Abstract
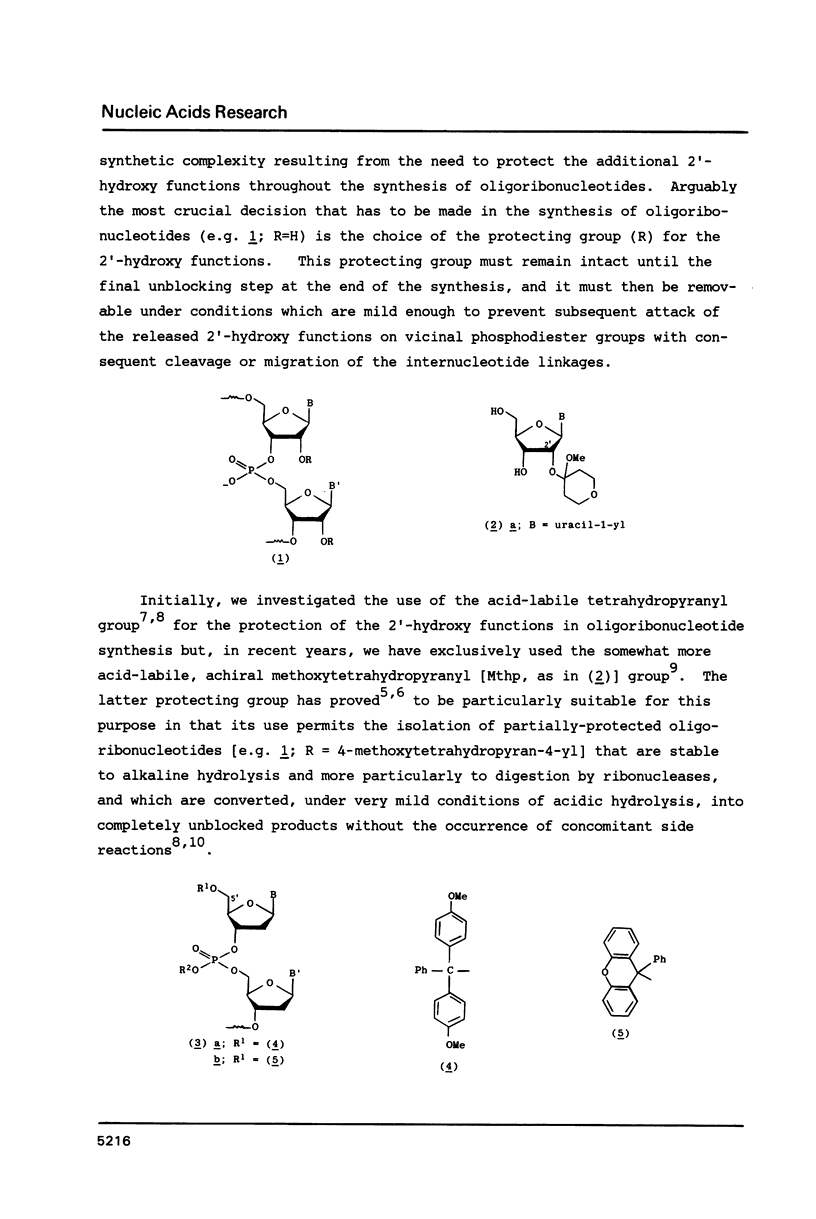
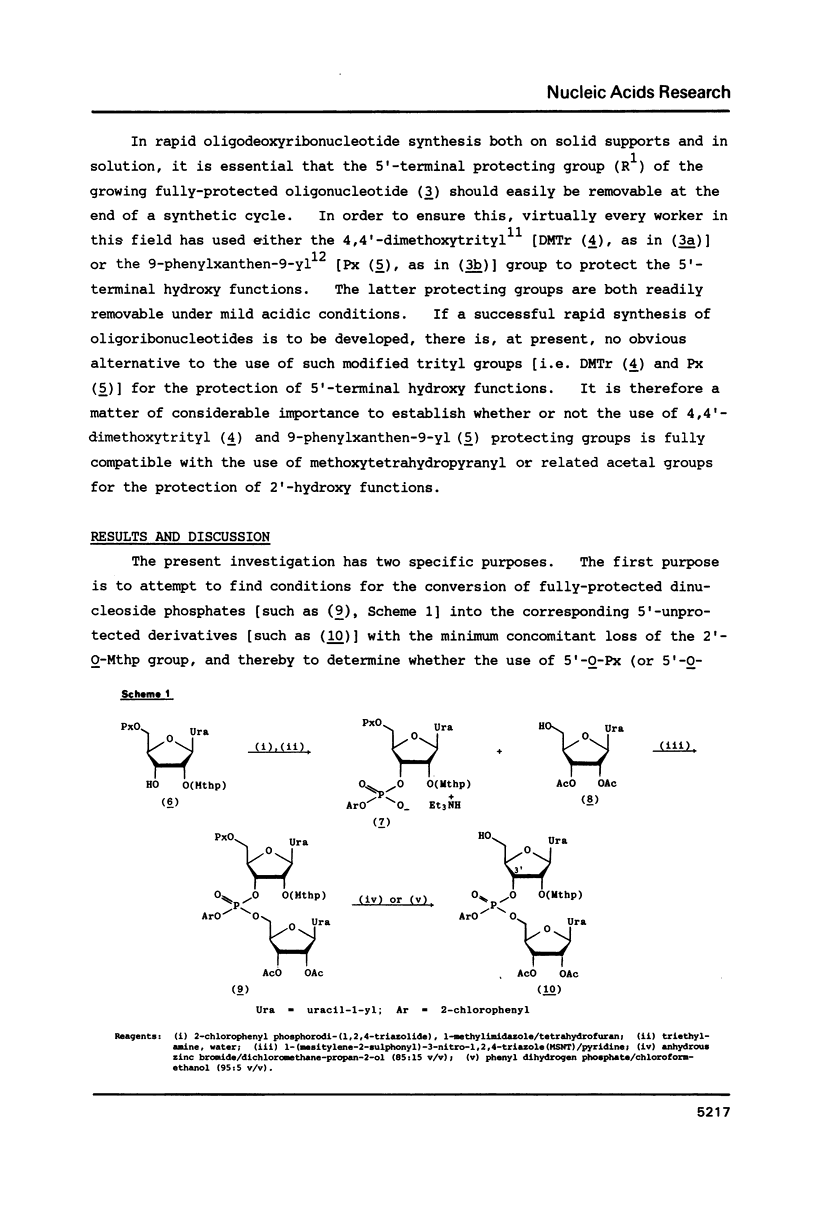
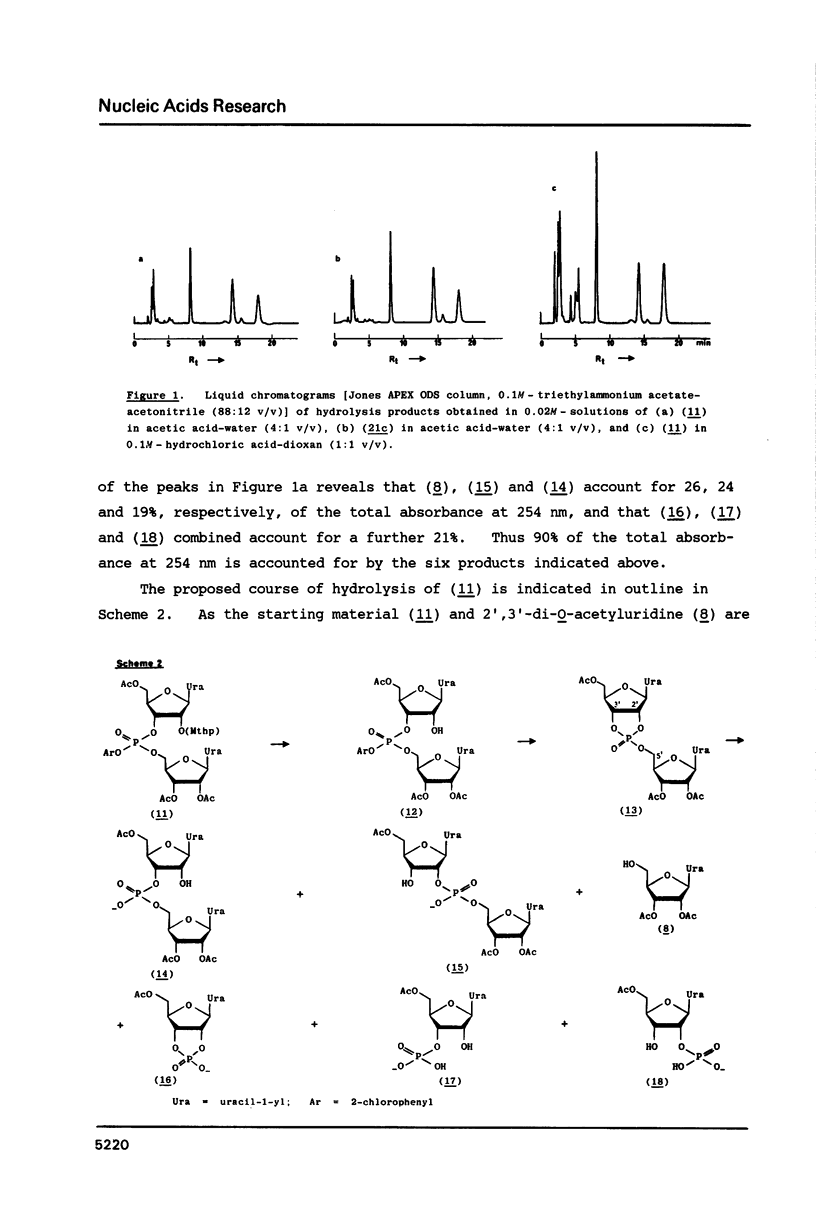
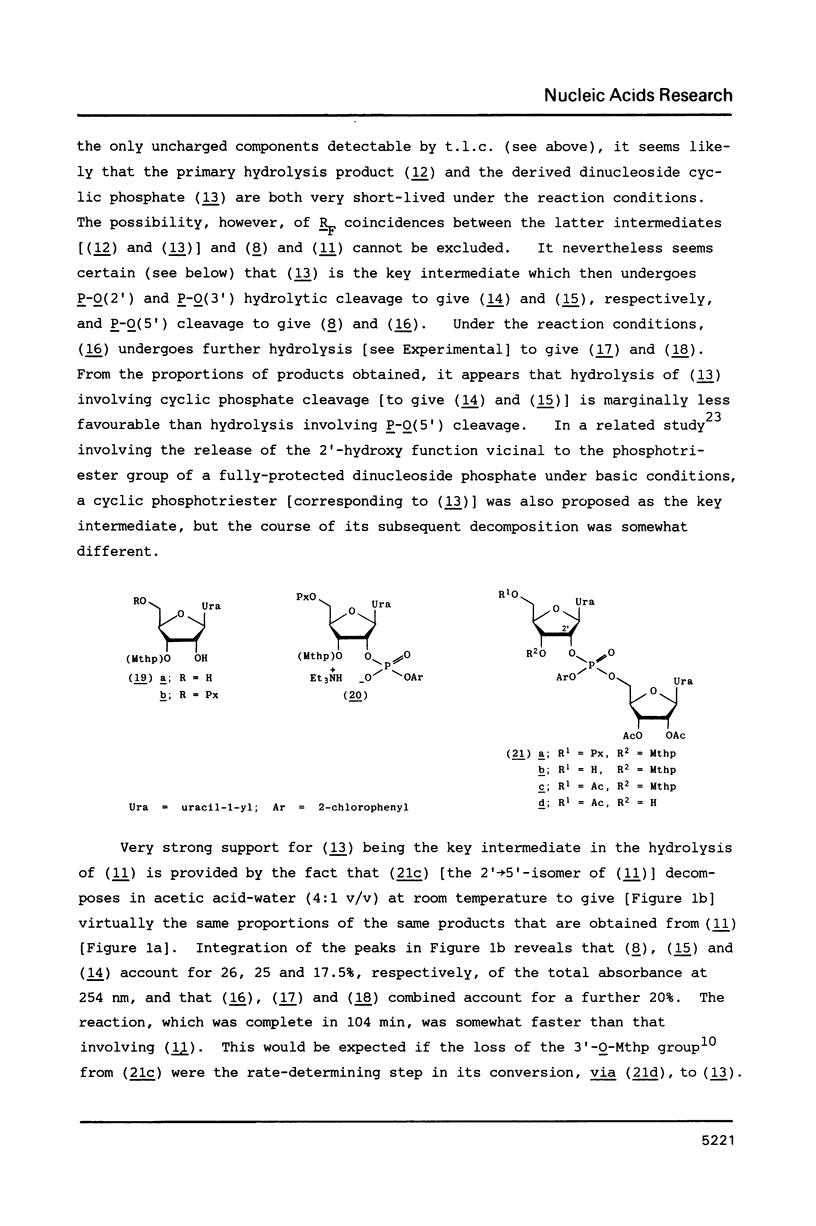
When 2'-O-methoxytetrahydropyranyl-5'-O-(9-phenylxanthen-9-yl) uridylyl-(3'----5')-(2',3'-di-O-acetyluridine) 2-chlorophenyl ester (9) is treated with zinc bromide in dichloromethane-propan-2-ol (85:15 v/v) at room temperature, under stringently anhydrous conditions, the corresponding 5'-unblocked dinucleoside phosphate (10) is obtained in 86% isolated yield; however, when no special precautions are taken to exclude moisture, (10) is obtained in only 72% yield. The removal of the 5'-O-(9-phenylxanthen-9-yl) protecting group from (10) with a protic acid (phenyl dihydrogen phosphate) appears to be much less selective and efficient. 80% Acetic acid promoted removal of the methoxytetrahydropyranyl protecting group from the isomeric fully-protected uridylyl-(3'----5')- and uridylyl-(2'----5')-uridine derivatives [(11) and (21c), respectively] leads to virtually identical mixtures [Figures 1a and 1b, respectively] of the partially-protected dinucleoside phosphates [(14) and (15)], 2',3'-di-O-acetyluridine (8), 5'-O-acetyluridine 2',3'-cyclic phosphate (16), and 5'-O-acetyluridine 2'(3')-phosphates [(18) and (17)].
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Griffin B. E., Jarman M., Reese C. B. The synthesis of oligoribonucleotides. IV. Preparation of dinucleoside phosphates from 2',5'-protected ribonucleoside derivatives. Tetrahedron. 1968 Jan;24(2):639–662. doi: 10.1016/0040-4020(68)88015-9. [DOI] [PubMed] [Google Scholar]
- Markiewicz W. T., Biała E., Adamiak R. W., Grześkowiak K., Kierzek R., Kraszewski A., Stawiński J., Wiewiórowski M. Further studies on oligoribonucleotide synthesis. Nucleic Acids Symp Ser. 1980;(7):115–127. [PubMed] [Google Scholar]
- RAMMLER D. H., KHORANA H. G. A new approach to the specific synthesis of the C3'-C5' inter-ribonucleotide linkage. Biochem Biophys Res Commun. 1962 Apr 3;7:147–150. doi: 10.1016/0006-291x(62)90164-x. [DOI] [PubMed] [Google Scholar]
- Reese C. B., Saffhill R., Sulston J. E. 4-methoxytetrahydropyran-4-yl. A symmetrical alternative to the tetrahydropyranyl protecting group. Tetrahedron. 1970 Feb;26(4):1023–1030. doi: 10.1016/s0040-4020(01)98779-4. [DOI] [PubMed] [Google Scholar]
- Reese C. B., Zard L. Some observations relating to the oximate ion promoted unblocking of oligonucleotide aryl esters. Nucleic Acids Res. 1981 Sep 25;9(18):4611–4626. doi: 10.1093/nar/9.18.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J. F., Wille-Hazeleger G., Burgers P. M., van Boom J. H. Neighbouring group participation in the unblocking of phosphotriesters of nucleic acids. Nucleic Acids Res. 1979;6(6):2237–2259. doi: 10.1093/nar/6.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]



