Abstract
Research into conditions that improve axon regeneration has the potential to open a new door for treatment of brain injury caused by stroke and neurodegenerative diseases of aging, such as Alzheimer, by harnessing intrinsic neuronal ability to reorganize itself. Elucidating the molecular mechanisms of axon regeneration should shed light on how this process becomes restricted in the postnatal stage and in the CNS and therefore could provide therapeutic targets for developing strategies to improve axon regeneration in the adult CNS. In this review, we first discuss the general view about nerve regeneration and the advantages of using C. elegans as a model system to study axon regeneration. We then compare the conserved regeneration patterns and molecular mechanisms between C. elegans and vertebrates. Lastly, we discuss the power of femtosecond laser technology and its application in axon regeneration research.
Key words: axon regeneration, C. elegans, genetics, femtosecond laser, neuronal circuits
Introduction
Axon regeneration is crucial for the restoration of functional neuronal circuits after trauma in both vertebrates and invertebrates. The injured axon initiates a new growth cone at the end of the proximal segment while the distal segment of the injured axon undergoes Wallerian degeneration. In some cases, the regenerating axon can fuse with the distal segment, preventing it from degeneration.1 Based on studies in a variety of organisms, it was found that although the capacity of neuronal regeneration should be universal, in reality, not all neurons successfully regenerate after injury. Furthermore, the regulation of axon regeneration may involve unique mechanisms that are distinct from axonal development. Thus, understanding the dichotomy of regeneration responses in different neuron types at different ages and the underlying mechanisms of regeneration may help develop therapeutic strategies for the treatments of neurodegenerative diseases as well as brain and spinal cord injuries. Caenorhabditis elegans has emerged as a powerful model for studying axon regeneration since the invention of the femtosecond laser system opens up the possibilities for high through-put genetic screens to identify novel genes involved in regulating axon regeneration.2 In this review, we first discuss the general view about nerve regeneration and the advantages of using C. elegans as a model system to study axon regeneration. We then compare the conserved regeneration patterns and molecular mechanisms between C. elegans and vertebrates. Lastly, we discuss the power of femtosecond laser technology and its application in axon regeneration research.
Distinct Regeneration Responses between CNS and PNS Neurons
The nervous system can be grossly divided, based on function and location, into two distinct parts, the central nervous system (CNS) and the peripheral nervous system (PNS) (Fig. 1). The different regeneration capacity of these two neuronal compartments has been noticed since the early 19th century.3 In contrast to the PNS, where injured neurons can regenerate robustly, neurons located within the CNS fail to regenerate after injury in adult warm-blooded vertebrates, including mammals and birds. Interestingly, mammals at embryonic or perinatal stages and some cold-blooded amphibians, such as newts, are capable of robust regeneration in both CNS and PNS neurons at all ages.4 The dichotomy in regeneration responses between CNS and PNS neurons may be attributed to both the lack of intrinsic axon growth promoting factors in CNS neurons as well as the inhibitory CNS environment.5
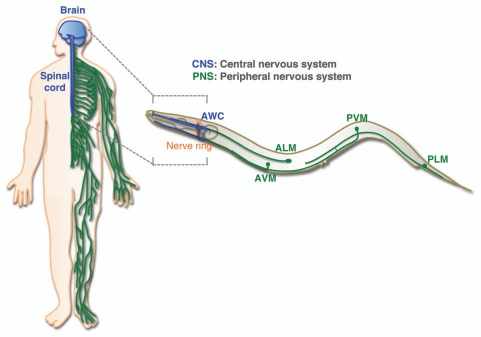
Figure 1.
Analogous counterparts of the human nervous systems in C. elegans. The human nervous system can be divided, based primarily on the locations, into the central nervous system (CNS) and the peripheral nervous system (PNS). The human CNS consists of the brain and the spinal cord while the rest of the nervous system belongs to the PNS. The nerve ring located at the head region of C. elegans is a CNS equivalent. Neurons within the nerve ring, such as AWC, display limited axon regeneration after injury. In contrast, neurons outside of the nerve ring, including ALM, can effectively regenerate axons after injury.
Although CNS neurons in the embryonic or perinatal stages in mammals are capable of robust regeneration, their adult counterparts are not. This developmental decline in regeneration capacity implies that the intrinsic growth program of adult CNS neurons does not support regeneration, which can be attributed to various cell autonomous factors.6–11 The noticeably reduced level of endogenous cAMP in adult neurons in comparison to those at younger stages limits the regeneration of adult CNS neurons.7 The effect of endogenous cAMP levels on axon regeneration has been extensively studied in the dorsal root ganglion (DRG) neurons. DRG neurons develop as bipolar sensory neurons, projecting neurites to both CNS and PNS regions. The inability of regeneration occurs in the CNS branch, but not the PNS branch. However, if the PNS and CNS branch are dissected sequentially, the severed PNS branch triggers the elevation of endogenous cAMP levels, which in turn allows the CNS branch to regrow extensively.8 The limited ability of mature CNS neurons to regenerate axons is also influenced by the diminished activity of the mammalian target of rapamycin (mTOR) pathway, which normally acts to regulate cell growth,9 and the reduced level of distinctive sets of gene expression regulated by the transcription factors, Krüppel-like factor 4 (KLF4) 10 and STAT3.11
The inhibitory CNS environment is the other key factor that contributes to the incapacity of regeneration in adult CNS neurons. By bridging a segment of peripheral nerve (PN) to the injured spinal cord, Aguayo and colleagues observed CNS neurons regenerate remarkably into the PN graft, which is permissive to axon regeneration. However, the regeneration is impeded once the regenerating axons reach the CNS region.12 This observation implies that the adult CNS environment is inhibitory to axon regeneration, which can be attributed to the presence of glial scars, myelin debris and several repulsive axon guidance cues.13 Glial scars form at the lesion site 14 d after injury.14,15 It not only acts as a physical barrier, but also secretes a number of extracellular matrix molecules, most notably chondroitin sulfate proteoglycans (CSPGs), that are inhibitory to axon regeneration.16–18 In addition, the removal of myelin debris after injury is considerably slow in the CNS region. The long lasting myelin debris induces the axon retraction, suggesting that myelin debris contains the inhibitory factors of axon regeneration.3 Subsequently, three major myelin-based inhibitors have been identified, including myelin-associated glycoprotein (MAG), Nogo and oligodendrocyte myelin glycoprotein (OMgp).19–25 Despite lack of sequence or structural similarity in these three molecules, they seem to share a common receptor, NgR1.25–28 The mechanisms underlying the inhibition of myelin-based factors to axon regeneration remain to be clarified due to the conflicting reports of regeneration obtained from the knockout mice of Nogo, MAG and NgR.29,30 Lastly, the repulsive axon guidance cues are likely another obstacle of axon regeneration in the CNS.31 Many of the guidance cues are downregulated once development is accomplished, however, some of the repellents, such as Netrin, Sema4D and Sema5A, remain expressed by adult oligodendrocytes.5,31–37 More interestingly, the expressions of Sema3A and Eph-B3 are induced in the fibroblasts and astrocytes after brain injury.38–40 The contribution of repulsive guidance factors to myelin-based regeneration block is revealed by the mild improvement of regeneration in ephrin-B3 knockouts, but the roles of other repellents remains unclear.37
The Advantages of the C. elegans Model in the Study of Axon Regeneration
Some progress has been made in understanding the dichotomy of neuronal regeneration between the CNS and the PNS in vertebrates based on studies mainly performed using the in vitro explant assays. However, reports of regeneration observed in mouse knockouts sometimes conflict with results obtained from the in vitro explant assays.30 Thus, it remains largely unknown how to overcome the inhibitory CNS environment to regenerate severed neuronal circuits. The challenge to the field is to develop a genetically tractable model system accessible to an optical scalpel that would enable us to perform high throughput and unbiased screens to identify novel molecules that regulate axon regeneration.
C. elegans is a great model system suitable for delineating the molecular mechanisms of regeneration for several reasons. First, C. elegans has a simple nervous system composed of 302 neurons with a complete map of all axon trajectories and synaptic connections. Second, many powerful genetic tools are available in worms to allow identification, characterization and testing of interactions between genes involved in axon regeneration. Third, many genes are highly conserved between C. elegans and humans. Thus, what we learn in C. elegans would be relevant to regeneration of the brain and spinal cord in humans. Fourth, the optimal size and transparency of nematodes allows us to visualize axon regeneration in live animals using time-lapse fluorescent microscopy as well as to cut any axon of interest using femtosecond laser technology. Most importantly, many intriguing regeneration patterns and molecular mechanisms are conserved between C. elegans and vertebrates (Table 1), suggesting that we may use C. elegans as a model to facilitate our understanding of nerve regeneration in humans.
Table 1.
The conserved patterns and molecular mechanisms of axon regeneration between vertebrates and C. elegans
| Regeneration patterns | Vertebrates | C. elegans |
| Dichotomy of regeneration ability between CNS and PNS neurons Yes3–5 | Yes41,42 | |
| Developmental decline of intrinsic axon growth ability | Yes43 | Yes41,42,44 |
| Manner of regeneration influenced by the proximity of the injury to the cell body | Yes45–48 | Yes42 |
| Synaptic branch negatively influences the intrinsic growth state of injured neurons | Yes49 | Yes41 |
| Regenerating axon fused with the disconnected distal axon segment | ND | Yes1 |
| Molecular mechanisms of axon regeneration | Vertebrates | C. elegans |
| Ephrin signaling inhibits axon regeneration | Yes37,40 | Yes41 |
| cAMP signaling promotes axon regeneration | Yes7,8,49–52 | Yes53 |
| p38 MAP kinase pathway promotes axon regeneration | Yes54–56 | Yes44 |
| mTOR signaling enhances axon regeneration | Yes9 | ND |
| klf-4 restricts axon regeneration | Yes10 | ND |
ND, not determined.
The Conserved Patterns of Axon Regeneration between C. elegans and Vertebrates
The most striking resemblance between C. elegans and vertebrates is the dichotomy of regeneration ability between CNS and PNS neurons. In vertebrates, PNS neurons display robust axon regeneration after injury while CNS neurons exhibit limited axon regeneration.5 In C. elegans, the nerve ring in the head is considered an equivalent of the vertebrate CNS. Neurons located within the nerve ring, such as amphid neurons AWC, AWB and ASH, are incapable of regenerating axons, while a variety of different PNS neurons, such as touch neurons (AVM, ALM, PVM and PLM), regenerate axons extensively after laser axotomy (Fig. 1 and unpublished observations).41,42
Also, both C. elegans and vertebrates display a decline in axon regeneration capacity in advacing development. Studies of spinal cord regeneration in vertebrates show that chick and opossum exhibit effective repair after spinal cord injury at the embryonic stage, but gradually lose the regeneration ability at the postnatal stage.43 Similarly, it was demonstrated in C. elegans that ALM, AVM and DD/VD neurons regenerate significantly longer axons at larva stages than adult stages.41,42,44 In addition, regeneration at larva stages display less guidance errors.41,42 In early development, AVM neurons first project an axon ventrally to the ventral nerve cord before extending anteriorly along the ventral midline to the nerve ring. The chance for a regenerating AVM axon to reach the ventral nerve cord following injury at the ventral projection of the AVM axon decreases as development advances.42
Another conserved regeneration pattern between C. elegans and vertebrates is that the proximity of axonal injury to the cell body determines the regeneration responses. When the axon is transected at different positions, the farther the lesion is made away from the cell body the greater the chance injured neurons initiate growth cones from the proximal end of the injured axon.42,45–48
Furthermore, it was also found that, the synaptic branch has a negative influence on axon regeneration in vertebrates and C. elegans. Preconditioning lesions in the dorsal root ganglion (DRG) neurons in vertebrates, in which a lesion is made at the peripheral branch 1 or 2 weeks prior to the central branch injury, results in an increase of regeneration capacity of the central branch in DRG neurons.49 The effects of cutting the synaptic branch in C. elegans are analogous to ‘preconditioning lesion’ paradigms in the dorsal root ganglion (DRG) neurons in vertebrates. In C. elegans, the touch neuron PLM projects an axon anteriorly at a lateral position and branches collaterally to form synapses with the ventral nerve cord. In contrast to the robust regeneration observed after axotomy that is proximal to the PLM axon branch, almost no regrowth occurs that is distal to the branch following axotomy.41 Strikingly, if the branch and the distal axon are cut sequentially, the distal axon becomes capable of regrowth.41 These observations suggest that the synaptic branch somehow controls the intrinsic growth state of injured neurons and has an inhibitory effect on distal axon regeneration.
The ultimate goal of regeneration is to repair the damaged neuronal circuit and restore its function. There are many ways to reconnect the injured neuron to its target. For example, a regenerating axon can either follow the original axonal trajectory or take an ectopic path to reach the target. Axonal fusion is the former way of re-establishing the connection by a regenerating axon that directly bridges between the proximal and distal segments of the original axon. However, axon fusion is only described in C. elegans and other invertebrates, like crayfish, earthworms and leech.1,53,57–60
Shared Molecular Mechanisms of Axon Regeneration between C. elegans and Vertebrates
As mentioned earlier, repulsive axon guidance cues existing in the CNS environment block axon regeneration. Ephrin, a myelin-based inhibitor that prevents axon regeneration of CNS neurons in mice, has also been reported for its negative influence on axon regeneration in C. elegans (Table 1).37,41 In worms lacking VAB-1 Eph receptors, regenerating PLM axons display significantly less misguidance phenotypes than those in wild-type animals.41 PLM axons of vab-1 mutants regrew more linearly and stayed closer to their original trajectory after injury.41 In addition, transgenic animals expressing a myristoylated Ephrin receptor (MYR::VAB-1) in PLM neurons displayed significantly less anterior regrowth than wild-type animals. These studies suggest a negative role for Ephrin signaling in both axon guidance and outgrowth during regeneration. Interestingly, it was further shown that Ephrin signaling regulates axon guidance in regeneration through a kinaseindependent mechanism while regulating axon outgrowth in regeneration through a kinase-dependent mechanism.41
In addition to the extracellular inhibitors, intrinsic factors are important in controlling the process of axon regeneration. The endogenous level of cAMP has been correlated with the regeneration capacity in both vertebrates and C. elegans (Table 1).8,49,50,53 Under the conditioning lesion, in which the peripheral branch of DRG neurons was cut prior to the central branch lesion, the intrinsic cAMP level of DRG neurons was elevated after the conditioning lesion, leading to efficient axon regeneration in the central branch of DRG neurons.49 Elevation of cAMP levels using cAMP analog or rolipram that prevents cAMP breakdown is sufficient to alter the regeneration response of DRG neurons after injury.8,51,52 In C. elegans, the elevated cAMP level due to the loss of neuronal phosphodiesterase, pde-4, promotes axon regeneration as judged by faster growth cone formation and higher growth rate in injured axons.53
Another class of intrinsic factors that regulates axon regeneration is the mitogen-activated protein (MAP) kinase cascade, which includes MAPKKK dlk-1, MAPKK mkk-4 and p38 MAPK pmk-3 (Table 1). dlk-1, an important regulator of presynaptic development, was identified in a genetic screen for genes involved in spontaneous axon regeneration after progressive axon fragmentation induced by neuronal β-spectrin unc-70 mutation in C. elegans.61,62 In dlk-1 mutants, transected axons were able to develop the transient filopodia at the tip similar to wild-type animals. However, they never formed a growth cone and exhibited only limited axon regeneration. In contrast, overexpression of dlk-1 not only accelerates growth cone formation, but also ameliorates the morphology and behavior of growth cones in C. elegans.44 Like dlk-1, mkk-4 and pmk-3 mutants fail to initiate axon regeneration after injury in C. elegans. However, dlk-1 overexpression is unable to bypass the requirement of mkk-4 and pmk-3, suggesting that mkk-4 and pmk-3 act downstream of dlk-1 in the same genetic pathway to regulate axon regeneration. p38 MAP kinase has been previously shown to regulate the remodeling of microtubules and local protein synthesis that are essential for growth cone formation in vertebrates.54,55 The application of the p38 inhibitor significantly eliminated the growth cone formation and axon regeneration in the cultured DRG neurons.56 These observations indicate an evolutionarily conserved role of the MAP kinase cascade in axon regeneration from C. elegans to vertebrates.
Cell growth control genes have been recently identified as important regulators of axon regeneration (Table 1).9 It was shown that mTOR signaling is downregulated and global protein translation is reduced in injured adult RGC neurons, which may contribute to their regeneration failure.9 Moreover, growth cone formation is severely affected in the cultured DRG neurons upon rapamycin, the mTOR (mammalian target of rapamycin) inhibitor, treatment.56 Strikingly, deletion of PTEN (phosphatase and tensin homolog) and tuberous sclerosis complex 1, two negative regulators of the mTOR pathway, enables axons to overcome inhibition and regenerate after injury.9 Although the effect of the mTOR pathway on axon regeneration in C. elegans has yet to be determined, the restricted neuronal expression of the PTEN homolog, DAF-18, in a subset of amphid neurons located in the nerve ring in the head suggests that PTEN may not have a strong role in inhibiting CNS regeneration in nematodes.63
The progressive decline in axon growth ability in advancing development implies expression of differential sets of genes in neurons at various ages. Comparison of gene expression profiles of RGC neurons between embryonic and postnatal stages identified the zinc-finger transcription factor, KLF-4, as an important regulator of axon regeneration.10 Overexpression of KLF-4 limits the ability of RGCs to extend neurites during development as well as reduces axon regeneration after injury.10 KLF-4 belongs to Krüppel-like factor family, which consists of 17 related transcription factors with similar DNA binding domains as well as divergent activation and repression domains.64 Krüppel-like factors can be clustered into six groups based on amino acid similarity. KLFs within the same group display similar effects on axon growth ability, suggesting an association between axon growth regulatory ability and protein architecture.10 For example, KLF-9, containing similar functional domains as KLF-4, acts to inhibit axon outgrowth, while KLF-6 and KLF-7, which can be clustered into another group, act positively to enhance axon growth ability.10 Interestingly, the expression curve of different KLFs coincides with the developmental decline in axon growth ability. Embryonic RGCs express high levels of axon growth-promoting KLFs, such as KLF-6 and KLF-7, whereas postnatal RGCs express high levels of axon growth-inhibiting KLFs, such as KLF-4 and KLF-9.10 C. elegans contains at least three Krüppellike factors (klf-1, klf-2 and klf-3). It would be important to determine whether they function similarly to their mammalian counterparts in regulating axon growth ability.
Development of Femtosecond Laser Technology for Axon Regeneration Studies
Laser ablation has been applied to study the function of specific cells in C. elegans for decades.65 By killing individual or groups of cells with a UV laser, the subsequent abnormalities observed in development or behavior would provide answers to how cells interact with each other. However, the conventional UV laser system is not focused enough to injure only subcellular structures. It also generates more collateral damage to the surrounding tissues due to the higher pulse energy (0.5 µJ) induced by the longer pulse duration on the order of nanosecond or picosecond.65–68 The invention of the near-infrared (NIR; 780–800 nm) femtosecond laser system brings the surgical precision of laser ablation from the cellular level (∼10 µm) to the subcellular level (<1 µm) (Fig. 2).2 The femtosecond laser system permits subcellular neuronal dissections and minimizes the collateral damage to the nearby tissue through the combination of low pulse energy (10–40 nJ), ultrashort pulse duration (100–200 fs), and nonlinear energy deposition.68,69 Energy deposition by nonlinear absorption restricts damage to a focal point.69 At the focal point of the NIR laser plasma, temperatures can reach 10,000 K, the plasma temperature on the surface of the sun. But 1 µm away from the focal point, the temperature rise is only 10°C and lasts for just 1 µs so the heat dissipation is minimal. Femtosecond laser pulses can be delivered in either megahertz (MHz) mode or kilohertz (kHz) mode, depending on the repetition rate.41,70 When the laser pulses are delivered at a high repetition rate (MHz mode), the latter pulses arrive before heat dissipates from the earlier pulses, resulting in heat accumulation and collateral damage. In contrast, when the laser is controlled at a low repetition rate (kHz mode), the latter pulses reach the focal point after heat generated from the earlier pulses is already dissipated, limiting damage to just the focal point.69 Thus, development of femtosecond laser technology greatly enhances the precision of laser surgery, enabling us to injure axons without damaging the soma.
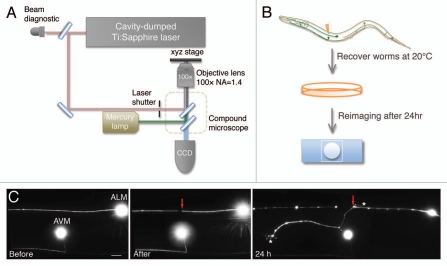
Figure 2.
Study of axon regeneration using femtosecond laser axotomy and live imaging. (A) The experimental setup of femtosecond laser surgery includes two parts: the laser system and the fluorescent imaging microscope. Single tightly-focused laser pulses generated by the cavity-dumped Ti:sapphire laser oscillator provides 5–30 nJ in pulse energy, 100 fs in pulse duration and 200 kHz in repetition rate. The quality of the laser plasma is monitored by the beam diagnostic. A mechanical shutter is used to control the entry of the laser beam into the fluorescent imaging objective. The laser plasma is tightly focused onto targeted axons using a 100×, 1.4 NA oil-immersion objective. The microscope is equipped with a CCD camera, which is used to visualize target neurons, verify surgery and image the regeneration process. We use the position of the AVM cell body as a reference point to mark the injury site in the ALM axon. (B) Individual animals were mounted on 2% agar pads, anesthetized with 3 mM sodium azide, and the targeted neuron was imaged before and after laser axotomy using a CCD camera. Worms were recovered from the sodium azide treatment, placed on a fresh plate with bacterial food and reimaged after 24 h. (C) Femtosecond laser axotomy creates a small break in the ALM axon, which triggers robust regeneration within 24 h in wild-type animals. The red arrow indicates the lesion site and the asterisk denotes the terminus of the regenerating axon. Scale bar represents 10 µm.
Concluding Remarks
In addition to the femtosecond laser surgery, the microfluidic device has been recently developed as a minimally invasive tool to precisely manipulate nematodes and their microenvironment for various neurobiological studies, including axon regeneration. To substitute the conventional way of immobilizing worms, such as anesthesia on agar pads, microfluidic devices immobilize worms in a chemical-free manner through thermal or mechanical mechanisms.71–73 By trapping the worm in a cooling liquid or a pressurized chamber, microfluidic devices effectively avoid the adverse effect of anesthesia on axon regeneration and minimize the time for worms to recover after laser surgery.74 Furthermore, in combination with the interface to multi-well plates containing compounds or an RNAi library, microfluidic devices allow high-throughput screens for novel genes involved in axon regeneration and drugs that interact with axon regeneration in live animals.71,74
Elucidating the cellular and molecular basis for axon regeneration should shed light on how this process becomes restricted in the postnatal stage and in the CNS and therefore can provide therapeutic targets for developing strategies to improve axon regeneration in the adult CNS. It is also hoped that these studies can open a new door for treatment of neurodegenerative diseases as well as brain and spinal cord injury by harnessing the intrinsic neuronal ability to reorganize itself.
Acknowledgments
We thank anonymous reviewers for useful comments. This work was funded by grants to C.C. from the following: the Whitehall Foundation, the March of Dimes Foundation, and the CIHR grant RMF-82501.
References
- 1.Neumann B, Nguyen KC, Hall DH, Ben-Yakar A, Hilliard MA. Axonal regeneration proceeds through specific axonal fusion in transected C. elegans neurons. Dev Dyn. 2011;240:1365–1372. doi: 10.1002/dvdy.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A. Neurosurgery: functional regeneration after laser axotomy. Nature. 2004;432:822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- 3.Ry C. Degeneration and Regeneration of the Nervous System. Oxford Univ Press; 1928. [Google Scholar]
- 4.Chernoff EA, Sato K, Corn A, Karcavich RE. Spinal cord regeneration: intrinsic properties and emerging mechanisms. Semin Cell Dev Biol. 2002;13:361–368. doi: 10.1016/S1084952102000927. [DOI] [PubMed] [Google Scholar]
- 5.Case LC, Tessier-Lavigne M. Regeneration of the adult central nervous system. Curr Biol. 2005;15:749–753. doi: 10.1016/j.cub.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 7.Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/S0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 9.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc Natl Acad Sci USA. 2011;108:6282–6287. doi: 10.1073/pnas.1015239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 13.Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci. 2006;361:1565–1574. doi: 10.1098/rstb.2006.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudge JS, Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci. 1990;10:3594–3603. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 16.Fitch MT, Silver J. Glial cell extracellular matrix: boundaries for axon growth in development and regeneration. Cell Tissue Res. 1997;290:379–384. doi: 10.1007/s004410050944. [DOI] [PubMed] [Google Scholar]
- 17.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/S0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 18.Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res. 2002;137:313–332. doi: 10.1016/S0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- 19.Berry M. Post-injury myelin-breakdown products inhibit axonal growth: an hypothesis to explain the failure of axonal regeneration in the mammalian central nervous system. Bibl Anat. 1982;23:1–11. [PubMed] [Google Scholar]
- 20.McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-X. [DOI] [PubMed] [Google Scholar]
- 21.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 22.Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 23.GrandPré T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 24.Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, et al. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 25.Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 26.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 27.Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/S0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 28.Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 29.Woolf CJ. No Nogo: now where to go? Neuron. 2003;38:153–156. doi: 10.1016/S0896-6273(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 30.Zheng B, Atwal J, Ho C, Case L, He XL, Garcia KC, et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci USA. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Winter F, Oudega M, Lankhorst AJ, Hamers FP, Blits B, Ruitenberg MJ, et al. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175:61–75. doi: 10.1006/exnr.2002.7884. [DOI] [PubMed] [Google Scholar]
- 32.Manitt C, Colicos MA, Thompson KM, Rousselle E, Peterson AC, Kennedy TE. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J Neurosci. 2001;21:3911–3922. doi: 10.1523/JNEUROSCI.21-11-03911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Löw K, Culbertson M, Bradke F, Tessier-Lavigne M, Tuszynski MH. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28:1099–1108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldberg JL, Vargas ME, Wang JT, Mandemakers W, Oster SF, Sretavan DW, et al. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci USA. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasterkamp RJ, De Winter F, Giger RJ, Verhaagen J. Role for semaphorin III and its receptor neuropilin-1 in neuronal regeneration and scar formation? Prog Brain Res. 1998;117:151–170. doi: 10.1016/S0079-6123(08)64014-5. [DOI] [PubMed] [Google Scholar]
- 39.Pasterkamp RJ, Giger RJ, Ruitenberg MJ, Holtmaat AJ, De Wit J, De Winter F, et al. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- 40.Miranda JD, White LA, Marcillo AE, Willson CA, Jagid J, Whittemore SR. Induction of Eph B3 after spinal cord injury. Exp Neurol. 1999;156:218–222. doi: 10.1006/exnr.1998.7012. [DOI] [PubMed] [Google Scholar]
- 41.Wu Z, Ghosh-Roy A, Yanik MF, Zhang JZ, Jin Y, Chisholm AD. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling and synaptic branching. Proc Natl Acad Sci USA. 2007;104:15132–15137. doi: 10.1073/pnas.0707001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabel CV, Antoine F, Chuang CF, Samuel AD, Chang C. Distinct cellular and molecular mechanisms mediate initial axon development and adultstage axon regeneration in C. elegans. Development. 2008;135:1129–1136. doi: 10.1242/dev.013995. [DOI] [PubMed] [Google Scholar]
- 43.Ferretti P, Zhang F, O'Neill P. Changes in spinal cord regenerative ability through phylogenesis and development: lessons to be learnt. Dev Dyn. 2003;226:245–256. doi: 10.1002/dvdy.10226. [DOI] [PubMed] [Google Scholar]
- 44.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330:254–256. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- 46.Goslin K, Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol. 1989;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi K, Kawai-Hirai R, Ishikawa K, Takata K. Reversal of neuronal polarity characterized by conversion of dendrites into axons in neonatal rat cortical neurons in vitro. Neuroscience. 2002;110:7–17. doi: 10.1016/S0306-4522(01)00592-9. [DOI] [PubMed] [Google Scholar]
- 48.Bradke F, Dotti CG. Differentiated neurons retain the capacity to generate axons from dendrites. Curr Biol. 2000;10:1467–1470. doi: 10.1016/S0960-9822(00)00807-1. [DOI] [PubMed] [Google Scholar]
- 49.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/S0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 50.Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/S0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 51.Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/S0896-6273(02)00702-X. [DOI] [PubMed] [Google Scholar]
- 52.Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci USA. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci. 2010;30:3175–3183. doi: 10.1523/JNEUROSCI.5464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewcock JW, Genoud N, Lettieri K, Pfaff SL. The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron. 2007;56:604–620. doi: 10.1016/j.neuron.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 55.Campbell DS, Holt CE. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron. 2003;37:939–952. doi: 10.1016/S0896-6273(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 56.Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, et al. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoy RR, Bittner GD, Kennedy D. Regeneration in crustacean motoneurons: evidence for axonal fusion. Science. 1967;156:251–252. doi: 10.1126/science.156.3772.251. [DOI] [PubMed] [Google Scholar]
- 58.Birse SC, Bittner GD. Regeneration of giant axons in earthworms. Brain Res. 1976;113:575–581. doi: 10.1016/0006-8993(76)90058-5. [DOI] [PubMed] [Google Scholar]
- 59.Deriemer SA, Elliott EJ, Macagno ER, Muller KJ. Morphological evidence that regenerating axons can fuse with severed axon segments. Brain Res. 1983;272:157–161. doi: 10.1016/0006-8993(83)90373-6. [DOI] [PubMed] [Google Scholar]
- 60.Macagno ER, Muller KJ, DeRiemer SA. Regeneration of axons and synaptic connections by touch sensory neurons in the leech central nervous system. J Neurosci. 1985;5:2510–2521. doi: 10.1523/JNEUROSCI.05-09-02510.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, et al. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120:407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 62.Hammarlund M, Davis WS, Jorgensen EM. Mutations in beta-spectrin disrupt axon outgrowth and sarcomere structure. J Cell Biol. 2000;149:931–942. doi: 10.1083/jcb.149.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brisbin S, Liu J, Boudreau J, Peng J, Evangelista M, Chin-Sang I. A role for C. elegans Eph RTK signaling in PTEN regulation. Dev Cell. 2009;17:459–469. doi: 10.1016/j.devcel.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 64.Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sulston JE, White JG. Regulation and cell autonomy during postembryonic development of Caenorhabditis elegans. Dev Biol. 1980;78:577–597. doi: 10.1016/0012-1606(80)90353-X. [DOI] [PubMed] [Google Scholar]
- 66.Oraevsky AA, Silva LBD, Rubenchik AM, Feit MD, Glinsky ME, Perry MD, et al. Plasma mediated ablation of biological tissues with nanosecond-to-femtosecond laser pulses: relative role of linear and nonlinear absorption. IEEE J Sel Top Quantum Electron. 1996;2:801–809. doi: 10.1109/2944.577302. [DOI] [Google Scholar]
- 67.Khodjakov A, La Terra S, Chang F. Laser microsurgery in fission yeast; role of the mitotic spindle midzone in anaphase B. Curr Biol. 2004;14:1330–1340. doi: 10.1016/j.cub.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 68.Yanik MF, Cinar H, Cinar HN, Gibby A, Chisholm AD, Jin Y, et al. Nerve regeneration in Caenorhabditis elegans after femtosecond laser axotomy. IEEE J Sel Top Quantum Electron. 2006;12:1283–1291. doi: 10.1109/JSTQE.2006.879579. [DOI] [Google Scholar]
- 69.Chung SH, Mazur E. Femtosecond laser ablation of neurons in C. elegans for behavioral studies. Appl Phys, A Mater Sci Process. 2009;96:335–341. doi: 10.1007/s00339-009-5201-7. [DOI] [Google Scholar]
- 70.Bourgeois F, Ben-Yakar A. Femtosecond laser nanoaxotomy properties and their effect on axonal recovery in C. elegans. Opt Express. 2008;16:>5963. doi: 10.1364/OE.16.005963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rohde CB, Zeng F, Gonzalez-Rubio R, Angel M, Yanik MF. Microfluidic system for on-chip high-throughput whole-animal sorting and screening at subcellular resolution. Proc Natl Acad Sci USA. 2007;104:13891–13895. doi: 10.1073/pnas.0706513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo SX, Bourgeois F, Chokshi T, Durr NJ, Hilliard MA, Chronis N, et al. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nat Methods. 2008;5:531–533. doi: 10.1038/nmeth.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeng F, Rohde CB, Yanik MF. Sub-cellular precision on-chip small-animal immobilization, multi-photon imaging and femtosecond-laser manipulation. Lab Chip. 2008;8:653–656. doi: 10.1039/b804808 h. [DOI] [PubMed] [Google Scholar]
- 74.Ben-Yakar A, Chronis N, Lu H. Microfluidics for the analysis of behavior, nerve regeneration and neural cell biology in C. elegans. Curr Opin Neurobiol. 2009;19:561–567. doi: 10.1016/j.conb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]