Abstract
Integrin alpha9 (ITGA9) is one of the less studied integrin subunits that facilitates accelerated cell migration and regulates diverse biological functions such as angiogenesis, lymphangiogenesis, cancer cell proliferation and migration. In this work, integrin alpha9 expression and its epigenetic regulation in normal human breast tissue, primary breast tumors and breast cancer cell line MCF7 were studied. It was shown that integrin alpha9 is expressed in normal human breast tissue. In breast cancer, ITGA9 expression was downregulated or lost in 44% of tumors while another 45% of tumors showed normal or increased ITGA9 expression level (possible aberrations in the ITGA9 mRNA structure were supposed in 11% of tumors). Methylation of ITGA9 CpG-island located in the first intron of the gene was shown in 90% of the breast tumors with the decreased ITGA9 expression while no methylation at 5′-untranslated region of ITGA9 was observed. 5-aza-dC treatment restored integrin alpha9 expression in ITGA9-negative MCF7 breast carcinoma cells, Trichostatin A treatment did not influenced it but a combined treatment of the cells with 5-aza-dC/Trichostatin A doubled the ITGA9 activation. The obtained results suggest CpG methylation as a major mechanism of integrin alpha9 inactivation in breast cancer with a possible involvement of other yet unidentified molecular pathways.
Key words: integrin alpha9, ITGA9, breast cancer, expression, methylation, bisulfite sequencing
Introduction
Integrins are transmembrane glycoprotein receptors responsible for cell-cell and cell-matrix interactions.1,2 At the cell surface, integrins not only keep the structural organization of continuous matrix-cytoskeleton network but participate in cell signaling and regulation of cell proliferation, adhesion and migration.3 In carcinogenesis, integrins play an important role in cancer angiogenesis4 and metastasis,5 revealing them as potential targets for novel anti-tumor and anti-angiogenesis therapies based on chemical inhibitors or antagonists against different integrin subunits.6–8
Structurally, integrins are heterodimer molecules composed of two transmembrane glycoprotein subunits α and β (α,β). Up to date, there are 18 α and 8 β integrin subunits forming 24 different integrin molecules with their own function in normal and pathological cell physiology.9
One of the less studied integrin subunits is integrin alpha9 (ITGA9, NM_002207) initially cloned from the lung and colon human cDNA libraries.10 Integrin alpha9 subunit interacts only with beta1 subunit generating α9β1 heterodimer, which is expressed in many cell types such as epithelial cells, neutrophiles, hepatocytes, muscle and endothelial cells.10–13 ITGA9 expression is essential for the vital activity of the organism; mice homozygous for a null mutation in the alpha9 subunit gene die between 6 and 12 d of age.14
Integrin α9β1 is a receptor for thrombospondin-1 (THBS1),15 ADAM12/ADAM1516 and nerve growth factor (NGF),17 interacts with vascular cell adhesion molecule 1 (VCAM1),18 fibronectin,19 tenascin C,20 osteopontin,21 VEGF-C, -D22 and VEGF-A23 isoforms. Functionally, integrin α9β1 is involved in angiogenesis and lymphangiogenesis, proliferation and migration of the different cells24–26 playing an important role both in normal physiology and different pathological processes including carcinogenesis.
Unfortunately, there are not so abundant data on the integrin alpha9 (ITGA9) expression in tumor tissues. It was shown that ITGA9 is expressed in melanoma cells,27 aberrantly upregulated in small-cell lung cancers, both cell lines and primary tumors28 and medulloblastoma cells,29 the expression level of integrin α9β1 on astrocytomas is correlated with increased grade of this brain tumor and is highest on glioblastoma, whereas normal astrocytes do not express this integrin,30 ITGA9 is absent in normal adults colon tissues but it is expressed in 6 of 10 primary colon adenocarcinomas and 2 of 7 colon adenocarcinoma cell lines (Caco-2 and T84).31 According to another study, ITGA9 has significantly higher expression levels in colorectal tumors with high microsatellite instability (11 tumors of 42) vs. colorectal tumors with low or null microsatellite instability (31 tumors of 42).32 Molecular mechanisms of ITGA9 activation in cancer cells remain unclear; however the effect may unfavorably contribute to the cancer prognosis supporting ITGA9 as a potential target for anti-integrin therapy.
From the other side, extensive search of genetic/epigenetic aberrations of ITGA9 by NotI-micorarray identified frequent (more than in 30%) aberrations (deletions, methylation) in kidney, lung, breast, ovary, cervical, prostate and colorectal cancer,33,34 in 45–55% of head and neck squamous cell carcinomas35 and 65% of uterine cervical carcinomas (41% deletion and 24% methylation).36 A possibility of ITGA9 CpG-island methylation was verified in cervical33 and colorectal34 tumors by methylspecific PCR and bisulfite sequencing.
In breast tumors, integrin α9β1 expression was shown immunohistochemically in 23 of 90 cases (26%)37 but it is complicated to interpret the data in terms of increase/decrease of ITGA9 expression in breast cancer because of absence of the data on its basic expression in normal human breast tissue.
In this study, we have investigated an integrin alpha9 expression in normal human breast tissue, primary breast tumors and breast carcinoma cell line MCF7 and check a possible epigenetic regulation of ITGA9 expression in breast cancer.
Results
ITGA9 expression in human breast tumors.
Multiplex and TaqMan-based quantitative Real-Time RT-PCR analysis was used to determine integrin alpha9 (ITGA9) expressions in human breast tumors with GAPDH and b-actin as the reference genes, respectively. Tumor and control samples were matched pairs for each patient with malignancy—from the central part of tumor and a more distant part of the breast, accordingly. Tissue samples obtained from the patients undergoing cosmetic surgery were designated as normal breast tissue. Two different primer pairs for different regions of the gene (amplified DNA fragments of 810 bp and 1100 bp) (Materials and Methods and Fig. 3A) were used to study ITGA9 expression by multiplex RT-PCR. Two normal breast samples and 38 matched pairs (tumor and control) were analyzed (Fig. 1).
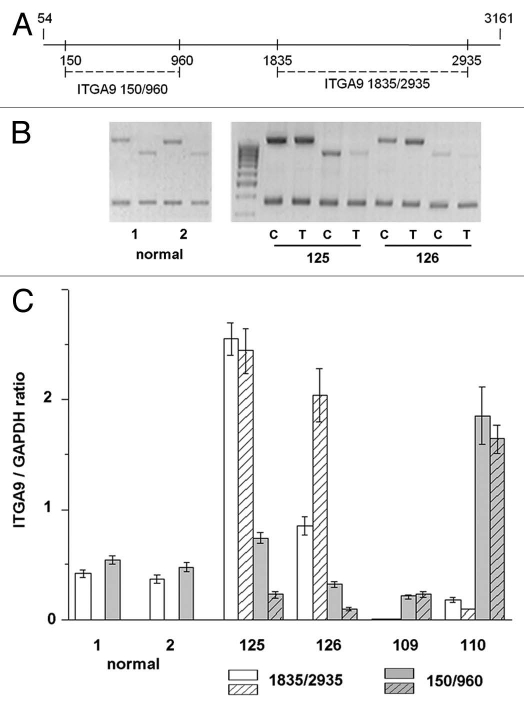
Figure 3.
Multiplex RT-PCR analysis of ITGA9 expression with different primer pairs. (A) Scheme of the primers used in the study. (B) Representative gel from multiplex RT-PCR. DNA fragments amplified with two different primers pairs are shown. GAPDH expression was used as an internal standard. (C) ITGA9 expression levels normalized to that of GAPDH (TotalLab Programme). The graph shows the mean expression levels from triplicate experiments (± SD) (OriginPro 8.1). 1 and 2, normal breast tissue samples; 109, 110, 125 and 126, breast tumors; C and T, control and tumor breast tissue (match pair for each patient), respectively.
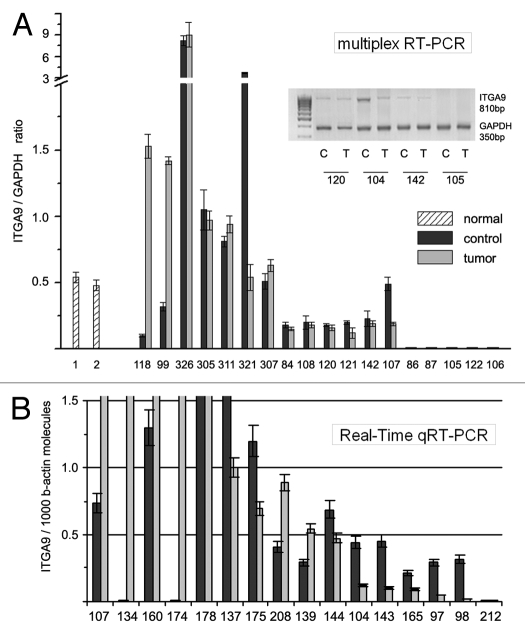
Figure 1.
ITGA9 expression in normal and tumor human breast tissue. (A) Multiplex RT-PCR. Intensity of the amplified ITGA9 fragments normalized to that of GAPDH (TotalLab Programme). Representative gel from multiplex RT-PCR is shown (inset). (B) Quantitative Real-Time RTPCR. ITGA9 expression normalized to 1000 b-actin molecules. The graph shows the mean expression levels from triplicate experiments (± SD) (OriginPro 8.1). 1 and 2, normal breast tissue; 84–32, breast tumors.
According to multiplex RT-PCR, the ITGA9 was expressed in normal human breast tissue (Fig. 1A). However, in breast tumors, ITGA9 expression was heterogeneously changed—there were both samples with significantly increased or decreased (up to complete disappearance) ITGA9 expression level. These results were confirmed by TaqMan-based qRT-PCR for the same breast tumor and normal clinical samples and additional 20 matched pairs were studied (Fig. 1B). To analyze the obtained data, more than 2-fold expression change was taken as reliable in comparative analysis of ITGA9 expression in tumor and control breast tissues. It was found that ITGA9 expression was normal or increased in 45% of breast tumors (17 of 38 samples) and decreased or absent in another 44% of tumors (Fig. 2).
Figure 2.
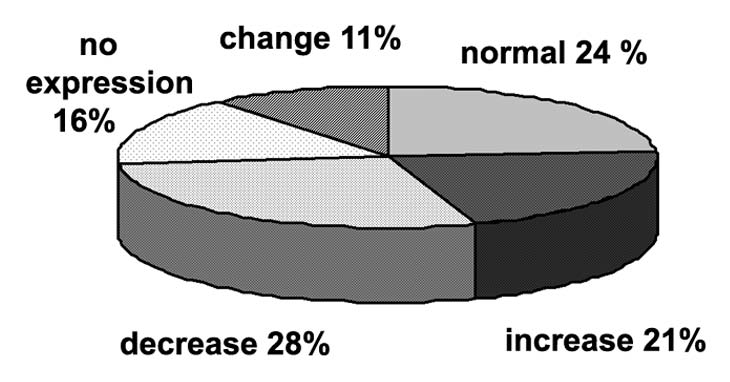
Diagram of subdivision of patients for different groups in dependency of ITGA9 expression change in breast tumors.
It is important to note that for most of the analyzed samples, similar ITGA9 expression levels were shown using both primers pairs. However, 11% of samples (4 of 38 samples) showed a different ITGA9 expression in dependence from the primer pairs used (Fig. 3). It indirectly supports an involvement of genetic changes (deletions, mutations) or pathological alternative splicing in ITGA9 function in breast cancer. Also, the fact underlines the importance of experimental design and primers/antibodies choice in ITGA9 investigation because it could contribute to the obtained results.
Methylation status of ITGA9 CpG-island in breast tumors.
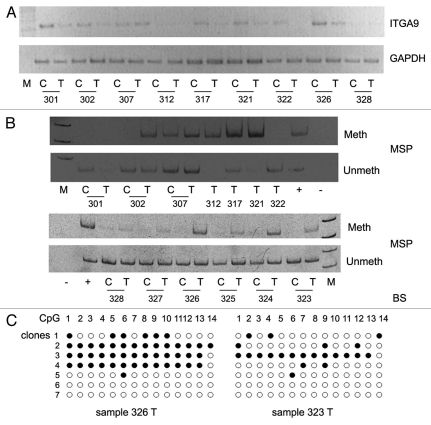
On the next step, DNA methylation status of ITGA9 CpG-island (located at the first intron of the gene) was assessed by methyl-specific PCR (MSP) and bisulfite sequencing. In total, 12 breast tumors with decreased or absent ITGA9 expression and one tumor with normal expression level (sample 307) were studied (Fig. 4).
Figure 4.
Methylation of ITGA9 CpG-island in primary human breast tumors. (A) ITGA9 expression in breast tumors; a representative gel from multiplex RT-PCR. (B) Methyl-specific PCR on ITGA9 CpG-island. (C) Bisulfite sequencing. 301–328, breast tumors; C and T, control and tumor breast tissue (match pair for each patient); +, positive PCR control; −, negative PCR control; M, DNA marker; Meth and Unmeth, primers for methylated or un-methylated DNA sequence, respectively.
Hypermethylation of ITGA9 GpC-island located in the first intron of the gene was shown in 90% of the breast tumors with the decreased ITGA9 expression (Fig. 4A) while no methylation at 5′-untranslated region of ITGA9 was observed in the same samples. Control sequencing of the DNA fragments amplified with methyl-specific primers and bisulfite sequencing confirmed high methylation of the CpG-island in these samples (although there were unmethylated clones as well possibly due to the presence of normal cells in the total tumor clinical sample) (Fig. 4B).
The obtained results suggest hypermethylation of CpG-island as a main molecular mechanism of downregulation of ITGA9 expression in breast tumors. However, the presence of ITGA9 expression in some heavy-methylated breast tumors (samples 307 and 326) and its complete absence in tumor with non-methylated CpG region (sample 322) indicate a complexity of the process and an existence of another molecular mechanisms contributing to the regulation of ITGA9 expression in breast cancer.
Restoration of ITGA9 expression in breast carcinoma MCF7 cells in vitro.
To confirm hypermethylation as a main regulatory mechanism for ITGA9 inactivation in breast cancer, experiments on ITGA9 re-activation in breast cancer cells MCF7 in vitro were done.
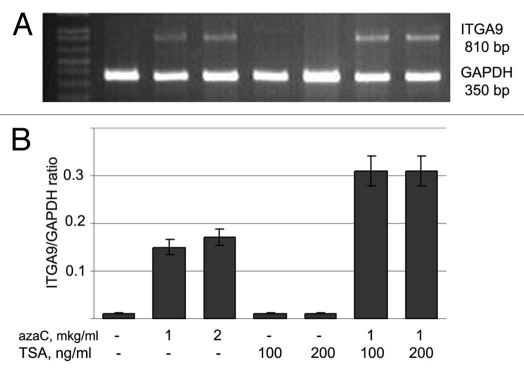
At the first step, we showed that MCF7 cells practically do not express ITGA9 and CpG-island of the gene is almost completely methylated in the cells according bisulfite sequencing (data not shown). For the functional experiment in vitro, MCF7 cells were treated with DNA demethylating agent 5-deozyazacytidine (5-aza-dC) or hystone-deacetylase inhibitor Trichostatin A (TSA) or both. ITGA9 expression level was then determined by multiplex RT-PCR with the primers for 810 bp amplified DNA fragment (Fig. 5).
Figure 5.
Activation of ITGA9 expression in MCF7 breast carcinoma cells by 5-deoxyazacytidine treatment. (A) Representative gel showing ITGA9 amplified by multiplex RT-PCR. (B) Intensity of the amplified ITGA9 DNA fragments normalized to that of GAPDH (TotalLab Programme). Bars represent the mean ± SD from triplicate experiments (OriginPro 8.1). The lane order on the gel corresponds to the bars on the histogram. 5-aza-dC, 5-azadeoxycytidine; TSA, Trichostatin A.
It was shown that the 5-aza-dC treatment activated the ITGA9 expression in breast cancer cells MCF7 (near the 10-fold) but TSA treatment did not influence the expression. Combined 5-aza-dC/TSA treatment increased the activating effect up to 20-fold supporting a possible involvement of some other molecular pathways in the regulation of ITGA9 expression in breast cancer.
Taken together, our results indicate that integrin alpha9 expression is heterogeneously changed in human breast tumors and hypermethylation of ITGA9 CpG-island could be a major mechanism of integrin alpha9 inactivation in breast cancer with a possible involvement of other unidentified yet molecular pathways.
Discussion
One of the important results of the study is detection of ITGA9 expression in normal human breast tissue that was not shown earlier. The fact is of significance in its own right and logically corresponds to the literature data on ITGA9 expression in different cell types—the integrin subunit is expressed in epithelial and smooth muscle cells,10 on neutrophils12,18 and human polymorphonuclear leukocytes,13 which are constituent parts of breast tissue clinical sample. Thus, an expression of ITGA9 in normal breast tissue could be stated as a baseline to estimate its possible changes in different pathological conditions.
Interestingly, different levels of ITGA9 expression in the control breast samples (normal counterparts of the matched pairs) were shown. There were both samples with the increased and decreased ITGA9 expression, and not always it coincided with ITGA9 expression in the tumor counterpart. Possibly, the control breast tissues are already affected by the disease or inversely, some pre-tumor changes occur in the breast tissue. The results suppose that a possibility to use a normal-looking counterpart as a normal tissue (in comparative study) should be preliminary tested for each experimental system.
According our RT-PCR data, ITGA9 expression in breast tumors is changed bi-directionally—21% of tumors showed significantly increased ITGA9 level and 44% of the studied tumors showed decreased ITGA9 expression (up to complete absence). The obtained results supplement the data on ITGA9 expression in 26% of breast tumors showed by immunohistochemistry.37
Here, two different aspects of the changes are to be discussed. From the one side, an activation of ITGA9 expression was shown for different human tumors and cancer cells—small-cell lung cancer,28 medulloblastoma cells,29 astrocytomas and glioblastoma.30 From the other side, frequent genetic/epigenetic aberrations (deletions, methylation) of ITGA9 were identified in different epithelial cancers using NotI-microarray.33–36 Taken together, the presented data suppose an existence of different molecular pathways participating in the regulation of ITGA9 expression in cancer and resulting in the heterogeneity of primary tumors based on ITGA9 expression level. In fact, it was shown for different cell lines in vitro that colon adenocarcinoma Caco-2 and T84 cells express the integrin alpha9 subunit while the five other colon carcinoma cell lines tested were negative for its expression,31 two glioblastoma cell lines, LN229 and LN18 are alpha9beta1 integrin positive and negative, respectively.30
Our results are in concordance with these data and show that along with activation of ITGA9 expression in some breast tumors there are groups of patients with absent (16% of tumors) or significantly decreased (28% of tumors) ITGA9 expression. If the first group seems to consist of the tumors with genetically eliminated ITGA9 (deletions, mutations), the second group of tumors should have a functional ITGA9 gene and some epigenetic mechanisms are possibly involved in the attenuation of ITGA9 expression in the cells. Earlier, ITGA9 CpG-island methylation was indeed shown in more than 30% of cervical33 and colorectal34 tumors. According to our data, hypermethylation of ITGA9 CpG-island could be an important mechanism for ITGA9 epigenetic inactivation in breast cancer responsible for its decreased expression in about 25% of primary breast tumors.
However the expression of ITGA9 in the samples with high methylation of ITGA9 CpG-island (samples 307 and 326) suggests an involvement of some antagonistic molecular regulators of ITGA9 expression as well. The hypothesis is supported by the further observation that the combined treatment of MCF7 cells with 5-aza-dC/TSA increased ITGA9 expression level even more (2-fold) compare with the aza-treatment alone. Possibly, an activation of some positive regulators of ITGA9 by Trichostatin A (TSA) treatment contributes to the 5-aza-dC-stimulated ITGA9 expression in MCF7 cells. Thus, the presented data suggest that some other molecular pathways could be involved in ITGA9 regulation in cancer along with the hypermethylation of its CpG-island.
A similar complex regulation of gene expression was shown for another member of the same integrin subfamily integrin alpha4beta1—aberrant DNA methylation of its promoter region results in integrin alpha4 silencing in 55% of cholangiocarcinomas,38 84.7% of 46 primary gastric tumors and 8 of 9 gastric cancer cell lines39 while TSA treatment upregulated integrin alpha4 expression in hepatocellular carcinoma cell line Hep3B.40
The obtained data show complexity of ITGA9 regulation in breast cancer cells that result in existence of ITGA9-expressing or ITGA9-non-expressing breast tumors. Because of the epigenetic inactivation of ITGA9 in the last group, one can assume that the patients will not benefit from anti-integrin therapy and epigenetics drugs (like anti-methylation agents or HDAC inhibitors) while the first group could be taken into consideration for those treatments. In these terms, ITGA9 expression level could be an important diagnostic marker for novel epigenetic drugs or anti-integrin-based antimetastatic therapies.
Materials and Methods
Reagents.
TRIZOL reagent was from Invitrogen, M-MLV Reverse Transcriptase and RQ1 RNase free DNase were from Promega and 5-aza-dC and TSA were from Sigma.
Patients and tissue samples.
A total of 38 patients with breast cancer and two individuals without malignancy (undergoing cosmetic surgery) were studied. All samples were obtained from primary breast tumors during the radical surgery at Central Municipal Hospital N1, Novosibirsk, Russia, “snap-frozen” in liquid nitrogen and stored at −70°C. Regions were manually dissected from the frozen blocks to provide a consistent tumor cell content of more than 70% in tissues used for analysis. The prevalent histological type of tumors was duct infiltrating cancer of different degree of malignancy. Most patients were at the second stage of malignancy progression according the formula TxNxMx. All patients gave written informed consent. The study protocol has been approved by the Local Ethics Committee in accordance with the Helsinki Declaration of 1975.
RT-PCR analyses of ITGA9 expression.
Total RNA was extracted from the cells using the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. DNase treatment was done using RQ1 RNase free DNase I (Promega) at 37°C for 30 min with subsequent RNA precipitation with isopropanol. cDNA was synthesized from 1–2 µg of total RNA using oligo-dT primers and M-MTLV reverse transcriptase (Promega) according to the manufacturer's protocol and 1/10th of the product was subjected to PCR analysis.
The following conditions were used for multiplex RT-PCR: 94°C for 4 min, 94°C for 30 sec, 61°C (for ITGA9 1835/2935) or 64°C (for ITGA9 150/960) for 60 sec and 72°C for 1 min, with a final elongation step at 72°C for 10 min using a Tercik PCR machine (DNA-Technology). The total reaction volume was 10 µl. ITGA9 1100 bp and 810 bp DNA fragments were amplified for 39 and 37 cycles, respectively; GAPDH (housekeeping gene) was amplified for 27 cycles. The amplified products were separated on 1.0% agarose gels. The gels were scanned using the “DNA Analyzer” system and ITGA9 expression levels were estimated from the intensity of the amplified ITGA9 DNA fragment normalized against the intensity of GAPDH (TotalLab program, Nonlinear Dynamics). The PCR primers used for human ITGA9 and GAPDH were as follows: ITGA9-1835/2935-F, 5′-CCT CTG ACA CCA GTT CTC CGC-3′; ITGA9-1835/2935-R, 5′-GCC TCG AAG ACC ACC GTCA-3′; ITGA9-150/960-F, 5′-GAC CCG CAG CGC CCC G-3′; ITGA9-150/960-R, 5′-GCG CAC AAG GAG GAG CCG-3′; GAPDH-F, 5′-GGG CGC CTG GTC ACAA-3′; GAPDH-R, 5′-AAC ATG GGG GCA TCA GCA GA-3′.
Quantitative real-time RT-PCR (qRT-PCR) was performed using the ABI PRISM 7000 Sequence Detector (AppliedBiosystems) and the ITGA9 TaqMan Assay (Medigen) under the following conditions: 50°C for 2 min, 94°C for 10 min, followed by 40 cycles at 94°C for 15 sec and 60°C for 1 min. The total reaction volume was 25 µl. b-actin was used as the housekeeping gene. The PCR primers and TaqMan probes used were: ITGA9-F, 5′-GTT GGT GGG AAT CCT CAT CTT C-3′; ITGA9-R, 5′-TTT GTA CCT TCG GCG AAA GAA-3′, ITGA9-probe, 5′-FAM-TGG CCG TGC TGC TCT GGA AGA TG-TAMRA-3′; b-actin-F, 5′-GGC ACC CAG CAC AAT GAA G-3′; b-actin-R, 5′-GCC GAT CCA CAC GGA GTA CT-3′; b-actin-probe, 5′-FAM-TCA AGA TCA TTG CTC CTC CTG AGC GC-TAMRA-3′.
Genomic DNA isolation and bisulfite conversion.
Genomic DNA was isolated from the tissue samples using E.Z.N.A. DNA isolation kit and bisulfite conversation of the genomic DNA was performed by E.Z.N.A. DNA methylation kit (Zymo Research) according to the manufacturer's instructions.
Methyl-specific PCR.
Methyl-specific PCR for ITGA9 fragment amplification was performed with primers specific to the methylated (Met) and unmethylated (Unmeth) DNA sequences of ITGA9 CpG island. Blood gDNA treated with SssI-methyltransferase (NewEngland Biolabs) was used as a positive control for Met-primers. The following conditions were used for PCR: 95°C for 4 min, 95°C for 30 sec, 60–65°C for 30 sec and 72°C for 30 sec, with a final elongation step at 72°C for 7 min, 45 cycles. Reaction mixture contained 1x DreamTaq buffer, 0.2 mM dNTPs, 0.4 µM primers, 3.5% DMSO, 50–100 ng of bisulfite converted DNA and 1.25 U of DreamTaq DNA-polymerase (Fermentas). The total reaction volume was 30 µl; 10 µl of the amplified products were separated on 10% PAAG and visualized by ethidium bromide staining.
The PCR primers were as follows: ITGA9-Met-F, 5′-TGG AGT ATT TTT ACG ATA ATA CGC-3′; ITGA9-Met-R, 5′-AAA AAC CGA AAA AAC GAC GA-3′, (116 bp); ITGA9-Unmeth-F, 5′-TGG AGT ATT TTT ATG ATA ATA TGT GT-3′; ITGA9-Unmeth-R, 5′-AAA AAA AAC CAA AAA AAC AAC AAC-3′, (119 bp).
Bisulfite sequencing.
Amplification of the ITGA9 DNA fragment for bisulfite sequencing was done using bisulfite-treated gDNA and primers specific for ITGA9 CpG-island sequence (ITGA9-F, 5′-CCC TGG GGT CCC AGC CCA GAG-3′; ITGA9-R, 5′-GAG AGG CTA TAC TCC TTC CTC AG-3′). The following conditions were used for PCR: 94°C for 2 min, 94°C for 30 sec, 56°C for 30 sec and 72°C for 1 min, with a final elongation step at 72°C for 10 min, 35 cycles. The total reaction volume was 30 µl. The PCR products were purified using a DNA Clean and Concentrator Kit (Zymo Research Corporation) and cloned in TOPO-vector using a TOPO TA Cloning Kit for Sequencing (Invitrogen BV) according to the manufacturer's instructions. Plasmid DNA was isolated using a Zyppy Plasmid Miniprep Kit (Zymo Research Corporation) according to the manufacturer's protocol. Sequencing was performed using a BigDye Terminator Cycle Sequencing Ready Reaction kit v1.1 and ABI Prism 3100 Genetic Analyzer (Applied Biosystems) according to the manufacturer's protocol. Eight to 10 clones were analyzed for each sample.
Cell lines, cell culture and 5-aza-dC/TSA treatment.
The MCF7 human breast cancer cell line was obtained from MTC (Karolinska Institute). Cells were maintained in Iscove's Modified Dulbecco's medium (IMDM) supplemented with \2 mM L-glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin, and 10% FBS at 37°C in a humidified 5% CO2 incubator. Deoxyazacytidine (5-aza-dC, 1 or 2 µg/ml) or Trichostatin A (TSA, 100 or 200 ng/ml) treatment was done by the incubation with the cells for 72 h or 24 h, respectively. For combined treatment, the cells were incubated with 5-aza-dC (1 µg/ml) for 48 h after that TSA (100 or 200 ng/ml) was added for additional 24 h. Cells were harvested for analysis using trypsin/EDTA.
Acknowledgments
We thank Drs. A. Proskura, O. Goldinshtein, V. Titova for the assistance with the clinical tissue samples collecting, Dr. Z. Shevchuk for her help with bisulfite sequencing, A. Grigoriev for the help with the manuscript preparation. The work was supported by the research grant from Russian Foundation for Basic Research (RFBR 09-04-01599a); Karolinska Institute; ERZ was supported by the Swedish Institute, the Swedish Cancer Society and the Swedish Research Council. EVG was recipient of fellowship from the Concern Foundation in Los Angeles and the Cancer Research Institute in New York.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.0106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soung YH, Clifford JL, Chung J. Crosstalk between integrin and receptor tyrosine kinase signaling in breast carcinoma progression. BMB Rep. 2010;43:311–318. doi: 10.5483/BMBRep.2010.43.5.311. [DOI] [PubMed] [Google Scholar]
- 4.Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol. 2004;64:207–238. doi: 10.1016/S0070-2153(04)64009-9. [DOI] [PubMed] [Google Scholar]
- 5.Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Chui WK, Ho PC. Integrin targeted drug and gene delivery. Expert Opin Drug Deliv. 2010;7:159–171. doi: 10.1517/17425240903468696. [DOI] [PubMed] [Google Scholar]
- 7.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rüegg C, Alghisi GC. Vascular integrins: therapeutic and imaging targets of tumor angiogenesis. Recent Results Cancer Res. 2010;180:83–101. doi: 10.1007/978-3-540-78281-0_6. [DOI] [PubMed] [Google Scholar]
- 9.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer EL, Ruegg C, Ferrando R, Pytela R, Sheppard D. Sequence and tissue distribution of the integrin alpha 9 subunit, a novel partner of beta 1 that is widely distributed in epithelia and muscle. J Cell Biol. 1993;123:1289–1297. doi: 10.1083/jcb.123.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young BA, Taooka Y, Liu S, Askins KJ, Yokosaki Y, Thomas SM, et al. The cytoplasmic domain of the integrin alpha9 subunit requires the adaptor protein paxillin to inhibit cell spreading but promotes cell migration in a paxillin-independent manner. Mol Biol Cell. 2001;12:3214–3225. doi: 10.1091/mbc.12.10.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mambole A, Bigot S, Baruch D, Lesavre P, Halbwachs-Mecarelli L. Human neutrophil integrin {alpha}9{beta}1: up-regulation by cell activation and synergy with {beta}2 integrins during adhesion to endothelium under flow. J Leukoc Biol. 2010;88:321–327. doi: 10.1189/jlb.1009704. [DOI] [PubMed] [Google Scholar]
- 13.Shang T, Yednock T, Issekutz AC. alpha9beta1 integrin is expressed on human neutrophils and contributes to neutrophil migration through human lung and synovial fibroblast barriers. J Leukoc Biol. 1999;66:809–816. doi: 10.1002/jlb.66.5.809. [DOI] [PubMed] [Google Scholar]
- 14.Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/MCB.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staniszewska I, Zaveri S, Del Valle L, Oliva I, Rothman VL, Croul SE, et al. Interaction of alpha9beta1 integrin with thrombospondin-1 promotes angiogenesis. Circ Res. 2007;100:1308–1316. doi: 10.1161/01.RES.0000266662.98355.66. [DOI] [PubMed] [Google Scholar]
- 16.Eto K, Puzon-McLaughlin W, Sheppard D, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin alpha9beta1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem. 2000;275:34922–34930. doi: 10.1074/jbc.M001953200. [DOI] [PubMed] [Google Scholar]
- 17.Staniszewska I, Sariyer IK, Lecht S, Brown MC, Walsh EM, Tuszynski GP, et al. Integrin alpha9 beta1 is a receptor for nerve growth factor and other neurotrophins. J Cell Sci. 2008;121:504–513. doi: 10.1242/jcs.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taooka Y, Chen J, Yednock T, Sheppard D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145:413–420. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 20.Yokosaki Y, Palmer EL, Prieto AL, Crossin KL, Bourdon MA, Pytela R, et al. The integrin alpha 9 beta 1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem. 1994;269:26691–26696. [PubMed] [Google Scholar]
- 21.Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S, et al. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274:36328–36234. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 22.Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, et al. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J Biol Chem. 2007;282:15187–15196. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- 24.Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiber TD, Steinl C, Essl M, Abele H, Geiger K, Müller CA, et al. The integrin alpha9beta1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation. Haematologica. 2009;94:1493–1501. doi: 10.3324/haematol.2009.006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta SK, Vlahakis NE. Integrin α9β1. Unique signaling pathways reveal diverse biological roles. Cell Adhes Migr. 2010;4:194–198. doi: 10.4161/cam.4.2.10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lydolph MC, Morgan-Fisher M, Høye AM, Couchman JR, Wewer UM, Yoneda A. Alpha9beta1 integrin in melanoma cells can signal different adhesion states for migration and anchorage. Exp Cell Res. 2009;315:3312–3324. doi: 10.1016/j.yexcr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Hibi K, Yamakawa K, Ueda R, Horio Y, Murata Y, Tamari M, et al. Aberrant upregulation of a novel integrin alpha subunit gene at 3p21.3 in small cell lung cancer. Oncogene. 1994;9:611–619. [PubMed] [Google Scholar]
- 29.Fiorilli P, Partridge D, Staniszewska I, Wang JY, Grabacka M, So K, et al. Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation. Lab Invest. 2008;88:1143–1156. doi: 10.1038/labinvest.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown MC, Staniszewska I, Lazarovici P, Tuszynski GP, Del Valle L, Marcinkiewicz C. Regulatory effect of nerve growth factor in alpha9beta1 integrin-dependent progression of glioblastoma. Neuro-oncol. 2008;10:968–980. doi: 10.1215/15228517-2008-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basora N, Desloges N, Chang Q, Bouatrouss Y, Gosselin J, Poisson J, et al. Expression of the alpha9beta1 integrin in human colonic epithelial cells: resurgence of the fetal phenotype in a subset of colon cancers and adenocarcinoma cell lines. Int J Cancer. 1998;75:738–743. doi: 10.1002/(SICI)1097-0215(19980302)75:5<738::AIDI°C12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Ortega P, Moran A, Fernandez-Marcelo T, De Juan C, Frias C, Lopez-Asenjo JA, et al. MMP-7 and SGCE as distinctive molecular factors in sporadic colorectal cancers from the mutator phenotype pathway. Int J Oncol. 2010;36:1209–1215. doi: 10.3892/ijo_00000604. [DOI] [PubMed] [Google Scholar]
- 33.Pavlova TV, Kashuba VI, Muravenko OV, Yenamandra SP, Ivanova TA, Zabarovskaia VI, et al. Technology of analysis of epigenetic and structural changes of epithelial tumors genome with NotI-microarrays by the example of human chromosome. Mol Biol (Mosk) 2009;43:313–320. doi: 10.1134/S0026893309020137. [DOI] [PubMed] [Google Scholar]
- 34.Gerashchenko GV, Gordiyuk VV, Skrypkina IY, Kvasha SM, Kolesnik OO, Ugryn DD, et al. Screening of epigenetic and genetic disturbances of human chromosome 3 genes in colorectal cancer. Ukr Biokhim Zh. 2009;81:81–87. [PubMed] [Google Scholar]
- 35.Ghosh A, Ghosh S, Maiti GP, Sabbir MG, Zabarovsky ER, Roy A, et al. Frequent alterations of the candidate genes hMLH1, ITGA9 and RBSP3 in early dysplastic lesions of head and neck: Clinical and prognostic significance. Cancer Sci. 2010;101:1511–1520. doi: 10.1111/j.1349-7006.2010.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitra S, Mazumder Indra D, Bhattacharya N, Singh RK, Basu PS, Mondal RK, et al. RBSP3 is frequently altered in premalignant cervical lesions: clinical and prognostic significance. Genes Chromosomes Cancer. 2010;49:155–170. doi: 10.1002/gcc.20726. [DOI] [PubMed] [Google Scholar]
- 37.Arihiro K, Kaneko M, Fujii S, Inai K, Yokosaki Y. Significance of alpha 9 beta 1 and alpha v beta 6 integrin expression in breast carcinoma. Breast Cancer. 2000;7:19–26. doi: 10.1007/BF02967183. [DOI] [PubMed] [Google Scholar]
- 38.Uhm KO, Lee JO, Lee YM, Lee ES, Kim HS, Park SH. Aberrant DNA methylation of integrin alpha4: a potential novel role for metastasis of cholangiocarcinoma. J Cancer Res Clin Oncol. 2010;136:187–194. doi: 10.1007/s00432-009-0646-9. [DOI] [PubMed] [Google Scholar]
- 39.Park J, Song SH, Kim TY, Choi MC, Jong HS, Kim TY, et al. Aberrant methylation of integrin alpha4 gene in human gastric cancer cells. Oncogene. 2004;23:3474–3480. doi: 10.1038/sj.onc.1207470. [DOI] [PubMed] [Google Scholar]
- 40.Lin KT, Yeh SH, Chen DS, Chen PJ, Jou YS. Epigenetic activation of alpha4, beta2 and beta6 integrins involved in cell migration in trichostatin A-treated Hep3B cells. J Biomed Sci. 2005;12:803–813. doi: 10.1007/s11373-005-9005-2. [DOI] [PubMed] [Google Scholar]