Swindle J, Hedgpeth J, Jaenisch R, Weissman IL. T-cell receptor-driven lymphomagenesis in mice derived from a reprogrammed T cell. Proc Natl Acad Sci USA. 2010;107:18939–18943. doi: 10.1073/pnas.1013230107. [DOI] [PMC free article] [PubMed] [Google Scholar]