Abstract
Elucidating the response of breast cancer cells to chemotherapeutic and hormonal based drugs and radiation is clearly important as these are common treatment approaches. Signaling cascades often involved in chemo-, hormonal- and radiation resistance are the Ras/PI3K/PTE N/Akt/mTO R, Ras/Raf/MEK/ERK and p53 pathways. In the following studies we have examined the effects of activation of the Ras/PI3K/PTE N/Akt/mTO R cascade in the response of MCF-7 breast cancer cells to chemotherapeutic- and hormonal-based drugs and radiation. Activation of Akt by introduction of conditionally-activated Akt-1 gene could result in resistance to chemotherapeutic and hormonal based drugs as well as radiation. We have determined that chemotherapeutic drugs such as doxorubicin or the hormone based drug tamoxifen, both used to treat breast cancer, resulted in the activation of the Raf/MEK/ERK pathway which is often associated with a proproliferative, anti-apoptotic response. In drug sensitive MCF-7 cells which have wild-type p53; ERK, p53 and downstream p21Cip-1 were induced upon exposure to doxorubicin. In contrast, in the drug resistant cells which expressed activated Akt-1, much lower levels of p53 and p21Cip1 were induced upon exposure to doxorubicin. These results indicate the involvement of the Ras/PI3K/PTE N/Akt/mTO R, Ras/Raf/MEK/ERK and p53 pathways in the response to chemotherapeutic and hormonal based drugs. Understanding how breast cancers respond to chemo- and hormonal-based therapies and radiation may enhance the ability to treat breast cancer more effectively.
Key words: Akt, ERK, mTOR, chemotherapeutic drugs, radiation
Introduction
Signal transduction cascades downstream of epidermal growth factor (EGF) receptor (EGFR) isoforms have been associated with breast cancer development and resistance to anticancer agents.1–5 Among the signaling pathways downstream of the EGFR, the Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways have been shown to regulate apoptosis and their deregulation is often implicated in malignant transformation.5–11 The PI3K p110 catalytic subunit gene (PIK3CA) is one of the most frequently mutated genes in breast cancer.12–15
Phosphatidylinositol (PI) (3,4)P2 and PI(3,4,5)P3 produced by class 1A PI3Ks recruit phosphoinositide dependent kinase-1 (PDK1) as well as Akt isoforms to the plasma membrane by interacting with their pleckstrin homology (PH) domains.16–18 Colocalization of PDK1 with Akts at the plasma membrane causes PDK1 to phosphorylate Akts at a threonine residue (T308),19,20 and a serine residue (S473). Akt clearly plays important roles in the regulation of cell growth and its deregulation is often linked with malignant transformation.21–34
Activation of PDK1 and Akt by class 1A PI3Ks is negatively regulated by phosphatase and tensin homolog deleted on chromosome ten (PTEN).8,15 PTEN removes phosphate groups from PI(3,4)P2 and PI(3,4,5)P3 added by PI3K as well as from tyrosine phosphorylated proteins including focal adhesion kinase (FAK) and Shc.8,10,15
Diverse mechanisms regulate PTEN expression.5,35,36 These range from gene deletion, alterations in mRNA splicing, subcellular localization or epigenetic mechanisms which prevent PTEN transcription. Mutations have been reported to occur at PTEN in breast cancer at varying frequencies (5–21%). While PTEN is deleted in certain cancers, loss of heterozygosity (LOH) is probably a more common genetic event (30%) leading to changes in PTEN expression.35,37 PTEN promoter methylation leads to low PTEN expression.35 In one study, 26% of primary breast cancers had low PTEN levels which correlated with lymph node metastases and poor prognoses.38 PTEN has both plasma membrane and nuclear localized activities. Disruption of PTEN activity by various mechanisms could have vast effects on different processes affecting cancer and drug resistance.39–43
A consequence of impaired PTEN expression is elevated activation of Akt. One downstream molecule of mTOR is ribosomal S6 kinase (p70S6K). This kinase regulates the efficiency of translation of certain mRNAs and also functions in a negative feedback loop to control Akt activity.5,15,44,45 Akt, mTOR and p70S6K activation have been associated with a more severe prognosis in breast and other cancers.38,44,46–53 High levels of activated Akt expression have been associated with both chemo- and hormonal resistance in breast cancer.47,48,54 Indeed some studies have evaluated the effectiveness of targeting mTOR in PTEN-negative cells.49 Cells which express high levels of activated Akt may be more sensitive to mTOR inhibitors and inhibition of mTOR activity by rapamycin may restore their sensitivity to chemo- and hormonal based therapies.49,55
Previously it was determined that mutated forms of Akt and PTEN can induce chemotherapeutic- and hormonal-based drug resistance in breast cancer.47,54,55 PTEN mutants which eliminate the lipid phosphatase activity will result in activated Akt expression which leads to drug resistance and sensitivity to the mTOR inhibitor rapamycin.55
After growth factor/cytokine/mitogen stimulation of the EGFR, the Ras/Raf/MEK/ERK pathway is also activated.10 The Ras/Raf/MEK/ERK pathway has been shown to play pivotal roles in chemotherapeutic drug resistance.5,56–59 This pathway can be activated by either mutations in upstream receptors or mutations in pathway components. We have shown that activated Ras and Raf genes will result in drug resistance of breast cancer cells.6 The roles of various chemotherapeutic- and hormonal-based drugs play in the activation of these pathways have not been well investigated. Inappropriate activation of these pathways could result in the generation of drug resistant cells as well as cancer initiating cells (CICs).60–69
In the following studies, the effects of Akt-1 activation on the response of breast cancer cells to chemotherapeutic- and hormonal-based drugs and radiation were examined as these three different approaches are used to treat breast cancer. Elevated Akt-1 expression resulted in resistance to doxorubicin, tamoxifen and radiation. Doxorubicin treatment resulted in the induction of the anti-apoptotic ERK molecule. Furthermore drug resistant cells displayed altered p53 and downstream p21Cip-1 expression. These results highlight the importance of the PI3K/PTEN/Akt/mTOR pathway in therapy resistance in breast cancer.
Results
Ectopic Akt-1 expression induces resistance of MCF-7 cells to tamoxifen.
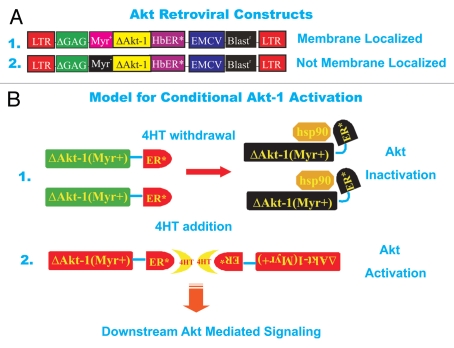
The activity of the PI3K/PTEN/Akt/mTOR cascade was manipulated in MCF-7 cells in order to determine how signals transduced by this pathway control the sensitivity of breast cancer cells to various therapies. We wanted to be able to turn on and off the expression of Akt-1 so MCF-7 cells were infected with retroviruses encoding Akt-1 genes (ΔAkt-1) under the control of the modified estrogen receptor hormone binding domain (ER*) which allows the Akt-1 gene to be turned on or off by 4-OH tamoxifen (4HT) addition or withdrawal respectively (see Fig. 1).70,71 Two different conditional Akt-1 viruses were used in these studies. Both modified Akt-1 viruses have the pleckstrin homology domain of Akt deleted (ΔPH), however, one virus has the v-Src myristoylation domain added [ΔAkt-1:ER*(Myr+)] which results in membrane localization of Akt-1, while the other “control” Akt-1 virus, lacks a functional v-Src myristoylation domain [ΔAkt-1:ER*(Myr−)] due to the change of an amino acid and hence is not membrane localized.
Figure 1.
Structures of Akt viruses used in this study and model for activation of Akt construct after 4HT treatment. (A) Conditional ΔAkt-1:ER*(Myr+) and (B) ΔAkt-1:ER*(Myr-) viruses which encode resistance to blasticidin. Fusion of two proteins yielded ΔAkt-1:ER*(Myr+) and (B) ΔAkt-1:ER*(Myr-). Human Akt-1 was modified by deleting amino acids 4 through 129, followed by replacement of D3 with glutamate and addition of a hemaggluinin subunit-1 (HA1) epitope to the carboxyl terminus to yield ΔAkt-1.17 The HA1 epitope consists of 17 amino acids with the sequence MAY PYD VPD YAS LGP GL. Amino acids YPY DVP DYA SL within this epitope correspond to amino acids 98 through 108 of HA1 from hemagglutinin-3/neuraminidase-2 (H3NS) stains of human influenza A virus. Deletion of amino acids 4 through 129 removes the pleckstrin homology (PH) domain from ΔAkt-1, which spans from amino acid 7 to amino acid 106. This domain mediates binding of Akt-1 to PI(4,5)P2 or PI(3,4)P3.16 The carboxyl terminal portion of ΔAkt-1:ER* is composed of a mutant murine ERα truncated at the N-terminus. This fragment is identical to amino acids 281 through 599 of murine ERα except that G525 is replaced by arginine. The portion of ERα fused to ΔAkt-1 contains the ligand binding domain, which spans from amino acid 306 to amino acid 556. However this ERα fragment lacks the DNA binding domain which spans from amino acid 184 to amino acid 266. Binding of 4HT to the ERα ligand binding domain of ΔAkt-1:ER* stimulates its Akt-1 activity. Replacement of G525 with arginine with the ERα fragment prevents binding of estrogen to ΔAkt-1:ER*. Replacement of the N-terminal methionine residue of ΔAkt-1:ER*with a myristoylation signal sequence yielded ΔAkt-1:ER*(Myr+). This myristoylation signal sequence corresponds to amino acids 1 though 15 of viral sarcoma (v-Src) from Schmidt-Ruppin subgroup A strain of Rous sarcoma virus. The v-Src myristoylation domain (Myr+) allows localization of the ΔAkt-1:ER*(Myr+) proteins to the lipid rich cell membrane. In contrast, ΔAkt-1:ER*(Myr-), has an alanine substitution in the v-Src Myr domain and is not myristoylated. Lack of myristoylation prevents ΔAkt-1:ER*(Myr-) from localizing to the plasma membrane. The genes encoding ΔAkt-1:ER*(Myr+) and ΔAkt-1:ER*(Myr-) were inserted into pWZLblast at the multiple cloning site (MCS) to yield ΔAkt-1:ER*(Myr+) ΔAkt-1:ER*(Myr-), respectively. The multiple cloning site of pWZLblast is situated between 5′ and 3′ Moloney Leukemia virus (MMLV) long-terminal repeats (LTRs). A MMLV retroviral packaging signal is located between the MMLV 5′LTR and the MCS. The gene encoding blasticidin S resistance from Bacillus cereus is positioned between the MCS and the MMLV 3′LTR. Blasticidin resistance is driven by an internal ribosomal entry site (IRES) from the Rueckert strain of encephalomyocarditis virus and encodes resistance to blasticidin in both Escherichia coli and mammalian cells. (B) Model for activation of conditional ΔAkt-1:ER*(Myr+) gene upon 4HT treatment. In the absence of 4HT, the ΔAkt-1:ER*(Myr+) proteins are associated with heat shock proteins such as Hsp90 and are inactive. In contrast, in the presence of 4HT, the ΔAkt-1:ER*(Myr+) proteins dimerize due to the binding of 4HT to the hormone binding domain of the modified estrogen receptor which binds 4HT 100-times more efficiently than β-estradiol (estrogen). This induces Akt activity and downstream signaling.
MCF-7 breast cancer cells were infected with retroviral stocks encoding both retroviruses and stably infected cells were isolated in the presence of the drug blasticidin as the blasticidin resistance gene is present in both retroviral constructs downstream of the modified Akt-1 genes (see Fig. 1). These infected cells are named MCF7/ΔAkt-1:ER*(Myr+) and MCF7/ΔAkt-1:ER*(Myr−).
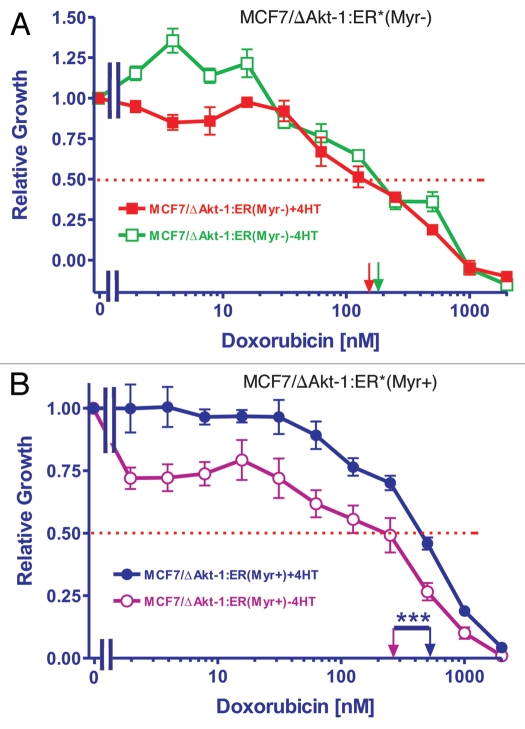
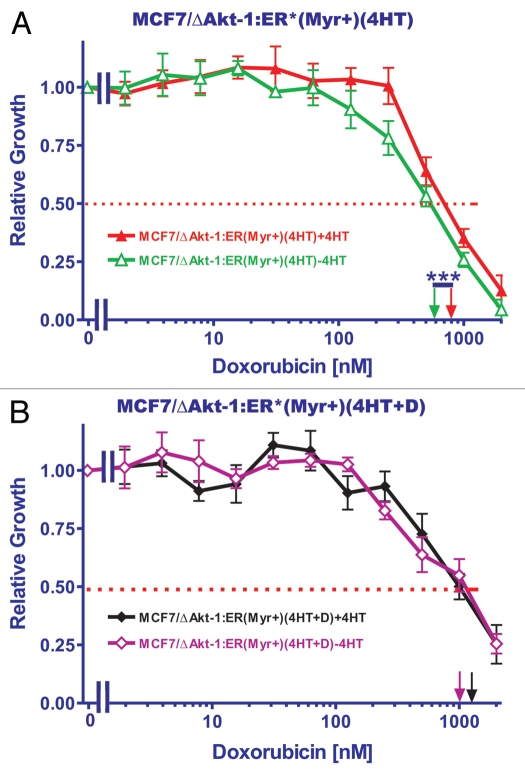
To examine the resistance of the cells to chemotherapeuticand hormonal-based drugs, the cells were plated in the presence of either doxorubicin or 4HT, two drugs both used to treat breast cancer. The IC50s in response to different concentrations of doxorubicin were examined in both MCF7/ΔAkt-1:ER*(Myr+) and MCF7/ΔAkt-1:ER*(Myr−) in the presence and absence of 4HT. MCF7/ΔAkt-1:ER*(Myr+) and MCF7/ΔAkt-1:ER*(Myr−) had doxorubicin IC50s of approximately 150 nM and 80 nM respectively in the absence of 4HT. However, when they were plated in the presence of 4HT, the IC50 for doxorubicin in MCF7/ΔAkt-1:ER*(Myr+) cells remained similar (150 nM) while the IC50 for doxorubicin in MCF7/ΔAkt-1:ER*(Myr−) cells decreased to less than 10 nM. These results indicated that initially the MCF7/ΔAkt-1:ER*(Myr+) cells were resistant approximately 15-fold and 2-fold more resistant to 4HT and doxorubicin than the MCF7/ΔAkt-1:ER*(Myr−) cells (Fig. 2).
Figure 2.
Requirement for membrane localization of Akt for resistance to tamoxifen. MTT was utilized to monitor the requirement of membrane localization of Akt for resistance to tamoxifen (4HT). MCF7/ΔAkt-1:ER*(Myr+) and MCF7/ΔAkt-1:ER*(Myr−) were plated in different concentrations of doxorubicin in either the presence and absence of 500 nM 4HT and proliferation was estimated by MTT analysis. The dotted arrow represents where 50% inhibition of growth intercepts with the X axis and is used to estimate the IC50. The statistical significance in (B) was determined by the unpaired t-test (***p < 0.0001).
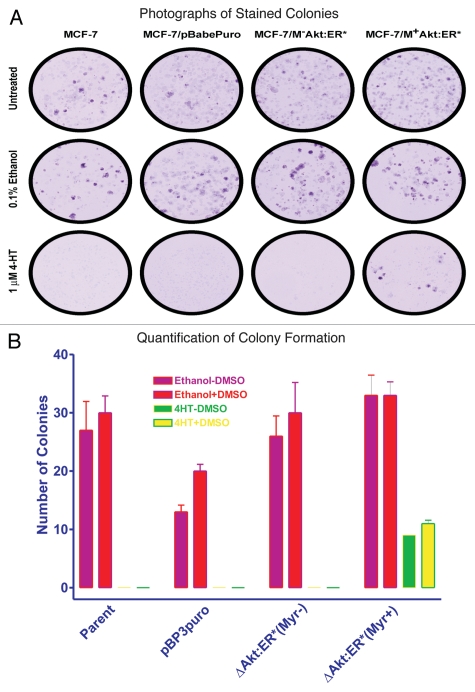
The effects of activated Akt-1 on the cloning efficiency in 4HT-containing medium were also examined (Fig. 3). In these experiments, 1,000 cells were plated in each well of a 6-well plate in triplicate and fed the indicated medium supplements for a period of 3 weeks. Fresh medium containing the drugs was provided to the cells every three days. Photographs of the giemsa-stained plates showing the colonies in the different culture conditions is presented in Figure 3A. When cells were cultured in either the presence of the solvent used to dissolve 4HT, ethanol or ethanol and DMSO, as an additional control, MCF-7, MCF7/ΔAkt-1:ER*(Myr+) and MCF7/ΔAkt-1:ER*(Myr−) cells had similar plating efficiencies (Fig. 3B). The efficiency of the empty retroviral vector control cell line, MCF-7/pBABEpuro was slightly lower under these conditions. However, much different results were observed when the cells were plated in the presence of 4HT. Namely, MCF7/ΔAkt-1:ER*(Myr+) cells had a higher cloning efficiency when plated in medium containing 1 µM 4HT than MCF7/ΔAkt-1:ER*(Myr−) cells. As controls, the plating efficiencies of MCF-7 and MCF7/pBABEpuro cells were also examined. When these negative controls were plated in the presence of 1 µM 4HT in 6-well plates, they did not grow.
Figure 3.
Effects of Akt activation on colony formation. Colony formation of control (MCF-7 and MCF7/pBABEpuro) and Akt transfected cells [MCF7/ΔAkt-1:ER*(Myr+) and MCF7/ΔAkt-1:ER*(Myr−)] in 4HT. MCF-7 cells transfected with the various Akt plasmids were plated in triplicate in 6-well plates. The following day, the cells were exposed to no drug, 0.1% ethanol, the vehicle used to dissolve 4HT, or 0.1% ethanol plus DMSO or 1,000 nM 4HT. (A) Photographs of colonies of cells stained with giemsa dye and cultured under the various conditions for 4 weeks. (B) Quantification of the number of colonies (n = 3) for each culture condition. Means and standard deviations of three culture plates for each condition are presented. The statistical significance in (B) between the number of colonies observed in MCF7/ΔAkt-1:ER*(Myr+) plated in 4HT compared with MCF-7, MCF7/pBABEpuro or MCF7/ΔAkt-1:ER*(Myr−) cells was determined by the unpaired t-test (p < 0.0036).
Increased Akt-1 expression in 4HT-selected MCF7/ΔAkt-1:ER*(Myr+) cells.
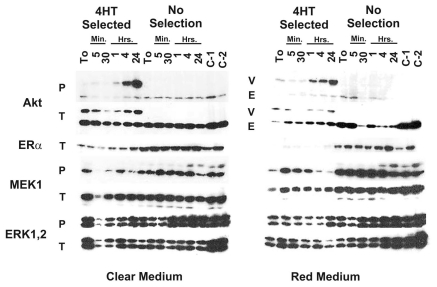
We next examined the effects of 4HT selection on Akt-1, MEK/ERK and ERα expression. In these experiments, we cultured MCF7/ΔAkt-1:ER*(Myr+) cells for 4 weeks in 4 different culture conditions (Fig. 4). Namely cells were cultured in phenol red free (clear) RPMI + 10% charcoal stripped (CS) FBS in the presence and absence of 4HT (Fig. 4A), and also in RPMI medium containing phenol red (red medium) and 10% FBS in the presence and absence of 4HT (Fig. 4B). Phenol red free medium and charcoal stripped FBS were used as they contain components which may affect ERα expression. Four days prior to the experiments presented in Figure 4, the culture medium was removed from the plates, the cell monolayers were washed with PBS twice and then the cells cultured in the either phenol red free medium containing 10% CS FBS (Fig. 4A) or phenol red containing medium containing 10% FBS (Fig. 4B). Cells were then stimulated with 4HT for the indicated time periods. In addition, two control cell lines were examined, MCF-7 cells containing GFPΔRaf-1:ER or ΔA-Raf:ER to examine the effects on ERK expression. In the 4HT-selected cells, there were higher levels of ΔAkt-1:ER* activity when the cells were stimulated with 4HT than in the non selected cells. In the population of non-selected cells, no ΔAkt-1:ER* activity was detected even after 4HT treatment. These experiments reflect a common occurrence in studies with retroviral-infected cells and also agree with the original MTT assays presented in Figure 2. Namely, in the absence of a biological selection, there may only be low levels of expression of the introduced gene encoded by the introduced provirus in the cells.
Figure 4.
Elevated Akt expression in 4HT-selected cells. MCF7/ΔAkt-1:ER*(Myr+) cells were cultured in the indicated conditions (+/- 4HT, in the presence and absence of phenol red containing medium) for 4 weeks. Cells were then collected after trypsinization and plated in phenol red free RPMI 1640 containing 10% FBS. After allowing the cells to adhere for 24 h, the treated with 1,000 nM 4HT for the indicated time periods. Protein lysates were prepared and subjected to protein gel blot analysis.
The effects of culturing cells in the presence and absence of 4HT and phenol red on the expression of ERα were also examined. When cells were cultured in the presence of 4HT, there were lower levels of ERα detected. Moreover, when cells were cultured in the presence of 4HT and phenol red (Fig. 4B), there were even lower levels of ERα detected.
The effects of ΔAkt-1:ER*(Myr+) activity on MEK1 and downstream ERK1,2 activation were examined. When ΔAkt-1:ER*(Myr+) was induced, there were lower levels of activated (phosphorylated) MEK1 and ERK1,2 detected, suggesting that Akt may suppress the activity of the Raf/MEK/ERK cascade as previously reported in references 72 and 73.
Isolation of drug resistant breast cancer cells.
We isolated drug resistant cells from MCF7/ΔAkt-1:ER*(Myr+) by continuously culturing them in either 500 nM 4HT or 500 nM 4HT+10 nM doxorubicin. In these experiments, higher cell concentrations (100,000 cells per well in a 6-well plate) were plated to allow the out-growth of drug resistant cells. These selected cells are named MCF7/ΔAkt-1:ER*(Myr+)(4HT)R and MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox)R. The resistance of these cells to doxorubicin was determined by MTT analysis that was performed in 500 nM 4HT (Fig. 5). The doxorubicin IC50 for the unselected MCF7/ΔAkt-1:ER*(Myr+) in the presence of 500 nM 4HT was approximately 100 nM. In contrast the doxorubicin IC50s for the MCF7/ΔAkt-1:ER*(Myr+)(4HT)R and MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox)R were approximately 10-fold higher, about 1,000 nM. Thus selection in medium containing 4HT, which resulted in increased Akt expression (see below) increased resistance to doxorubicin. Interestingly, selection in the combination of 4HT and doxorubicin did not increase the resistance of the MCF7/ΔAkt-1:ER*(Myr+) cells to doxorubicin compared with 4HT alone selection.
Figure 5.
Sensitivity of resistant cells to doxorubicin. The sensitivities of MCF7/ΔAkt-1:ER*(Myr+) which had been selected for 4 weeks in either 500 nM 4HT (A) or 500 nM 4HT + 100 nM Doxorubicin (B) was examined by MTT analysis. The statistical significance in (A) was determined by the unpaired t-test (***p < 0.0001). The statistical significance between the IC50s presented in (A and B) were determined by the unpaired t-test (***p < 0.0001).
Effects of 4HT and doxorubicin on gene expression.
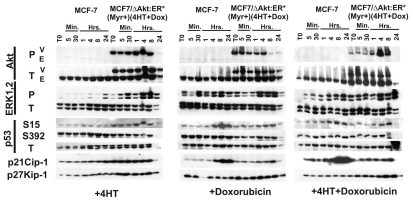
At this point, we chose to examine the effects of 4HT and doxorubicin treatment on gene expression in both uninfected MCF-7 and MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox)R cells. We examined how exposure to either 4HT or doxorubicin altered the expression of ERK1.2, Akt and p53-regulated genes (Fig. 6). In these experiments, MCF-7 cells and MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R were cultured in RPMI + 10% CS phenol red free medium for 4 d prior to the start of the experiments. Then the medium from the plates were removed, the monolayers washed twice with PBS and cultured in phenol red free medium lacking CS for 24 h. The cells were then stimulated with 4HT, doxorubicin or the combination of 4HT+doxorubicin for the indicated time periods. Treatment with 4HT, doxorubicin or 4HT+doxorubicin did not significantly induce Akt activation in MCF-7 cells. In contrast, the vector-derived (V) ΔAkt:ER*(Myr+) but not the endogenous (E) Akt was phosphorylated and activated from the T0 time point up until 8 h of treatment in MCF7/ΔAkt:ER*(Myr+)(4HT+Dox) R cells. After treatment with doxorubicin by itself, decreased levels of the activated vector-derived (V) ΔAkt:ER*(Myr+) were detected after 8 h. Thus in the MCF7/ΔAkt:ER*(Myr+) (4HT+Dox)R cells, there were high levels of the activated vector-derived (V) ΔAkt:ER*(Myr+) detected. These cells differed from the 4HT-selected MCF7/ΔAkt-1:ER*(Myr+) cells, as in these cells, which were not selected in the presence of doxorubicin, inducible vector-derived (V) ΔAkt:ER*(Myr+) was not detected (Fig. 4) whereas higher levels of vector-derived (V) ΔAkt:ER*(Myr+) were observed in MCF7/ΔAkt:ER*(Myr+) (4HT+Dox)R cells even at the T0 time point.
Figure 6.
Effects of drug resistance on ERK and p53 mediated gene expression. MCF-7 and MCF7/ΔAkt-1:ER*(Myr+)(4HT+DoxR) cells were collected after trypsinization and plated in phenol red free RPMI 1640 containing 10% FBS. After allowing the cells to adhere for 24 h, the monolayers were washed twice with PBS and then cultured in phenol red free RPMI 1640 lacking FBS for 24 h. Then the cells were treated with 1,000 nM 4HT, 100 nM doxorubicin or 1,000 nM 4HT and 100 nM doxorubicin for the indicated time periods. Protein lysates were prepared and subjected to protein gel blot analysis.
Activated ERK1,2 was induced by both 4HT and doxorubicin treatment in both MCF-7 and MCF7/ΔAkt:ER*(Myr+) (4HT+Dox)R cells, indicating that both 4HT and doxorubicin can induce a signaling pathway associated with pro-proliferative and anti-apoptotic effects. However, it should be pointed out that doxorubicin was a more potent and rapid inducer of ERK1,2 than 4HT.
Doxorubicin was a potent inducer of phosphorylation of p53 at S15 in MCF-7 cells. In contrast, not as much phosphorylation at S15 was detected in the MCF7/ΔAkt:ER*(Myr+) (4HT+Dox)R cells following doxorubicin treatment, although there was some induction of phosphorylation at S15 observed in MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R cells following 4HT and 4HT+doxorubicin treatment. Changes in the phosphorylation status of S392 or the levels of total p53 were not readily observed in either MCF-7 or MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R cells following 4HT, doxorubicin or 4HT+doxorubicin treatment.
p21Cip-1 was also induced in similar time periods after either doxorubicin or 4HT+doxorbubicin treatment of MCF-7 cells. In contrast, increased levels of p21Cip-1 were not detected in MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R cells following either doxorubicin or 4HT+doxorubicin treatment. The levels of p27Kip-1 were slightly higher in the MCF7/ΔAkt:ER*(Myr+) (4HT+Dox)R cells, however, they did not vary as dramatically as the levels of p21Cip-1 in MCF-7 cells following either doxorubicin or 4HT+doxorubicin treatment.
Effects of selection for 4HT + doxorubicin resistance on plating in different selective medium.
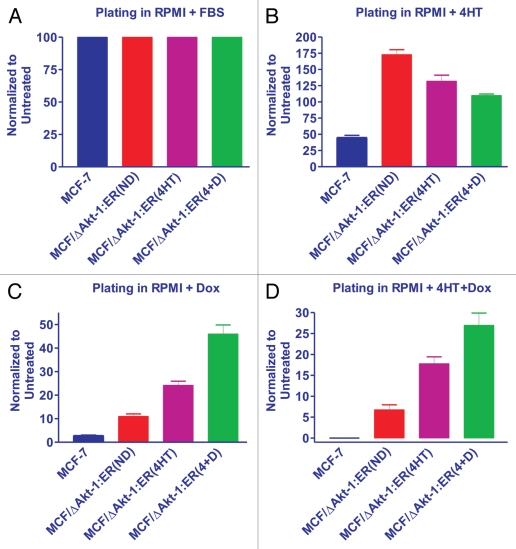
We examined the differential plating abilities of MCF-7 and MCF7/ΔAkt:ER*(Myr+)(4HT+Dox) R cells in the presence of no selective agent (RPMI + 10% FBS), 4HT, Doxorubicin (Dox) or 4HT+Doxorubicin (4+D). In these experiments, we compared doxorubicin-sensitive MCF-7 with the MCF7/ΔAkt:ER*(Myr+) (4HT+Dox)R cells which had been grown for 4 weeks in RPMI+FBS, RPMI+FBS+4HT or RPMI+4HT+Dox and then plated 10,000 cells in triplicate wells in a 6-well plate in RPMI+FBS, RPMI+FBS+4HT, RPMI+FBS+Dox or RPMI+FBS+4HT+Dox. Thus we examined how cells which were originally drug resistant would react when they were grown for 4 weeks in RPMI+FBS, RPMI+FBS+4HT or RPMI+FBS+4HT+Dox. The RPMI + FBS represent non-selective conditions and thus it is a measure of how the cells have retained their resistance. We normalized the number of colonies in each cell line to 100% when they were plated in RPMI + 10% FBS (Fig. 7A).
Figure 7.
Effects of Akt activation on colony formation in the presence and absence of doxorubicin and 4HT. MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox) cells were culture for 4 weeks in RPMI 10% FBS, labeled (ND = no drugs), RPMI + 10% FBS + 1,000 nM 4HT, labeled (4HT) or RPMI + 10% FBS + 1,000 nM 4HT + 100 nM doxorubicin, labeled (4+D). Colony counts were normalized to the number of colonies observed in untreated cells (n = 3). (A) Colony formation of MCF-7 and MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox) in RPMI + 10% FBS. (B) Colony formation of MCF-7 and MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox) in RPMI + 10% FBS + 1,000 nM 4HT. (C) Colony formation of MCF-7 and MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox) in RPMI + 10% FBS + 100 nM doxorubicin (Dox). (D) Colony formation of MCF-7 and MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox) in RPMI + 10% FBS + 100 nM 4HT + 100 nM doxorubicin (4HT+D).
When 10,000 MCF-7 cells were plated in the presence of 4HT, approximately 2.2-fold less colonies were observed than when they were plated in the absence of 4HT (RPMI + 10% FBS). Interestingly, when the MCF7/ΔAkt:ER*(Myr+) (4HT+Dox)R cells had been plated in RPMI + 10% FBS for 4 weeks were subsequently plated in medium containing 4HT, they maintained their resistance to 4HT as approximately 3.8-fold more colonies were observed than in the MCF-7 cells (Fig. 7B). When the MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R cells had been plated in either 4HT or 4HT+doxorubicin for 4 weeks were then plated in medium containing 4HT, they maintained their resistance to 4HT as approximately 2.9- and 2.4-fold respectively more colonies were observed than in the MCF-7 cells. Thus, the MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R remained their resistance to 4HT compared with MCF-7 cells.
Very few colonies were observed when MCF-7 cells were plated in medium containing 25 nM doxorubicin (Dox). When the drug resistant MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R cells were plated in medium with just doxorubicin, they were still resistant to doxorubicin, even in the absence of 4HT (Fig. 7C). The MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R which had been maintained in 4HT or 4HT+Dox were 2- and 4.5-fold more resistant to doxorubicin respectively than the MCF7/ΔAkt:ER*(Myr+)(4HT+Dox) R which had been maintained in RPMI + 10% FBS for 4 weeks.
Very few colonies were also observed when MCF-7 cells were plated in medium containing 4HT+Dox (Fig. 7D) When the drug-resistant MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R cells which had been cultured in no selective drugs for 4 weeks, were subsequently plated in medium with 4HT+Dox, they were resistant to 4HT+Dox as approximately 7-fold more colonies were observed than in MCF-7 cells. The MCF7/ΔAkt:ER*(Myr+)(4HT+Dox) R which had been maintained in 4HT or 4HT+Dox were more resistant to 4HT+doxorubicin than the MCF7/ΔAkt:ER*(Myr+) (4HT+Dox)R which had been maintained in RPMI + 10% FBS for 4 weeks as approximately 2.6- and 4-fold more colonies were observed.
Effects of activated Akt-1 expression on radio-sensitivity.
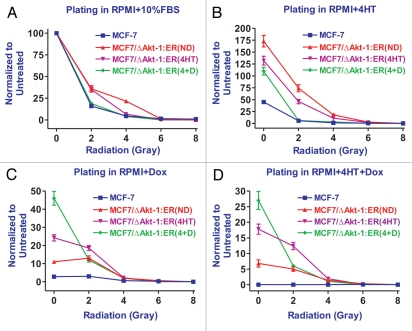
The effects of activated Akt-1 on the radio-sensitivity of MCF-7 cells were determined. These experiments were performed at the same time as those presented in Figure 7, except the cells were treated with 0, 2, 4, 6 and 8 grays of radiation. MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R cells which had been cultured in RPMI + FBS, 4HT or 4HT+Dox for 4 weeks prior to the start of the experiments were more resistant to 2 grays of radiation than MCF-7 cells cultured in 4HT+Dox when they were plated in either RPMI + FBS or 4HT containing medium (Fig. 8A and B). There was a 2.9-fold difference between MCF-7 and MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R cells cultured in 4HT+Dox which is difficult to see on this linear graph. Linear curves are presented as some data points, especially at the higher radiation doses, were zero. In general, the MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R cells cultured in 4HT+Dox had a lower plating efficiency than the MCF7/ΔAkt:ER*(Myr+)(4HT+Dox)R cultured in either RPMI + 10% FBS or 4HT medium which most likely reflect the negative effects of being cultured in medium with both 4HT and Dox. The difference in plating between the MCF7/ΔAkt:ER*(Myr+) (4HT+Dox)R cells cultured in 4HT+Dox and MCF-7 cells plated in either Dox or 4HT+Dox containing medium and exposed to 2 grays of radiation than MCF-7 cells is more dramatic as they are 4- and 60-fold more radio-resistant (Fig. 8C and D). These results reflected the fact that selection of cells in 4HT+Dox containing medium results in cells with increased resistance to Dox or 4HT+Dox. In general, doses of radiation greater than 2 gray essentially eliminated the colony formation of all the cells, regardless of whether they expressed activated Akt-1. In summary, activation of Akt-1 conferred resistance to radiation up to a dose of 2 gray.
Figure 8.
Effects of Akt activation on colony formation in the presence and absence of radiation, doxorubicin and 4HT. MCF7/ΔAkt-1:ER*(Myr+) (4HT+Dox) cells were culture for 4 weeks in RPMI 10% FBS, labeled (ND = no drugs), RPMI + 10% FBS + 1,000 nM 4HT, labeled (4HT) or RPMI + 10% FBS + 1,000 nM 4HT + 100 nM doxorubicin, labeled (4+D). Colony counts were normalized to the number of colonies observed in untreated cells (n = 3). (A) Colony formation of MCF-7 and MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox) in RPMI + 10% FBS. (B) Colony formation of MCF-7 and MCF7/ΔAkt-1:ER*(Myr+) (4HT+Dox) in RPMI + 10% FBS + 1,000 nM 4HT. (C) Colony formation of MCF-7 and MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox) in RPMI + 10% FBS + 100 nM doxorubicin (Dox). (D) Colony formation of MCF-7 and MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox) in RPMI + 10% FBS + 100 nM 4HT + 100 nM doxorubicin (4HT+D).
Discussion
In our studies, we examined the effects of doxorubicin, tamoxifen and radiation on MCF-7 and derivative cell lines which varied in their levels of activated Akt-1 expression. An advantage of our study is that all the cells had the same genetic background as they all were MCF-7 cells; however they differed in the levels of activated Akt-1 expression due to introduction of an activated Akt-1 gene as well as being selected under different culture conditions.
We have previously shown that introduction of dominant negative (DN) forms of PTEN into MCF-7 cells conferred resistance to doxorubicin and increased sensitivity to rapamycin. Furthermore, rapamycin could synergize with doxorubicin to lower its IC50.55 In the MCF-7 cells transfected cells with the PTEN(DN) genes, increased levels of activated Akt were detected. These results have clinical significance as the PI3K/PTEN/Akt/mTOR pathway is often activated in breast cancer by mutations at PIK3CA or multiple genetic mechanisms leading to dysregulation of PTEN. Furthermore, drug resistance frequently develops in breast cancer after chemo- or hormonal-based therapies. Doxorubicin (a.k.a Adriamycin) is frequently used to treat breast cancer patients. However, in drug resistant PTEN(DN)-transfected cells, they were hypersensitive to rapamycin.55 In the studies present in this report, increased expression of activated Akt-1 could result in the resistance of MCF-7 breast cancer cells to both chemotherapeutic drugs (doxorubicin) as well as hormonal based drugs (4HT).
In our studies, we have used conditional Akt-1 constructs to monitor the effects of activated Akt-1 on chemotherapeutic drug resistance and sensitivity to hormonal therapy. The set of paired Akt-1 constructs contained the activated Akt-1 gene fused to the hormone binding domain of the modified ER* which rendered its activity dependent upon the addition of 4HT to the media. Also in this pair of Akt-1 constructs, the pleckstrin homology (PH) of Akt-1 deleted. One Akt-1 construction in this pair can be conditionally-active as the modified ΔAkt-1 gene has the functional v-Src myristoylation domain (Myr+) added so that the ΔAkt-1:ER*(Myr+) is membrane-localized and active, while the ΔAkt-1:ER*(Myr−) has a mutation in the Myr sequence preventing its ability to be membrane-localized and is inactive. With these two Akt-1 constructs, we could determine that activation of Akt-1 and membrane localization was required for 4HT resistance.
An advantage of the MCF7/ΔAkt-1:ER*(Myr+) cells is that the activity of Akt-1 is inducible in the MCF7/ΔAkt-1:ER* by 4HT. A disadvantage is the effects that 4HT treatment will have on ER mediated gene expression in MCF-7 cells which are normally ER+. With the MCF7/ΔAkt-1:ER*(Myr+) cells, we could determine that activated Akt-1 also affected the expression of the MEK and ERK proteins as their expression increased upon Akt-1 activation (Figs. 4 and 6). Lower levels of activated MEK1 and ERK1/2 were detected in the 4HT-selected MCF7/ΔAkt-1:ER*(Myr+) cells than in the non-selected cells after addition of 4HT indicating that activated Akt suppressed MEK1 and downstream ERK as reported in other cell systems.72 Furthermore with the conditionally-active Akt, we could determine the effects of activation of Akt on the sensitivity of the cells to 4HT, doxorubicin and radiation.
These studies also indicate that doxorubicin and 4HT caused the induction of activated ERK1/2 in MCF-7 cells. We have previously observed that doxorubicin induced ERK activation in cytokine-dependent hematopoietic cells56 Estrogen is known to induce signaling pathways including the MAPK cascade in breast and other cell types.74–76 The mechanisms by which estrogen induces ERK are complex and it is not yet clear which ER (α or β) is involved. The effects of 4HT on ERK expression are not well elucidated and our studies point to the ability of 4HT to stimulate ERK phosphorylation at least at a low level after a prolonged exposure period.
Phosphorylation of p53 is one mechanism which regulates p53 activity.77 Chemotherapeutic drugs and radiation can induce p53 phosphorylation. We have previously demonstrated the induction of p53 after doxorubicin treatment of hematopoietic cells.56 In doxorubicin-sensitive MCF-7 cells, doxorubicin caused a dramatic increase in the levels of phosphorylated p53 at S15. Such an increase was not as dramatic in the drug resistant MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox) cells. In contrast, the levels of p53 phosphorylated at S392 were fairly constant. Phosphorylation of p53 at S15, inhibits its interaction with MDM2 which results in prevention of p53 degradation.78–81 Phosphorylation of p53 at 392 is associated with enhancing the DNA binding activity of p53.82 We observed a dramatic increase in phosphorylation of p53 at S15 but not S392 in MCF-7. In contrast, we did not observe a large increase in phosphorylation of p53 in response to doxorubicin in MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox) cells. We did not detect an increase in phosphorylation of p53 at S15 in response to 4HT in either MCF-7 or MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox) cells.
Previous studies have elucidated the key role of p53 in the induction of p21Cip-1 in response to chemotherapeutic drugs.83 p21Cip-1 induction by p53 can block cellular cycle progression and may in some cases result in cellular senescence.84 Although recent studies have indicated that p53 may block cellular senescence and lead instead to cellular quiescence.85–88 The levels of p21Cip-1 were increased in MCF-7 cells upon treatment with doxorubicin, in contrast such a dramatic increase in p21Cip-1 phosphorylation were not observed in MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox)R cells. Thus cell cycle progression is not as suppressed by doxorubicin induced p21Cip-1 expression in MCF7/ΔAkt-1:ER*(Myr+) (4HT+Dox)R cells as opposed to MCF-7 cells. These effects of doxorubicin were readily observed on the plating efficiency of MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox)R and MCF-7 cells. MCF-7 cells did not readily form colonies when they were plated in medium containing doxorubicin, while more colonies were recovered from MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox)R cells.
p27Kip-1 is also important in cell cycle progression and cellular senescence. However, p21Kip-1 appeared to be fairly constant in both MCF-7 and MCF7/ΔAkt-1:ER*(Myr+)(4HT+Dox)R cells.
We observed that doxorubicin treatment lead to the accumulation of p21Cip-1 in MCF-7 cells which have wild-type p53. In contrast in the drug resistant 4HT and doxorubicin selected MCF7/ΔAkt-1:ER*(Myr+)(4HT+DoxR), lower levels of S15-phosphorylated p53 and total p21Cip-1 were detected. Doxorubicin may induce reactive oxidative species (ROS), which, in turn, activate p53 and have effects on the induction of cellular senescence.89,90
The effects of Akt and p53 on sensitivity to radiation and the induction of cellular senescence of cells are being elucidated.91–98 In our studies, the activation of Akt-1 increased the radio resistance of MCF-7 cells, at least up to 2 grays. Some recent studies in other cancer types have shown the Akt expression can promote radio-resistance.99–104 In certain cases the radio resistance may be due to the increased Akt expression of the repair of double strand DNA breaks.99,100 However our studies are novel as we have investigated the effects of Akt-1 activation on sensitivity of breast cancer to radiation in combination with both hormonal and chemotherapy.
These results are relevant to potential cancer therapies as Akt is frequently activated by upstream PIK3CA or PTEN mutations or gene silencing. PTEN can be mutated or silenced by various mechanisms in human cancer and clearly this pathway plays important roles in breast and other cancers and the generation of cancer stem cells.105–110 Mutations occur which either delete the PTEN gene or alter its activity. Sometimes these mutations actually make the cells sensitive to Akt and mTOR inhibitors as the growth of the cells becomes dependent upon elevated Akt levels and downstream mTOR and p70S6K activities.49 Determining the activation status of the PI3K/PTEN/Akt/mTOR pathway may enhance the ability to treat breast cancer by various approaches, including chemotherapy, hormonal therapy and radio-therapy.
Materials and Methods
Cell culture.
MCF-7 cells were obtained from the American Type Culture Collection (ATCC) (Manassas, VA). Cell culture medium for MCF-7 cells consisted of Roswell Park Memorial Institute-1640 (RPMI 1640) medium (Invitrogen, Carlsbad, CA) supplemented with 10% (v/v) heat inactivated fetal bovine serum (FBS) as described in reference 6.
Akt plasmids.
MCF-7 cells were infected with the Akt-1 encoding viruses [ΔAkt-1:ER(Myr+) or ΔAkt-1:ER(Myr−)] as described in reference 71. Stably transfected MCF-7 cells were isolated after growth in 25 µg/ml blasticin (Invitrogen, Carlsbad, CA) for ΔAkt-1:ER(Myr+) or ΔAkt-1:ER(Myr−) as described in reference 71.
Analysis of sensitivity to doxorubicin and rapamycin.
Cells were seeded in 96-well cell culture plates (BD Biosciences) at a density of 5,000 cells/well in 100 µl/well of phenol red free RPMI-1640 containing 5% charcoal stripped (CS) FBS as described in reference 6. Cells were incubated for 1 d to permit cells to adhere to the bottom of each well. Cells were subsequently treated with serial 2-fold dilutions of doxorubicin, some in the presence of 500 nM 4HT. Cells were then incubated at 37°C for 4 d until the extent of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT, Sigma, St. Louis, MO) reduction in each well was quantified at 530 nm.6
Clonogenic assays and radiation treatment.
MCF-7 cells were collected and seeded in 6-well cell culture plates at densities of either 1,000 or 10,000 cells/well as described in reference 91. In the experiments to examine the effects of 4HT presented in Figure 3, the cells were allowed to adhere to the plates for 24 h and then treated with 1,000 nM 4HT, 1,000 nM estrogen or 0.1% DMSO or the various combinations. In the experiments to examine the effects of the 4HT, doxorubicin and radiation presented in Figures 7 and 8, the cells were plated in the 6-well plates for 24 h and then irradiated. Cells were irradiated with a Gammacell 40 (Atomic Energy of Canada Limited, Cs137 source). The cells were then cultured for 24 h before the addition of either 1,000 nM 4HT or 100 nM doxorubicin. Plates were incubated for 3–4 weeks and then stained with giemsa dye and colonies determined.
Western blot analysis.
Western blots were probed with antibodies specific for phospho and total Akt, MEK, ERK p53 and total p21Cip-1 and p27Kip-1 as previously described in reference 55. Antibodies used in this study were purchased from Cell Signaling (Beverly, MA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by a grant (R01CA098195) entitled Ras/Raf and PI3K/Akt Induced Breast Cancer Drug Resistance, from the US National Institutes of Health to J.A.M. This work was also supported in part by grants from: Fondazione Carisbo (to L.C.), MinSan 2008 entitled “Molecular therapy in paediatric sarcomas and leukemias against IGF-1 receptor system” (to A.M.M.), PRIN 2008 (to A.M.M.) and FIRB 2010 (RBAP10447J, to A.M.M. and L.C.). This work was also supported in part by a grant from the Italian Ministry of Health, Ricerca Finalizzata Stemness 2008 entitled “Molecular Determinants of Stemness and Mesenchymal Phenotype in Breast Cancer” (to M.L.). We thank Dr. Martin McMahon (UCSF) for generously providing the conditional ΔAkt-1:ER*(Myr+) and ΔAkt-1:ER*(Myr−) constructs.
References
- 1.Shelton JG, Steelman LS, Abrams SL, Bertrand FE, Franklin RA, McMahon M, et al. The epidermal growth factor receptor gene family as a target for therapeutic intervention in numerous cancers: what's genetics got to do with it? Expert Opin Ther Targets. 2005;9:1009–1030. doi: 10.1517/14728222.9.5.1009. [DOI] [PubMed] [Google Scholar]
- 2.Rexer BN, Engelman JA, Arteaga CL. Overcoming resistance to tyrosine kinase inhibitors: lessons learned from cancer cells treated with EGFR antagonists. Cell Cycle. 2009;8:18–22. doi: 10.4161/cc.8.1.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 4.Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MN, et al. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle. 2010;9:188–197. doi: 10.4161/cc.9.1.10407. [DOI] [PubMed] [Google Scholar]
- 5.McCubrey JA, Steelman LS, Kempf CR, Chappell W, Abrams SL, Stivala F, et al. Therapeutic Resistance Resulting from Mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR Signaling Pathways. J Cell Physiol. 2011 doi: 10.1002/jcp.22647. In press. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein-Oppenheimer CR, Henriquez-Roldan CF, Davis JM, Navolanic PM, Saleh OA, Steelman LS, et al. Role of the Raf signal transduction cascade in the in vitro resistance to the anticancer drug doxorubicin. Clin Cancer Res. 2001;7:2898–2907. [PubMed] [Google Scholar]
- 7.Davis JM, Navolanic PM, Weinstein-Oppenheimer CR, Steelman LS, Hu W, Konopleva M, et al. Raf-1 and Bcl-2 induce distinct and common pathways that contribute to breast cancer drug resistance. Clin Cancer Res. 2003;9:1161–1170. [PubMed] [Google Scholar]
- 8.Steelman LS, Bertrand FE, McCubrey JA. The complexity of PTEN: mutation, marker and potential target for therapeutic intervention. Expert Opin Ther Targets. 2004;8:537–550. doi: 10.1517/14728222.8.6.537. [DOI] [PubMed] [Google Scholar]
- 9.Luo J, Cantley LC. The negative regulation of phosphoinositide-3-kinase signaling by p85 and it's implication in cancer. Cell Cycle. 2005;4:1309–1312. doi: 10.4161/cc.4.10.2062. [DOI] [PubMed] [Google Scholar]
- 10.Steelman LS, Chappell WH, Abrams SL, Kempf CR, Long J, Laidler P, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging. 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappell WH, Steelman LS, Long JM, Kempf CR, Abrams SL, Franklin RA, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rational and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–164. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ligresti G, Militello L, Steelman LS, Cavallaro A, Basile F, Nicoletti F, et al. PIK3CA mutations in human solid tumors. Cell Cycle. 2009;8:1352–1358. doi: 10.4161/cc.8.9.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martelli AM, Evangelisti C, Chiarini F, McCubrey JA. The phosphatidylinositol-3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget. 2010;1:89–103. doi: 10.18632/oncotarget.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L, Vogt PK. Hot-spot mutations in p110alpha of phosphatidylinositol-3-kinase (pI3K): differential interactions with the regulatory subunit p85 and with RAS. Cell Cycle. 2010;9:596–600. doi: 10.4161/cc.9.3.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Cappellini A, Ognibene A, et al. The emerging role of the phosphatiylinositol-3-kinase/Akt/mammalian target of rapamycin signaling network in normal myelopoiesis and leukemogensis. Biochem Biophys Act. 2010;1803:991–1002. doi: 10.1016/j.bbamcr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 17.Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 18.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Cappellini A, Ognibene A, et al. The emerging role of the phosphatiylinositol-3-kinase/Akt/mammalian target of rapamycin signaling network in normal myelopoiesis and leukemogensis. Biochim Biophys Act. 2010;1803:991–1002. doi: 10.1016/j.bbamcr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/S0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 20.Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narasimhan SD, Mukhopadhyay A, Tissenbaum HA. InAKTivation of insulin/IGF-1 signaling by dephosphorylation. Cell Cycle. 2009;8:3878–3884. doi: 10.4161/cc.8.23.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brauer PM, Tyner AL. RAKing in AKT: a tumor suppressor function for the intracellular tyrosine kinase FRK. Cell Cycle. 2009;8:2728–2732. doi: 10.4161/cc.8.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasiprogram. Cell Cycle. 2010;9:3151–3156. doi: 10.4161/cc.9.16.13120. [DOI] [PubMed] [Google Scholar]
- 25.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 26.Demidenko ZN, Blagosklonny MV. At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence. Cell Cycle. 2009;8:1901–1904. doi: 10.4161/cc.8.12.8810. [DOI] [PubMed] [Google Scholar]
- 27.Demidenko ZN, Shtutman M, Blagosklonny MV. Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence. Cell Cycle. 2009;8:1896–1900. doi: 10.4161/cc.8.12.8809. [DOI] [PubMed] [Google Scholar]
- 28.Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–1895. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- 29.Blagosklonny MV. Aging-suppressants: Cellular senescence (hyperactivation) and its pharmacologic decleration. Cell Cycle. 2009;8:1883–1887. doi: 10.4161/cc.8.12.8815. [DOI] [PubMed] [Google Scholar]
- 30.Yang WL, Wu CY, Wu J, Lin HK. Regulation of Akt signaling activation by ubiquitination. Cell Cycle. 2010;9:486–497. doi: 10.4161/cc.9.3.10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lal MA, Bae D, Camilli TC, Patierno SR, Ceryak S. AKT1 mediates bypass of the G1/S checkpoint after genotoxic stress in normal human cells. Cell Cycle. 2009;8:1589–1602. doi: 10.4161/cc.8.10.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin J, Wang GL, Timchenko L, Timchenko NA. GSK3beta and aging liver. Aging. 2009;1:582–585. doi: 10.18632/aging.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gan B, DePinho RA. mTORC1 signaling governs hematopoietic stem cell quiescence. Cell Cycle. 2009;8:1003–1006. doi: 10.4161/cc.8.7.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging. 2010;2:344–352. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leslie NR, Foti M. Not in my genes: non-genomic loss of PTEN function in cancer. Trends Pharmacol Sci. 2011;32:131–140. doi: 10.1016/j.tips.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Sayed D, Abdellatif M. AKT-ing via microRNA. Cell Cycle. 2010;9:3213–3217. doi: 10.4161/cc.9.16.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh B, Ittmann MM, Krolewski JJ. Sporadic breast cancers exhibit loss of heterozygosity on chromosome segment 10q23 close to the Cowden disease locus. Genes Chromosomes Cancer. 1998;21:166–171. doi: 10.1002/(SICI)1098-2264(199802)21:2<166::AID-GCC13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 38.Tsutsui S, Inoue H, Yasuda K, Suzuki K, Higashi H, Era S, et al. Reduced expression of PTEN protein and its prognostic implications in invasive ductal carcinoma of the breast. Oncology. 2005;68:398–404. doi: 10.1159/000086981. [DOI] [PubMed] [Google Scholar]
- 39.Palomero T, Dominguez M, Ferrando AA. The role of the PTEN/AKT Pathway in NOTCH1-induced leukemia. Cell Cycle. 2008;7:965–970. doi: 10.4161/cc.7.8.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta A, Yang Q, Pandita RK, Hunt CR, Xiang T, Misri S, et al. Cell cycle checkpoint defects contribute to genomic instability in PTEN deficient cells independent of DNA DSB repair. Cell Cycle. 2009;8:2198–2210. doi: 10.4161/cc.8.14.8947. [DOI] [PubMed] [Google Scholar]
- 41.Diaz-Meco MT, Abu-Baker S. The Par-4/PTEN connection in tumor suppression. Cell Cycle. 2009;8:2518–2522. doi: 10.4161/cc.8.16.9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shan W, Liu J. Epithelial ovarian cancer: focus on genetics and animal models. Cell Cycle. 2009;8:731–735. doi: 10.4161/cc.8.5.7848. [DOI] [PubMed] [Google Scholar]
- 43.Poon JS, Eves R, Mak AS. Both lipid- and protein-phosphatase activities of PTEN contribute to the p53-PTEN anti-invasion pathway. Cell Cycle. 2010;9:4450–4454. doi: 10.4161/cc.9.22.13936. [DOI] [PubMed] [Google Scholar]
- 44.Martelli AM, Evangelisti C, Chappell W, Abrams SL, Bäsecke J, Stivala F, et al. Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia. 2011;25:1064–1079. doi: 10.1038/leu.2011.46. [DOI] [PubMed] [Google Scholar]
- 45.Steelman LS, Franklin RA, Abrams SL, Chappell W, Kempf CR, Bäsecke J, et al. Roles of the Ras/Raf/MEK/ERK Pathway in Leukemia Therapy. Leukemia. 2011 doi: 10.1038/leu.2011.66. In press. [DOI] [PubMed] [Google Scholar]
- 46.Bose S, Crane A, Hibshoosh H, Mansukhani M, Sandweis L, Parsons R. Reduced expression of PTEN correlates with breast cancer progression. Hum Pathol. 2002;33:405–409. doi: 10.1053/hupa.2002.124721. [DOI] [PubMed] [Google Scholar]
- 47.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- 48.Tokunaga E, Kimura Y, Mashino K, Oki E, Kataoka A, Ohno S, et al. Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer. 2006;13:137–144. doi: 10.2325/jbcs.13.137. [DOI] [PubMed] [Google Scholar]
- 49.deGraffenried LA, Friedrichs WE, Russell DH, Donzis EJ, Middleton AK, Silva JM, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin Cancer Res. 2004;10:8059–8067. doi: 10.1158/1078-0432.CCR-04-0035. [DOI] [PubMed] [Google Scholar]
- 50.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 51.Lin HJ, Hsieh FC, Song H, Lin J. Elevated phosphorylation and activation of PDK-1/AKT pathway in human breast cancer. Br J Cancer. 2005;93:1372–1381. doi: 10.1038/sj.bjc.6602862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klos KS, Wyszomierski SL, Sun M, Tan M, Zhou X, Li P, et al. ErbB2 increases vascular endothelial growth factor protein synthesis via activation of mammalian target of rapamycin/p70S6K leading to increased angiogenesis and spontaneous metastasis of human breast cancer cells. Cancer Res. 2006;66:2028–2037. doi: 10.1158/0008-5472.CAN-04-4559. [DOI] [PubMed] [Google Scholar]
- 53.Armour SM, Baur JA, Hsieh SN, Land-Bracha A, Thomas SM, Sinclair DA. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging. 2009;1:515–528. doi: 10.18632/aging.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faridi J, Wang L, Endemann G, Roth RA. Expression of Constitutively Active Akt-3 in MCF-7 Breast Cancer Cells Reverses the Estrogen and Tamoxifen Responsivity of these Cells in Vivo. Clin Cancer Res. 2003;9:2933–2939. [PubMed] [Google Scholar]
- 55.Steelman LS, Navolanic PN, Sokolosky M, Taylor JR, Lehmann BD, Chappell WH, et al. Suppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity of mTOR inhibitors. Oncogene. 2008;27:4086–4095. doi: 10.1038/onc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCubrey JA, Abrams SL, Ligresti G, Misaghian N, Wong ET, Basecke J, et al. Involvement of p53 and Raf/MEK/ERK pathways in hematopoietic drug resistance. Leukemia. 2008;22:2080–2090. doi: 10.1038/leu.2008.207. [DOI] [PubMed] [Google Scholar]
- 57.Abrams SL, Steelman LS, Shelton JG, Wong EW, Chappell WH, Basecke J, et al. The Raf/MEK/ERK pathway can govern drug resistance, apoptosis and sensitivity to targeted therapy. Cell Cycle. 2010;9:1781–1791. doi: 10.4161/cc.9.9.11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abrams SL, Steelman LS, Shelton JG, Chappell W, Basecke J, Stivala F, et al. Enhancing therapeutic efficacy by targeting non-oncogene addicted cells with combinations of signal transduction inhibitors and chemotherapy. Cell Cycle. 2010;9:1839–1846. doi: 10.4161/cc.9.9.11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steelman LS, Abrams SL, Shelton JG, Chappell WH, Bäsecke J, Stivala F, et al. Dominant roles of the Raf/MEK/ERK pathway in cell cycle progression, prevention of apoptosis and sensitivity to chemotherapeutic drugs. Cell Cycle. 2010;9:1629–1638. doi: 10.4161/cc.9.8.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 61.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/β-catenin signaling. PLoS Biol. 2009;7:1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Misaghian N, Ligresti G, Steelman LS, Bertrand FE, Bäsecke J, Libra M, et al. Targeting the leukemic stem cell—the holy grail of leukemia therapy. Leukemia. 2009;23:25–42. doi: 10.1038/leu.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buitenhuis M, Coffer PJ. The role of the PI3K-PKB signaling module in regulation of hematopoiesis. Cell Cycle. 2009;8:560–566. doi: 10.4161/cc.8.4.7654. [DOI] [PubMed] [Google Scholar]
- 64.Tamburini J, Green AS, Chapuis N, Bardet V, Lacombe C, Mayeux P, et al. Targeting translation in acute myeloid leukemia: a new paradigm for therapy? Cell Cycle. 2009;8:3893–3899. doi: 10.4161/cc.8.23.10091. [DOI] [PubMed] [Google Scholar]
- 65.Krymskaya VP, Goncharova EA. PI3K/mTORC1 activation in hamartoma syndromes: therapeutic prospects. Cell Cycle. 2009;8:403–413. doi: 10.4161/cc.8.3.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: thinking beyond rapamycin. Cell Cycle. 2009;8:3831–3837. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]
- 67.Sabisz M, Skladonowski A. Cancer stem cells and escape from drug-induced premature senescence in human lung tumor cells: implications for drug resistance and in vitro drug screening models. Cell Cycle. 2009;8:3208–3217. doi: 10.4161/cc.8.19.9758. [DOI] [PubMed] [Google Scholar]
- 68.McCubrey JA, Abrams SL, Stadelman K, Chappell WH, Lahair M, Ferland RA, et al. Targeting signal transduction pathways to eliminate chemotherapeutic drug resistance and cancer stem cells. Adv Enzyme Regul. 2010;50:285–307. doi: 10.1016/j.advenzreg.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCubrey JA, Chappell WH, Abrams SL, Franklin RA, Long JM, Sattler JA, et al. Targeting the cancer initiating cells: the Achilles' heel of cancer. Adv Enzyme Regul. 2011;51:152–162. doi: 10.1016/j.advenzreg.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Mirza AM, Kohn AD, Roth RA, McMahon M. Oncogenic transformation of cells by a conditionally active form of the protein kinase Akt/PKB. Cell Growth Differ. 2000;11:279–292. [PubMed] [Google Scholar]
- 71.Shelton JG, Steelman LS, Lee JT, Knapp SL, Blalock WL, Moye PW, et al. Effects of the RAF/MEK/ERK and PI3K/AKT signal transduction pathways on the abrogation of cytokine-dependence and prevention of apoptosis in hematopoietic cells. Oncogene. 2003;22:2478–2492. doi: 10.1038/sj.onc.1206321. [DOI] [PubMed] [Google Scholar]
- 72.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 73.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277:31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 74.Migliaccio A, Pagano M, Auricchio F. Immediate and transient stimulation of protein tyrosine phosphorylation by estradiol in MCF-7 cells. Oncogene. 1993;8:2183–2191. [PubMed] [Google Scholar]
- 75.Singh M, Sétáló G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh M, Sétáló G, Jr, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ashcroft M, Kubbutat MHG, Vousden KH. Regulation of p53 function and stability by phosphorylation. Mol Cell Biol. 1999;19:1751–1758. doi: 10.1128/mcb.19.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lees-Miller SP, Sakaguchi K, Ullrich SJ, Appella E, Anderson CW. Human DNA-activated protein kinase phosphorylates serines 15 and 37 in the amino-terminal transactivation domain of human p53. Mol Cell Biol. 1992;12:5041–5049. doi: 10.1128/mcb.12.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by mdm2. Cell. 1997;91:325–334. doi: 10.1016/S0092-8674(00)80416-X. [DOI] [PubMed] [Google Scholar]
- 80.Maclaine NJ, Hupp TR. The regulation of p53 by phosphorylation: a model for how distinct signals integrate into the p53 pathway. Aging. 2009;1:490–502. doi: 10.18632/aging.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang B, Vassilev LT. Reduced transcriptional activity in the p53 pathway of senescent cells revealed by the MDM2 antagonist nutlin-3. Aging. 2009;1:845–854. doi: 10.18632/aging.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keller DM, Zeng X, Wang Y, Zhang QH, Kapoor M, Shu H, et al. A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16 and SSRP1. Mol Cell. 2001;7:283–292. doi: 10.1016/S1097-2765(01)00176-9. [DOI] [PubMed] [Google Scholar]
- 83.Sankala HM, Hait NC, Paugh SW, Shida D, Lépine S, Elmore LW, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 84.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53 and p21(CIP1), but not p16(INK4a) Mol Cell. 2004;14:501–513. doi: 10.1016/S1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 85.Korotchkina LG, Demidenko ZN, Gudkov AV, Blagosklonny MV. Cellular quiescence caused by the Mdm-2 inhibitor nutlin-3A. Cell Cycle. 2009;8:3777–3781. doi: 10.4161/cc.8.22.10121. [DOI] [PubMed] [Google Scholar]
- 86.Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci USA. 2010;107:9660–9664. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging. 2010;2:924–935. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haferkamp S, Tran SL, Becker TM, Scurr LL, Kefford RF, Rizos H. The relative contributions of the p53 and pRb pathways in oncogene-induced melanocyte senescence. Aging. 2009;1:542–556. doi: 10.18632/aging.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging. 2010;2:471–474. doi: 10.18632/aging.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging. 2010;2:924–935. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lehmann BD, McCubrey JA, Jefferson HS, Paine MS, Chappell WH, Terrian DM. A dominant role for p53-dependent cellular senescence in radiosensitization of human prostate cancer cells. Cell Cycle. 2007;6:595–605. doi: 10.4161/cc.6.5.3901. [DOI] [PubMed] [Google Scholar]
- 92.Labi V, Villunger A. PUMA-mediated tumor suppression: A tale of two stories. Cell Cycle. 2010;9:4269–4275. doi: 10.4161/cc.9.21.13666. [DOI] [PubMed] [Google Scholar]
- 93.Hill R, Lee PW. The DNA-dependent protein kinase (DNA-PK): More than just a case of making ends meet? Cell Cycle. 2010;9:3460–3469. doi: 10.4161/cc.9.17.13043. [DOI] [PubMed] [Google Scholar]
- 94.Kim DH, Rho K, Kim S. A theoretical model for p53 dynamics: identifying optimal therapeutic strategy for its activation and stabilization. Cell Cycle. 2009;8:3707–3716. doi: 10.4161/cc.8.22.10023. [DOI] [PubMed] [Google Scholar]
- 95.Tao Y, Leteur C, Yang C, Zhang P, Castedo M, Pierre A, et al. Radiosensitization by Chir-124, a selective CHK1 inhibitor: effects of p53 and cell cycle checkpoints. Cell Cycle. 2009;8:1196–1205. doi: 10.4161/cc.8.8.8203. [DOI] [PubMed] [Google Scholar]
- 96.Dregalla RC, Zhou J, Idate RR, Battaglia CL, Liber HL, Bailey SM. Regulatory roles of tankyrase 1 at telomeres and in DNA repair: suppression of T-SCE and stabilization of DNA-PKcs. Aging. 2010;2:691–708. doi: 10.18632/aging.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anisimov VN. Metformin for aging and cancer prevention. Aging. 2010;2:760–774. doi: 10.18632/aging.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richardson RB. Ionizing radiation and aging: rejuvenating an old idea. Aging. 2009;1:887–902. doi: 10.18632/aging.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deng R, Tang J, Ma JG, Chen SP, Xia LP, Zhu XF, et al. PKB/Akt promotes DSB repair in cancer cells through upregulating Mre11 expression following ionizing radiation. Oncogene. 2011;30:944–955. doi: 10.1038/onc.2010.467. [DOI] [PubMed] [Google Scholar]
- 100.Mukherjee B, McEllin B, Camacho CV, Tomimatsu N, Sirasanagandala S, Nannepaga S, et al. EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res. 2009;69:4252–4259. doi: 10.1158/0008-5472.CAN-08-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xia S, Zhao Y, Yu S, Zhang M. Activated PI3K/Akt/COX-2 pathway induces resistance to radiation in human cervical cancer HeLa cells. Cancer Biother Radiopharm. 2010;25:317–323. doi: 10.1089/cbr.2009.0707. [DOI] [PubMed] [Google Scholar]
- 102.Krasny L. Shimony N, Tzukert K, Gorodetsky R, Lecht S, Nettelbeck DM, et al. An in-vitro tumour microenvironment model using adhesion to type I collagen reveals Akt-dependent radiation resistance in renal cancer cells. Nephrol Dial Transplant. 2010;25:373–380. doi: 10.1093/ndt/gfp525. [DOI] [PubMed] [Google Scholar]
- 103.Schuurbiers OC, Kaanders JH, van der Heijden HF, Dekhuijzen RP, Oyen WJ, Bussink J. The PI3-K/AKT-pathway and radiation resistance mechanisms in non-small cell lung cancer. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer. 2009;4:761–767. doi: 10.1097/JTO.0b013e3181a1084f. [DOI] [PubMed] [Google Scholar]
- 104.Kim TJ, Lee JW, Song SY, Choi JJ, Choi CH, Kim BG, et al. Increased expression of pAKT is associated with radiation resistance in cervical cancer. Br J Cancer. 2006;94:1678–1682. doi: 10.1038/sj.bjc.6603180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adams JR, Schachter NF, Liu JC, Zacksenhaus E, Egan SE. Elevated PI3K signaling drives multiple breast cancer subtypes. Oncotarget. 2011;2:435–447. doi: 10.18632/oncotarget.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pajic M, Kersbergen A, van Diepen F, Pfauth A, Jonkers J, Borst P, et al. Tumor-initiating cells are not enriched in cisplatin-surviving BRCA1; p53-deficient mammary tumor cells in vivo. Cell Cycle. 2010;9:3780–3791. doi: 10.4161/cc.9.18.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kemper K, Grandela C, Medema JP. Molecular identification and targeting of colorectal cancer stem cells. Oncotarget. 2010;1:387–395. doi: 10.18632/oncotarget.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vicente-Dueñas C, Abollo-Jiménez F, Ruiz-Roca L, Alonso-Escudero E, Jiménez R, Cenador MB, et al. The age of the target cell affects B-cell leukaemia malignancy. Aging. 2010;2:908–913. doi: 10.18632/aging.100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peng C, Chen Y, Li D, Li S. Role of Pten in leukemia stem cells. Oncotarget. 2010;1:156–160. doi: 10.18632/oncotarget.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bortolomai I, Canevari S, Facetti I, De Cecco L, Castellano G, Zacchetti A, et al. Tumor initiating cells: Development and critical characterization of a model derived from the A431 carcinoma cell line forming spheres in suspension. Cell Cycle. 2010;9:1194–1206. doi: 10.4161/cc.9.6.11108. [DOI] [PubMed] [Google Scholar]