Abstract
The goal of this review is to highlight how emerging new models of filopodia assembly which include tissue specific actin-bundling proteins could provide more comprehensive representations of filopodia assembly that would describe more adequately and effectively the complexity and plasticity of epithelial cells. This review also describes how the true diversity of actin bundling proteins must be considered to predict the far-reaching significance and versatile functions of filopodia in epithelial cells.
Key words: epithelial cell, fascin, villin, actin bundling, PIP2 binding, collective cell migration, microvilli, EMT, metastases
Introduction
Filopodia play an important role in epithelial cell-cell contact and cell migration.1 Efficient bundling of actin filaments within filopodia is essential for filopodia formation both in vitro2 and in cultured cells.3 Membrane deformation is energetically unfavorable and single actin filaments which are not adequately stiff to push the membrane buckle under the cell surface.4 Consequently it follows that tight parallel actin bundles are necessary for membrane protrusion and the mechanical stability of filopodia. 3 Fascin is the most widely examined actin-bundling protein that is enriched within filopodia and localizes to the entire length of the filopodia.5 However, fascin is not a ubiquitous protein. Particularly, fascin is not expressed in epithelial cells nor is it expressed in all carcinomas.6–10 So how are actin filaments bundled and filopodia assembled in cells that do not express fascin? Recent studies have identified tissue specific actin-bundling proteins like espin and villin that can assemble filopodia in the absence of fascin.
The routes of filopodia assembly are likely very complex, highly regulated and a single mechanism and a single actin-bundling protein is unlikely to account for the diverse structures, behaviors and functions of filopodia that are found in a wide range of cell types and organisms.11,12 Since numerous cells possess filopodia, which differ in length, thickness, dynamics, localization and origin, it has been suggested that by varying these properties of cell surface structures, the cell generates a range of discrete structures to perform specialized functions. We suggest that one way to generate this diversity would be through the recruitment of specific sets of actin-regulatory proteins which may be unique to the cell type or may be unique to the specialized function that these structures perform. Most current models of filopodia assembly for that reason, seem incomplete and based solely on the identification of a limited number of proteins. In this context, the role of tissue specific actin regulatory proteins and their role in the assembly of filopodia are particularly under-appreciated.
Filopodia are Associated with Unique Functions in Epithelial Cells
Filopodia are especially relevant for epithelial cells, where they are required to establish cell-cell contact between epithelial layers and to guide cell motility.13–16 Our basic understanding of the molecular mechanism(s) of filopodia assembly and functions of filopodia in epithelial cells nonetheless, remains quite rudimentary. Actin polymerized in filopodia has been shown to move and cluster integrins to spatially position integrins primed to probe the matrix at the very front of cell protrusion creating so-called “sticky fingers” along the leading edge of cells with sensory or exploratory functions.17–19 Accordingly in most cells filopodia are used to establish adhesion to the extracellular matrix and for cellular guidance. Since directional cues guide epithelial cell migration, filopodia play a major role in wound-healing, regeneration and maintenance of the epithelium.20–22 An impaired ability of epithelial cells for directional migration is linked to chronic inflammatory diseases which are in turn associated with an increased risk for carcinogenesis.23 Epithelial cell motility is plastic and complex. Epithelial cells can undergo epithelial-to-mesenchymal transition (EMT) and move as single cells. Alternatively, epithelial cells can move as intact clusters, sheets or tubes in a process called “collective cell migration.”24 Collective cell migration requires directed, coherent movement of cell groups that maintain constant positions while migrating.25 Collective cell migration is characterized by the movement of cells that maintain their polarized epithelial phenotype and remain connected by apical cell-cell junctions.25 Therefore, as opposed to single cell migration, collectively migrating cells do not develop a retracting uropod but exert pulling forces on their neighboring cells thus, keeping their positions within the collective structure while moving as 2-D sheets or 3-D strands. Collective cell migration is a conserved morphogenetic event that shapes embryos and tissue in both vertebrate and invertebrate systems. This type of cell migration is observed in the caudal migration of lateral line primordial in zebrafish,26 border cell migration in Drosophila ovaries,27 dorsal closure in Drosophila embryos,28 wound healing in epithelial monolayers29 and in vitro models of mammary gland branching morphogenesis.30 When epithelial cells migrate as groups of cells or collectively as sheets of cells rather than as single cells they can form intermittent or less stable or repetitive short-lived contacts with other cells in the group; alternatively they can maintain intact and stable cell-cell adhesions and cell-cell communication. Whether cells move as tightly adherent sheets or loosely associated with occasional contact but inherent polarity also determines a different migratory mechanism.31–33 Cell-cell junctions can form de novo and resolve again thus, single and collective migration modes are also interconvertible.24 The current thinking is that there may be a combination of localized (for leader cell) and collective signaling (for the remainder of the cells in the group) modes that work to move the loosely adherent or collective sheet of cells forward.34,35 It is speculated that collective sheets may even have different guidance properties relative to solitary migrating cells.35–37 Since single-cell and collective migration modes serve mutually exclusive functions during morphogenesis, tissue regeneration and, in pathological conditions of the epithelium, the role of filopodia in regulating single and collective migration is expected to be similarly complex and likely distinct from what is described for most mesenchymal cells. How epithelial cells migrate directionally in the context of complex multicellular organisms during development or in disease and how they use filopodia to guide such cell migration however, also remains undetermined.
Epithelial tumors cause more than 85% of all cancer-related deaths. Abundant filopodia are a characteristic of invasive, metastatic cancer cells and the number of filopodia correlates with their invasiveness.38,39 Similar to the developmental processes cancer cell migration can occur as single-cell migration (amoeboid or mesenchymal, solitary or in Indian files) or it can occur as collective cell migration (in cell sheets, strands, tubes or clusters).24,40–44 Furthermore, by altering cell-cell and cell-extracellular matrix adhesions cancer cell invasion modes can be altered from multicellular strands to amoeboid dissemination.41,45,46 Tumor cells choose different migratory mechanisms and the resulting diversity and plasticity contributes to the difficulty of treating metastasis.41 The role of filopodia in these diverse and complex cell migratory mechanisms and their contribution to cancer metastases however remains unknown.
In addition to their role in cell motility, filopodia in epithelial cells also align cells correctly to adhere to their target partner and to close the gap between epithelial sheets, a process called “adhesion zippering.” In this process filopodia interdigitate allowing E-cadherins to interact at sites of contact which then mature into adherens junctions through recruitment of catenins and alignment of the plasma membranes, thereby ‘zipping’ the two epithelial sheets together.20,47 Adhesion zippering is very well described in epithelial sheet fusion in C. elegans,48,49 during dorsal closure of Drosophila embryos20,47 and in cultured epithelial cells undergoing in vitro wound closure.21,50,51 Contractile filopodia also tether lens and retina to coordinate epithelial invagination highlighting the role of filopodia in similar morphogenetic events in vertebrates.52 It is speculated that in these unique situations, filopodia function as mechanosensitive organelles to control cell-cell communication and cell-cell adhesion.13,14,53,54
Several viruses and bacteria that infect epithelial cells, bind at filopodial tips which participate in their internalization and spread in host cells.55–57 Viruses are often associated with the dense microvilli of polarized epithelial cells but also with the filopodia of both polarized and nonpolarized cells.56,58–61 Transport of viruses along filopodia during the attachment and entry process has been documented for a number of viruses.55,62–64 Filopodia have been shown to act as cellular tentacles that serve to increase the efficiency of uptake of pathogens.65 Recent evidence suggests that viruses can themselves activate filopodia formation to enhance viral uptake.55,63,66 Since a majority of HIV transmissions occur across a mucosal surface such as the anorectal mucosa, vaginal mucosa and less frequently, the oral mucosa, several recent studies have demonstrated how profoundly and preferentially epithelial tissue are targeted during all stages of HIV-1 infections and how microvilli/filopodia-like structures are involved in cell-to-cell transmission of HIV virus in epithelial cells.67 Similarly, epithelial wounding has been shown to facilitate papillomavirus infection, the etiological agent of epithelial tumors and cancers.68 Prevotella intermedia group bacteria associated with oral and gastrointestinal tract disease pathogenesis also use filopodia and lamellipodia to adhere and invade epithelial cells.69 Enteropathogenic Escherichia coli, Shigella, Salmonella and Citrobacter rodentium, which colonize the gut, have all been shown to contain a WxxxE motif which promotes filopodia assembly by activating Cdc42 to regulate the adhesion, spread and survival of these enteropathogens in host epithelial cells.57,70–74 Interestingly, a similar process has also been described for the retrograde transport of activated epidermal growth factor (EGF) receptors on adenocarcinoma cells.75
In Epithelial Cells the Same Actin Bundling Protein Could Assemble Microvilli and Filopodia
Parallel, bundled actin filaments found in cellular protrusions such as microvilli and filopodia are ubiquitous in eukaryotes, but are particularly well-developed and functionally significant in epithelial cells.76 Actin-polymerization and actin-crosslinking provide the driving force for the formation of these cell surface structures, which can be rapidly extended and retracted thus, modifying cell morphology and function in response to extracellular stimuli or intracellular signaling.77 The molecular mechanism for the assembly and turnover of microvilli and filopodia in fact share several similarities namely, both contain parallel actin bundles; actin nucleation is essential for the assembly of both these structures; the bundles are formed by actin assembly that begins near an electron-dense complex at the tip; the bundles taper, that is the number of filaments decrease as one approaches the bundle tip; bundles are polarized with the barbed ends located at the bundle tip nearest to the plasma membrane; bundle production is independent of a specific type of crosslinker (multiple actin bundling proteins associated with microvilli and filopodia); the length of the filaments appears to be independent of filament number; the actin bundles must remain at constant length yet treadmill; the microvilli is embedded in the terminal web, while most filopodia are embedded in a lamellipodium.12,78–88 Since crosslinked bundles of actin filaments in a wide variety of cells from different organisms share common features it has been hypothesized that a common mechanism to construct initial actin bundles exists in all cells.86 In this context it is worth mentioning that apical filopodia of certain cell types have been proposed to be precursors of more specialized protrusions such as brush border microvilli or the sensory stereocilia of the inner ear hair cell.86 Villin and fascin are actin bundling proteins that are associated with parallel actin bundles in microvilli and filopodia.5,89–96 It follows then that actin-bundling proteins in epithelial cells could build parallel bundled structures in both the microvilli and filopodia. Furthermore, their physiological function may be determined not by their association with one organelle or the other but by their functional characteristics. Most actin bundling proteins are associated with multiple cellular protrusions which suggests that similar actin bundling events regulated by these proteins may occur in many of these cell surface structures.95 The epithelial cell specific actin-bundling protein villin is associated with microvilli, lamellipodia, microspikes and filopodia.89–91,97–99 Espin is associated with microvilli, stereocilia and filopodia.88,100,101 Similarly, the actin-bundling protein fascin is associated with microvilli; stereocilia, filopodia and lamellipodia; and in transformed epithelial cell lines that do express fascin, it is associated with cell-cell adherens junctions.88,95,96,102–104 The initial bundles assembled in these structures are also continuously modified. For instance, although intestinal and renal epithelial microvilli maintain constant length after initial assembly, the assembly and turnover of microvilli is dynamic, which implies ongoing assembly and disassembly and utilization of actin bundles within these structures.79,105,106 Perhaps the best-studied examples are the shortening of microvilli during starvation and after treatment with cyclohexamide or lectins and re-elongation of the microvilli following the removal of these stimuli or when G-actin is added to intact brush borders of intestinal epithelial cells.107–110 Other similarities between microvilli and filopodia involve the role of Arp2/3. Components of the Arp2/3 actin nucleating complex are expressed early in the time course of microvilli assembly when the cells are not polarized and their levels decrease significantly as cells become polarized and develop well-ordered microvilli.111 Consistent with that, RNAi-mediated knockdown of Arp2 has no effect on microvilli assembly.12 Likewise, while the convergent elongation model predicts that Arp2/3 nucleates actin assembly in filopodia, Arp2/3 is in fact excluded from established filopodia.82,112 Moreover, Arp2/3-deficient cells can effectively assemble filopodia.113 These examples highlight the similarities in the morphology, composition, assembly and, dynamics of microvilli and filopodia. Future studies with actin bundling proteins e.g., villin, fascin and, espin could help identify if there is a common set of proteins but different strategies to assemble the microvilli and filopodia in epithelial cells or if there is a shared molecular mechanism in which actin-bundling proteins play the same role in both microvilli and filopodia assembly and regulation. This is particularly relevant since cell surface structures are no longer being classified based on their morphological characteristics, but are increasingly being identified by the molecular machinery that regulates their assembly.
In Epithelial Cells, Multiple Actin Bundling Proteins Could Be Involved in Filopodia Assembly
Most bundled actin filament containing structures found in vivo are assembled by two or more actin crosslinking proteins.76,114 In microvilli of intestinal epithelial cells there are three actin crosslinkers per bundle, villin, fimbrin and espin;115 while in stereocilia there is fimbrin/plastin and espin.76 The biological role of actin crosslinking proteins is best described in D. melanogaster where oogenesis and bristle formation have been extensively studied over many years. Five actin crosslinking proteins are associated with D. melanogaster morphogenesis: singed (fascin), α-actinin, filamin, forked and quail (villin-like). Deletion or mutations in any one of these five proteins is associated with an abnormal phenotype (Table 1).116–123 A polarized distribution of two or more actin bundling proteins during cell motility is likewise documented.124 It is clear from studies done with Dictyostelium that even simple organisms such as amoeba require a whole spectrum of actin-binding proteins to perform basic cellular functions. Therefore, it is not unlikely that in eukaryotic cells, filopodia could be assembled by multiple actin bundling proteins.
Table 1.
Multiple actin bundling proteins are required by D. melanogaster during oogenesis and bristle formation
| Protein | Mutant phenotype |
| Fascin (Singed) | Short gnarled bristles; female sterility |
| Quail (Villin like) | Female sterility; disruption of follicle cells |
| Forked | Gnarled bristles |
| α-Actinin | Lethal; flightless, thoracic muscle, tubular flight muscle, leg muscle abnormalities |
| Filamin | Female sterility |
Most models of filopodia identify two actin bundling protein in filopodia namely, fascin and IRSp53. It may be noted that neither of these proteins is expressed in normal epithelial cells. On the other hand, most mammalian cells express additional actin crosslinking proteins such as α-actinin, filamin, T-plastin, espin, fimbrin and villin many of which have been localized to the filopodia.5,91,125–129 At least two of these actin-bundling proteins are expressed in epithelial cell and have been associated with both the microvilli and filopodia, namely, espin and villin.88,90–92,100 In the absence of fascin, some of these actin crosslinkers can also rescue filopodia assembly.5,88 Therefore, it is still uncertain whether fascin is sufficient for filopodia formation or if multiple actin bundling proteins share this role. It is also noteworthy that fibroblasts from fascin-1 deficient mice still extend filopods, suggesting that fascin is not the sole regulator of filopodia assembly.130 It seems probable then, that while fascin may be the main actin crosslinking protein in cells that do express fascin, other actin crosslinkers are likely used in specialized cells including epithelial cells that do not express fascin or IRSp53;90 or in specific conditions, such as the recruitment of filamin to filopodia induced by Wnt5a signaling.127 It is also possible that different sets of proteins form filopodia by related mechanisms depending on which proteins are present and/or active within a particular cell type.
There are discrete stages for most actin bundle formation in vivo. In almost all instances, two or more actin-bundling proteins localize sequentially and have non-redundant functions that are required to generate maximally cross-linked and maximally rigid actin bundles in vivo93,119,131 (Table 1). In general, one actin crosslinking protein is required to hold adjacent filaments together during the initial stages of elongation and the second is required to zipper together the filaments in tightly packed, well-ordered bundles. Such two-step actin assembly has been described in the development of microvilli in intestinal epithelial cells,93,132,133 in the assembly of stereocilia131 and, in the development of Drosophila bristles.134 In other instances, such as the colon adenocarcinoma cell line, Caco-2 there is a downregulation of fascin and an upregulation of villin and α-actinin as cells assemble well structured and functional microvilli.111 Could multiple actin bundling proteins have a similar function in the assembly of filopodia? In a recent study, a non-homogenous distribution of actin bundling proteins within the filopodia was described with α-actinin localized to the middle section of the filopodia, coronin 1 associated with the base of the filopodia and T-plastin associated with the entire length of the filopodia except the tip.129 Similarly, espin has been shown to localize to the the proximal part of the filopodia.5 In such models, a temporally regulated, sequential non-overlapping function of each of these proteins during actin filament bundling in filopodia can be envisioned. Interestingly only two actin bundling proteins are described so far that associate with the entire length of the filopodia namely fascin and the epithelial cell specific actin-bundling protein villin.82,90,91 There is also a distinct possibility that actin bundling proteins rapidly exchange between actin filaments within the filopodia to regulate filopodia turnover and dynamics.5,135 The types and numbers of F-actin bundling proteins and the number of actin filaments bundled would greatly affect the persistence length and/or stiffness of filopodia assembled by these proteins.11 These factors in turn, could influence how filopodia respond to chemical or mechanical cues and how downstream signals are activated in these cells. This would be consistent with the emerging concept that molecular characteristics of the filopodia may be determined by functional characteristics of the cell and the task executed by these cell surface protrusions.
Comparison of Filopodia Assembled in Epithelial Cells by Villin and Fascin
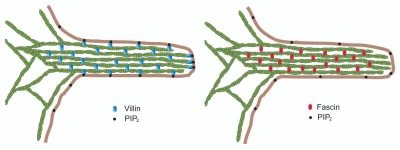
In this review we highlight the function of epithelial cell-specific actin-bundling proteins like villin, which can assemble actin bundles in microspikes and filopodia of migrating epithelial cells that lack fascin.90,91 The role of villin in the assembly and maintenance of the microvilli has been known for over three decades.92,132 However, villin also regulates cell migration and in vivo villin is required for the reorganization of the actin cytoskeleton during changes in cellular plasticity, cell motility, cell morphogenesis and wound healing.99,106,136–142 Villin is unique among the actin-bundling proteins and in fact among all actin-binding proteins in that it can cap, sever, nucleate and bundle actin filaments. These unique properties of villin may contribute to the diverse functions performed by villin in epithelial cells. Furthermore, the actin regulatory functions of villin are modified in response to changes in calcium, tyrosine phosphorylation and ligand binding {phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2], PLC-γ1 and actin} demonstrating villin's ability to dynamically reorganize the actin cytoskeleton.106,137,138 Studies done in our laboratory have shown that villin's ability to bind the plasma membrane [through its PtdIns(4,5)P2-binding domains] and simultaneously bundle actin filaments drives the emergence of filopodia (Fig. 1).143,144 Our recent findings illustrate that the convergence of different signaling cascades spatially restrict villin activity to areas of high PtdIns(4,5)P2 and high F-actin concentration to assemble filopodia.90 Furthermore, mutants of villin that fail to bind PtdIns(4,5)P2 or fail to bundle actin and assemble filopodia disrupt directional cell migration.90
Figure 1.
In epithelial cells villin and fascin may have non-overlapping functions in filopodia assembly. Villin can nucleate, cap, sever and bundle actin filaments. Villin also binds PtdIns(4,5)P2. Fascin is an actin-bundling protein that does not bind PtdIns(4,5)P2. This predicts that filopodia assembled by villin and fascin could be molecularly and functionally distinct. In epithelial cells that do express villin and fascin, villin may be associated with more dynamic actin bundles that undergo rapid turnover in response to extracellular cues. By contrast, fascin could be associated with more stable and rigid actin bundles within the filopodia.
Fascin-1 is expressed most abundantly in brain, ovary and testis; at the cellular level it is found in neuronal and glial cells, microcapillary endothelial cells and antigen-presenting dendritic cells.145–147 However, fascin is absent from normal epithelial cells.148,149 Moreover fascin expression is restricted to specific tissues during development.150 By contrast villin is more widely expressed in epithelial cells (Table 2). Recently, expression of fascin has been reported in some transformed epithelial cell lines and in some carcinomas.151–154 However, not all transformed epithelial cell lines or carcinomas express fascin.6–10 Studies done in our laboratory suggest that in such cells, villin is the principal actin-bundling protein that assembles filopodia.90,91
Table 2.
Comparison of villin and fascin and their role in filopodia assembly and turnover in normal and transformed epithelial cells
| Characteristics | Villin | Fascin |
| Expression | Expressed in epithelial cells of the gastrointestinal tract; urogenital tract; exocrine glands of endodermic lineage, brush cells, merkel cells, osteocytes, taste receptor cells. Expressed in all carcinomas and intestinal metaplasia. | Expressed in neuronal, glial, endothelial and dendritic cells. Not expressed in epithelial cells. Expressed in some transformed epithelial cell lines and some carcinomas such as squamous cell carcinomas. |
| Expression in transformed epithelial cell lines | Expression increases as cells become polarized and differentiated. | Expression decreases as cells become polarized and differentiated. |
| Localization | Associated with microvilli and terminal web in polarized epithelial cell lines. | Associated with cell-cell contacts in transformed epithelial cell lines. |
| Metastasis | Associated with EMT. | Not associated with EMT. |
| Filopodia location | Assembles filopodia at leading edge. | Assembles filopodia at leading edge of mesenchymal cells and in carcinomas may assemble filopodia at cell-cell contacts. |
| Filopodia dynamics | May regulate dynamics and turnover of filopodia. | May assemble rigid filaments in filopodia and regulate stability of filopodia. |
| Actin Dynamics | Can nucleate, cap, sever and bundle actin filaments. | Can bundle actin filaments. |
| Capping activity | Could cap filaments to regulate filopodia assembly and turnover. | Has no actin capping function. |
| Nucleation activity | Could nucleate actin assembly in filopodia. | Has no actin nucleating function. |
| PtdIns(4,5)P2 | Binds PtdIns(4,5)P2 and could generate evaginations or stabilize protrusions of filopodia by PtdIns(4,5)P2 clustering. | Does not bind PtdIns(4,5)P2. |
Both villin and fascin are associated with parallel actin bundles in the non-adherent more stable structures of the microvilli as well as in the adherent, more dynamic structures of the filopodia.89–91,95–99 However, villin is also associated with the less organized actin network in membrane ruffles and lamellipodia.98,99,136,142 Villin can cap, nucleate, sever and bundle actin while fascin only bundles actin filaments (Fig. 2). Villin can associate with PtdIns(4,5)P2 and the plasma membrane, another function not shared by fascin (Fig. 1).98,143 The diverse biochemical properties and the distinct intracellular distribution of villin and fascin suggests that it is unlikely that in epithelial cells that do express both these proteins they have redundant overlapping functions (Table 2). The most compelling data supporting this hypothesis comes from studies done in Drosophila where both fascin (singed) and villin (quail) have been shown to have nonoverlapping essential functions and both proteins are required for actin bundle assembly (Table 1).155 Deletion of either fascin or villin in Drosophila results in abnormal egg chambers and female sterility due to abnormal assembly of actin bundles in the nurse cells demonstrating that both proteins are required for bundle assembly and have distinct roles in bundle organization.118 A model of fascin and villin in Drosophila predicts that villin crosslinks F-actin loosely and then fascin organizes them into larger more compact bundles.119 Consistent with that, biophysical studies have identified the extreme mechanical stiffness of actin bundles assembled by fascin-1 compared with other actin crosslinking proteins as well as identified the unique and unusual effects of fascin-1 on the rigidity of actin bundles.5,156,157 In contrast, villin is associated with the assembly of loose poorly organized actin bundles.158–160 Furthermore, recent studies with the villin knockout mice have demonstrated that rather than just assemble actin bundles, villin may in fact be required to dynamically reorganize the actin bundles in response to cell signaling, cell injury and cell stress.106 It may be noted that unlike fascin, the actin bundling function of villin can be modified in response to cell signaling.106,137,138 This suggests that in epithelial cells that do express both villin and fascin, villin may be associated with more dynamic actin bundles that require rapid turnover in response to extracellular cues while fascin may be associated with more rigid, stable actin bundles within the filopodia. This predicts that filopodia assembled by villin and fascin could be not only molecularly but also functionally distinct. Further, this predicts that in carcinomas that do express villin and fascin, the dynamics of fascin and villin mediated filopodia might be significantly different and may be used by the cancer cells to assemble filopodia differing in dynamics, length and/or lifetime to regulate the different steps of tumorigenesis. Fascin and its actin bundling function are transcriptionally regulated, since fascin is generally absent from normal epithelial cells and is only expressed in some transformed epithelial cells. One can speculate then that in transformed epithelial cells that do express both villin and fascin, villin could be the first protein that is recruited to assemble filopodia to move cells prior to transcriptional upregulation of fascin.
Figure 2.
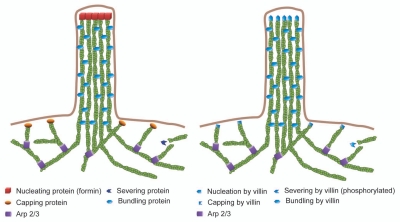
Model for filopodia assembly. This model describes the function of actin regulatory proteins including actin nucleating, capping, severing and bundling proteins during filopodia assembly. The figure on the right shows putative functions that could be attributed to tissue-specific actin capping, nucleating, severing and bundling proteins like villin during filopodia assembly.
EMT is strongly implicated in metastases but fascin is excluded from cells that display molecular signatures of EMT.161 Moreover, while some primary tumors are fascin 1-positive, all of the distant metastases have been found to be fascin 1-negative suggesting a tight and specific regulation of fascin 1 expression during tumorigenesis, which excludes cells that have undergone EMT.161 Collective cell migration is characteristic of certain types of cancers including high and intermediate differentiated lobular breast cancer, epithelial prostate cancer, large cell lung cancer and squamous cell carcinomas.11,25,162 The association of fascin with these types of cancers particularly squamous cell carcinomas and not those that have undergone EMT indicates that fascin may regulate collective cell migration in these types of cancers. Collective cell migration may represent an efficient migration strategy that allows translocation of heterogeneous sets of cells thereby disseminating cells of different clonality and function within one functional unit. This hypothesis is further supported by the observation that in transformed epithelial cell lines fascin is localized to areas of cell-cell contact and cell-cell adhesion.163,164 It is conceivable then that the more rigid filopodia assembled by fascin at cell-cell contacts function as mechanosensitive organelles while the more dynamic filopodia assembled by villin at the leading edge of cells function as chemosensitive organelles. It is worth noting that cell-cell junctions can form de novo and resolve again, demonstrating that individual and collective cell migration modes are interconvertible.24 Moreover cancer cell invasion modes can be altered by modifying cell-cell and cell-extracellular matrix adhesions.41,45,46 This would be congruent with the transcriptional regulation of fascin expression in some transformed epithelial cells.111 We suggest that such new models could help explain the adaptability of cell movement, the plasticity of epithelial cells, and significance of multiple actin bundling proteins villin and fascin in the filopodia of transformed epithelial cells.
Actin Bundling Proteins Like Villin That Can Also Nucleate and Cap Actin Filaments and their Role in Filopodia Assembly
A major unanswered question in understanding filopodia assembly has been how they are initiated. Currently there are two models that describe how filopodia are assembled in all mammalian cells. Filopodia in migrating cells have been proposed to be formed primarily by reorganization of the dendritic actin network of the lamellipodium112 (Fig. 2). It has been suggested that Arp2/3-mediated nucleation of actin filaments plays a major role in generating the parallel bundles of the filopodia.82 Further, this model suggests that a subset of Arp2/3 nucleated filaments are capped and clustered by Ena/VASP proteins Dia2 and myosin-X and as these filaments rapidly elongate they are bundled by fascin to generate filopodia.165 However, Arp2/3 is absent from established filopodia, which suggests that Arp2/3 may participate in the initiation but not in the steady-state elongation of these structures.112 More importantly, mammalian cells depleted of Arp2/3 complex can assemble filopodia demonstrating that Arp2/3 is dispensable for filopodia formation.113 The second model proposes that filopodia are formed through the direct polymerization of parallel actin filaments by members of the formin family together with Ena/VASP.166–168 Such filopodia are not derived from the underlying lamellipodial network but are formed de novo. The synergistic role of Ena/VASP and formins in this model has not been adequately interpreted, although it is postulated that Ena/VASP proteins assemble filopodia via their F-actin bundling activity.169 Both these models may be relevant. Migrating cells also display parallel actin bundles in microspikes which remain embedded within the lamellipodia during cellular protrusion.170 Microspikes can evolve to filopodia when they protrude beyond the leading edge.82 Additionally, migrating cells display parallel actin bundles in retraction fibers which are left behind when lamellipodium retract. Generally speaking, microspikes, filopodia and the retraction fibers are inter-convertible organelles although the molecular origins of these structures are not well understood. It has been suggested that some of these structures could be assembled by the convergent-elongation mechanism while others may arise from formin-mediated actin nucleation, which would explain the existence of multiple mechanisms to initiate filopodia assembly in cells. As an alternative, it has been suggested that tissue specific actin nucleating proteins could regulate the assembly of parallel actin bundles in filopodia11,12,171 (Fig. 2). One such protein could be villin, which can nucleate and bundle actin filaments.90,91,172
In intestinal cell lines as well as in enterocytes of the small and large intestine, the expression of villin increases as cells form long, well developed microvilli.137,138 In vitro, simple mixtures of villin and F-actin form uniformly polarized bundles similar to those seen in the ultrastructural analysis of microvilli.173 Overexpression of villin in fibroblasts and other villin-null cells reorganizes the actin cytoskeleton, which includes a loss of stress fibers and an assembly of parallel actin bundles in microvilli-like structures or in microspike and filopodia.89,91,97 Thus, villin has the ability to recruit actin from other microfilament structures to facilitate the formation of bundled actin structures. Villin is the first microvillar protein that is detected in the early stages of development in the immature chicken and mouse digestive tract and shows a transient elevation prior to the formation of the microvilli suggesting that villin plays a significant role in microvillar formation, maturation and/or maintenance.132,133 Moreover, the molecular basis for the localization of villin at this time, when there is little actin-based cytoskeleton, has led to the suggestion that villin interacts with the apical plasma membrane to nucleate actin and assemble the microvilli.132 As an actin filament nucleating and severing protein villin can accelerate actin assembly.174,175 Based on this, a membrane-associated actin-nucleating and actin-crosslinking role for villin has been hypothesized for several decades.93,132 Studies done in our lab and other have clearly demonstrated that in epithelial cells villin is a central player at the crossroads of signaling network that link the actin cytoskeleton with membrane dynamics.90,91,98,136,143 Most importantly, studies done in our lab have described how spatial organization of parallel actin bundles whose growth generates membrane deformation during cell motility are assembled by tissue-specific actin bundling proteins like villin.90 It is plausible then that villin's ability to nucleate and bundle actin filaments contributes to its function in filopodia assembly (Fig. 2).
Filopodia formation is thought to be dependent, in part, upon the relative activities of actin-capping and anti-capping proteins.176–178 It has also been shown that parallel actin bundle formation can be shifted to dendritic network formation by actin capping proteins.112 In fact, Mejillano and colleagues have demonstrated that depleting cells of capping proteins results in a dramatic explosion of filopodia while reinstating capping proteins back results in lamellipodia assembly indicating the role of capping proteins on cellular protrusions.179 The localization of actin bundling proteins like villin to both the lamellipodia and the filopodia and the fact that villin can cap and bundle actin filaments adds to the complexity of filopodia assembly in epithelial cells and the role of actin crosslinkers like villin in the assembly of such structures (Fig. 2). While such new models are emerging, more detailed studies are required to describe the molecular mechanism of filopodia assembly in the majority of mammalian cells that either do not use Arp2/3 or do not express fascin or have more complex and heterogeneous filopodia that fulfill multiple functions such as the filopodia in epithelial cells.
Filopodia Assembly by Actin Bundling Proteins That Can Also Interact with the Plasma Membrane
Recent studies have demonstrated that proteins that lack actin regulatory functions but interact with membrane lipids are sufficient to facilitate filopodia assembly. E.g., overexpression of the lipid phosphates related protein-1, LPR1 in HeLa and Cos-7 cells is associated with a large increase in the number of peripheral and dorsal filopodia.180 Similarly, the inverse BAR (I-BAR) domain of IRSp53 protein, which binds phosphoinositide-rich membranes with high affinity induces membrane protrusion.181,182 I-BAR/IM domains do not crosslink actin filaments but induce PtdIns(4,5) P2 clustering which results in membrane bending due to electrostatic interactions. Moreover, these proteins remain dynamically associated with the inner leaflet of the membrane as a consequence they also stabilize these membrane protrusions.183 IRSp53 localizes to filopodia.184 The ectopic expression of IRSp53 induces filopodia184–189 and knockdown of IRSp53 diminishes the ability of cells to develop filopodia.190,191 These studies reveal that direct membrane deformations without actin bundles can form filopodia. Many of the I-BAR domain proteins, however, harbor protein-protein interaction domains indicating that a strong association between the plasma membrane and actin dynamics is nonetheless required to sustain these membrane protrusions. Moreover, actin filaments are present in the majority of I-BAR-induced filopodia and may be essential for their long-term maintenance.3 However, what is noteworthy is that actin filaments themselves do not drive the protrusion but only provide mechanical support to the membrane deformations and/or stabilize the membrane protrusions. These recent findings highlight how little we know still about the molecular mechanisms that assemble filopodia.
It has also been suggested that transient changes in membrane curvature such as those caused by PtdIns(4,5)P2 clustering can cause filopodia assembly by activating pathways that trigger actin polymerization.192 It is hypothesized that the concentration of the actin filaments assembled and bundled by PtdIns(4,5)P2 clustering would curve the membrane locally. It has also been speculated that if the barbed end of actin filaments are kept together by a crosslinker that stays close to the growing filament tips, then the filaments would grow in the direction of protrusion and the corresponding bending force could be generated by the polymerization ratchet mechanism.193–195 These could be alternative mechanisms for filopodia assembly that are employed by actin bundling proteins that also bind PtdIns(4,5)P2 such as α-actinin, villin and espin90,196,197 (Figs. 1 and 2).
Acknowledgments
The work described in this review was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant DK-54755 (to S.K.).
Abbreviations
- EMT
epithelial-mesenchymal transition
- PIP2
phosphatidylinositol-4,5-bisphosphate
References
- 1.Kraikivski P, Slepchenko BM, Novak IL. Actin bundling: initiation mechanisms and kinetics. Phys Rev Lett. 2008;101:128102. doi: 10.1103/PhysRevLett.101.128102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vignjevic D, Yarar D, Welch MD, Peloquin J, Svitkina T, Borisy GG. Formation of filopodia-like bundles in vitro from a dendritic network. J Cell Biol. 2003;160:951–962. doi: 10.1083/jcb.200208059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang C, Hoelzle M, Disanza A, Scita G, Svitkina T. Coordination of membrane and actin cytoskeleton dynamics during filopodia protrusion. PLoS ONE. 2009;4:5678. doi: 10.1371/journal.pone.0005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mogilner A, Rubinstein B. The physics of filopodial protrusion. Biophys J. 2005;89:782–795. doi: 10.1529/biophysj.104.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vignjevic D, Kojima S, Aratyn Y, Danciu O, Svitkina T, Borisy GG. Role of fascin in filopodial protrusion. J Cell Biol. 2006;174:863–875. doi: 10.1083/jcb.200603013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal B, Ludwig OJ, Collins BT, Cortese C. Immunostaining as an adjunct to cytology for diagnosis of pancreatic adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1425–1431. doi: 10.1016/j.cgh.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Al-Saad S, Al-Shibli K, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic impact of NFkappaB p105, vimentin, E-cadherin and Par6 expression in epithelial and stromal compartment in non-small-cell lung cancer. Br J Cancer. 2008;99:1476–1483. doi: 10.1038/sj.bjc.6604713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soukup V, Babjuk M, Duskova J, Pesl M, Szakaczova M, Zamecnik L, et al. Does the expression of fascin-1 and tumor subclassification help to assess the risk of recurrence and progression in t1 urothelial urinary bladder carcinoma? Urol Int. 2008;80:413–418. doi: 10.1159/000132700. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi H, Shimizu M, Ban S, Koyama I, Hatori T, Fujita I, et al. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2009;33:1164–1172. doi: 10.1097/PAS.0b013e3181a162e5. [DOI] [PubMed] [Google Scholar]
- 10.Iguchi T, Yamashita N, Aishima S, Kuroda Y, Terashi T, Sugimachi K, et al. A comprehensive analysis of immunohistochemical studies in intrahepatic cholangiocarcinoma using the survival tree model. Oncology. 2009;76:293–300. doi: 10.1159/000207506. [DOI] [PubMed] [Google Scholar]
- 11.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 12.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-110. [DOI] [PubMed] [Google Scholar]
- 13.Raich WB, Agbunag C, Hardin J. Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr Biol. 1999;9:1139–1146. doi: 10.1016/S0960-9822(00)80015-9. [DOI] [PubMed] [Google Scholar]
- 14.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/S0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 15.Millard TH, Martin P. Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development. 2008;135:621–626. doi: 10.1242/dev.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahri S, Wang S, Conder R, Choy J, Vlachos S, Dong K, et al. The leading edge during dorsal closure as a model for epithelial plasticity: Pak is required for recruitment of the Scribble complex and septate junction formation. Development. 2010;137:2023–2032. doi: 10.1242/dev.045088. [DOI] [PubMed] [Google Scholar]
- 17.Galbraith CG, Yamada KM, Galbraith JA. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science. 2007;315:992–995. doi: 10.1126/science.1137904. [DOI] [PubMed] [Google Scholar]
- 18.Bridgman PC, Dave S, Asnes CF, Tullio AN, Adelstein RS. Myosin IIB is required for growth cone motility. J Neurosci. 2001;21:6159–6169. doi: 10.1523/JNEUROSCI.21-16-06159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidemann SR, Lamoureux P, Buxbaum RE. Growth cone behavior and production of traction force. J Cell Biol. 1990;111:1949–1957. doi: 10.1083/jcb.111.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood W, Martin P. Structures in focus-filopodia. Int J Biochem Cell Biol. 2002;34:726–730. doi: 10.1016/S1357-2725(01)00172-8. [DOI] [PubMed] [Google Scholar]
- 21.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/S0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 22.Yonemura S, Itoh M, Nagafuchi A, Tsukita S. Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci. 1995;108:127–142. doi: 10.1242/jcs.108.1.127. [DOI] [PubMed] [Google Scholar]
- 23.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 24.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 25.Friedl P, Hegerfeldt Y, Tusch M. Collective cell migration in morphogenesis and cancer. Int J Dev Biol. 2004;48:441–449. doi: 10.1387/ijdb.041821pf. [DOI] [PubMed] [Google Scholar]
- 26.Haas P, Gilmour D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell. 2006;10:673–680. doi: 10.1016/j.devcel.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Montell DJ. Developmental regulation of cell migration. Insight from a genetic approach in Drosophila. Cell Biochem Biophys. 1999;31:219–229. doi: 10.1007/BF02738240. [DOI] [PubMed] [Google Scholar]
- 28.Jacinto A, Woolner S, Martin P. Dynamic analysis of dorsal closure in Drosophila: from genetics to cell biology. Dev Cell. 2002;3:9–19. doi: 10.1016/S1534-5807(02)00208-3. [DOI] [PubMed] [Google Scholar]
- 29.Fenteany G, Janmey PA, Stossel TP. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr Biol. 2000;10:831–838. doi: 10.1016/S0960-9822(00)00579-0. [DOI] [PubMed] [Google Scholar]
- 30.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bronner-Fraser M. Neural crest cell migration in the developing embryo. Trends Cell Biol. 1993;3:392–397. doi: 10.1016/0962-8924(93)90089-J. [DOI] [PubMed] [Google Scholar]
- 32.Haga H, Irahara C, Kobayashi R, Nakagaki T, Kawabata K. Collective movement of epithelial cells on a collagen gel substrate. Biophys J. 2005;88:2250–2256. doi: 10.1529/biophysj.104.047654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 34.Matsubayashi Y, Ebisuya M, Honjoh S, Nishida E. ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr Biol. 2004;14:731–735. doi: 10.1016/j.cub.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 35.Rørth P. Collective guidance of collective cell migration. Trends Cell Biol. 2007;17:575–579. doi: 10.1016/j.tcb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, et al. Two distinct modes of guidance signalling during collective migration of border cells. Nature. 2007;448:362–365. doi: 10.1038/nature05965. [DOI] [PubMed] [Google Scholar]
- 37.Rørth P. Whence directionality: guidance mechanisms in solitary and collective cell migration. Dev Cell. 2011;20:9–18. doi: 10.1016/j.devcel.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 38.MacHesky LM. Lamellipodia and filopodia in metastasis and invasion. FEBS Lett. 2008;582:2102–2111. doi: 10.1016/j.febslet.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 39.Dasgupta S, Cushman I, Kpetemey M, Casey PJ, Vishwanatha JK. Prenylated c17orf37 induces filopodia formation to promote cell migration and metastasis. J Biol Chem. 2011;286:25935–25946. doi: 10.1074/jbc.M111.254599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 42.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 43.Grünert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4:657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- 44.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 45.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 46.Friedl P, Wolf K. Proteolytic and non-proteolytic migration of tumour cells and leucocytes. Biochem Soc Symp. 2003;70:277–285. doi: 10.1042/bss0700277. [DOI] [PubMed] [Google Scholar]
- 47.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–448. doi: 10.1016/S0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 48.Mohler WA, Simske JS, Williams-Masson EM, Hardin JD, White JG. Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr Biol. 1998;8:1087–1091. doi: 10.1016/S0960-9822(98)70447-6. [DOI] [PubMed] [Google Scholar]
- 49.Williams-Masson EM, Malik AN, Hardin J. An actinmediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development. 1997;124:2889–2901. doi: 10.1242/dev.124.15.2889. [DOI] [PubMed] [Google Scholar]
- 50.Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr Opin Cell Biol. 2001;13:76–84. doi: 10.1016/S0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 52.Chauhan BK, Disanza A, Choi SY, Faber SC, Lou M, Beggs HE, et al. Cdc42- and IRSp53-dependent contractile filopodia tether presumptive lens and retina to coordinate epithelial invagination. Development. 2009;136:3657–3667. doi: 10.1242/dev.042242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorfinkiel N, Blanchard GB, Adams RJ, Martinez Arias A. Mechanical control of global cell behaviour during dorsal closure in Drosophila. Development. 2009;136:1889–1898. doi: 10.1242/dev.030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacinto A, Wood W, Balayo T, Turmaine M, Martinez-Arias A, Martin P. Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol. 2000;10:1420–1426. doi: 10.1016/S0960-9822(00)00796-X. [DOI] [PubMed] [Google Scholar]
- 55.Smith JL, Lidke DS, Ozbun MA. Virus activated filopodia promote human papillomavirus type 31 uptake from the extracellular matrix. Virology. 2008;381:16–21. doi: 10.1016/j.virol.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiwari V, Oh MJ, Kovacs M, Shukla SY, Valyi-Nagy T, Shukla D. Role for nectin-1 in herpes simplex virus 1 entry and spread in human retinal pigment epithelial cells. FEBS J. 2008;275:5272–5285. doi: 10.1111/j.1742-4658.2008.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berger CN, Crepin VF, Jepson MA, Arbeloa A, Frankel G. The mechanisms used by enteropathogenic Escherichia coli to control filopodia dynamics. Cell Microbiol. 2009;11:309–322. doi: 10.1111/j.1462-5822.2008.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helenius A, Kartenbeck J, Simons K, Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980;84:404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duus KM, Lentchitsky V, Wagenaar T, Grose C, Webster-Cyriaque J. Wild-type Kaposi's sarcoma-associated herpesvirus isolated from the oropharynx of immune-competent individuals has tropism for cultured oral epithelial cells. J Virol. 2004;78:4074–4084. doi: 10.1128/JVI.78.8.4074-84.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith AE, Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 61.Nunbhakdi-Craig V, Craig L, Machleidt T, Sontag E. Simian virus 40 small tumor antigen induces deregulation of the actin cytoskeleton and tight junctions in kidney epithelial cells. J Virol. 2003;77:2807–2818. doi: 10.1128/JVI.77.5.2807-18.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170:317–325. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. Kaposi's sarcoma-associated herpes-virus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol-3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol. 2004;78:4207–4223. doi: 10.1128/JVI.78.8.4207-23.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zamudio-Meza H, Castillo-Alvarez A, Gonzalez-Bonilla C, Meza I. Cross-talk between Rac1 and Cdc42 GTPases regulates formation of filopodia required for dengue virus type-2 entry into HMEC-1 cells. J Gen Virol. 2009;90:2902–2911. doi: 10.1099/vir.0.014159-0. [DOI] [PubMed] [Google Scholar]
- 65.Kress H, Stelzer EH, Holzer D, Buss F, Griffiths G, Rohrbach A. Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc Natl Acad Sci USA. 2007;104:11633–11638. doi: 10.1073/pnas.0702449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 67.Pearce-Pratt R, Malamud D, Phillips DM. Role of the cytoskeleton in cell-to-cell transmission of human immunodeficiency virus. J Virol. 1994;68:2898–2905. doi: 10.1128/jvi.68.5.2898-2905.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 69.Gursoy UK, Kononen E, Uitto VJ. Prevotella intermedia ATCC 25,611 targets host cell lamellipodia in epithelial cell adhesion and invasion. Oral Microbiol Immunol. 2009;24:304–309. doi: 10.1111/j.1399-302X.2009.00510.x. [DOI] [PubMed] [Google Scholar]
- 70.Aiastui A, Pucciarelli MG, Garcia-del Portillo F. Salmonella enterica serovar typhimurium invades fibroblasts by multiple routes differing from the entry into epithelial cells. Infect Immun. 2010;78:2700–2713. doi: 10.1128/IAI.01389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bulgin R, Raymond B, Garnett JA, Frankel G, Crepin VF, Berger CN, et al. Bacterial guanine nucleotide exchange factors SopE-like and WxxxE effectors. Infect Immun. 2010;78:1417–1425. doi: 10.1128/IAI.01250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Higashide W, Dai S, Hombs VP, Zhou D. Involvement of SipA in modulating actin dynamics during Salmonella invasion into cultured epithelial cells. Cell Microbiol. 2002;4:357–365. doi: 10.1046/j.1462-5822.2002.00196.x. [DOI] [PubMed] [Google Scholar]
- 73.Simpson N, Shaw R, Crepin VF, Mundy R, FitzGerald AJ, Cummings N, et al. The enteropathogenic Escherichia coli type III secretion system effector Map binds EBP50/NHERF1: implication for cell signalling and diarrhoea. Mol Microbiol. 2006;60:349–363. doi: 10.1111/j.1365-2958.2006.05109.x. [DOI] [PubMed] [Google Scholar]
- 74.Bulgin RR, Arbeloa A, Chung JC, Frankel G. EspT triggers formation of lamellipodia and membrane ruffles through activation of Rac-1 and Cdc42. Cell Microbiol. 2009;11:217–229. doi: 10.1111/j.1462-5822.2008.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lidke DS, Lidke KA, Rieger B, Jovin TM, Arndt-Jovin DJ. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. J Cell Biol. 2005;170:619–626. doi: 10.1083/jcb.200503140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bartles JR. Parallel actin bundles and their multiple actin-bundling proteins. Curr Opin Cell Biol. 2000;12:72–78. doi: 10.1016/S0955-0674(99)00059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borisy GG, Svitkina TM. Actin machinery: pushing the envelope. Curr Opin Cell Biol. 2000;12:104–112. doi: 10.1016/S0955-0674(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 78.Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell. 2005;16:2443–2457. doi: 10.1091/mbc.E04-12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol. 2004;164:887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stidwill RP, Wysolmerski T, Burgess DR. The brush border cytoskeleton is not static: in vivo turnover of proteins. J Cell Biol. 1984;98:641–645. doi: 10.1083/jcb.98.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mooseker MS, Tilney LG. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol. 1975;67:725–743. doi: 10.1083/jcb.67.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, et al. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160:409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steketee MB, Tosney KW. Three functionally distinct adhesions in filopodia: shaft adhesions control lamellar extension. J Neurosci. 2002;22:8071–8083. doi: 10.1523/JNEUROSCI.22-18-08071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scott G, Leopardi S, Printup S, Madden BC. Filopodia are conduits for melanosome transfer to keratinocytes. J Cell Sci. 2002;115:1441–1451. doi: 10.1242/jcs.115.7.1441. [DOI] [PubMed] [Google Scholar]
- 85.Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DeRosier DJ, Tilney LG. F-actin bundles are derivatives of microvilli: What does this tell us about how bundles might form? J Cell Biol. 2000;148:1–6. [PMC free article] [PubMed] [Google Scholar]
- 87.Revenu C, Athman R, Robine S, Louvard D. The co-workers of actin filaments: from cell structures to signals. Nat Rev Mol Cell Biol. 2004;5:635–646. doi: 10.1038/nrm1437. [DOI] [PubMed] [Google Scholar]
- 88.Chou SW, Hwang P, Gomez G, Fernando CA, West MC, Pollock LM, et al. Fascin 2b is a component of stereocilia that lengthens actin-based protrusions. PLoS ONE. 2011;6:14807. doi: 10.1371/journal.pone.0014807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franck Z, Footer M, Bretscher A. Microinjection of villin into cultured cells induces rapid and long-lasting changes in cell morphology but does not inhibit cytokinesis, cell motility or membrane ruffling. J Cell Biol. 1990;111:2475–2485. doi: 10.1083/jcb.111.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.George SP, Chen H, Khurana S. Membrane-induced actin bundling is essential for directional cell migration. J Biol Chem. 2011 doi: 10.1242/jcs.116244. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.George SP, Wang Y, Mathew S, Srinivasan K, Khurana S. Dimerization and actin-bundling properties of villin and its role in the assembly of epithelial cell brush borders. J Biol Chem. 2007;282:26528–26541. doi: 10.1074/jbc.M703617200. [DOI] [PubMed] [Google Scholar]
- 92.Costa de Beauregard MA, Pringault E, Robine S, Louvard D. Suppression of villin expression by antisense RNA impairs brush border assembly in polarized epithelial intestinal cells. EMBO J. 1995;14:409–421. doi: 10.1002/j.1460-2075.1995.tb07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ezzell RM, Chafel MM, Matsudaira PT. Differential localization of villin and fimbrin during development of the mouse visceral endoderm and intestinal epithelium. Development. 1989;106:407–419. doi: 10.1242/dev.106.2.407. [DOI] [PubMed] [Google Scholar]
- 94.Ezzell RM, Leung J, Collins K, Chafel MM, Cardozo TJ, Matsudaira PT. Expression and localization of villin, fimbrin and myosin I in differentiating mouse F9 teratocarcinoma cells. Dev Biol. 1992;151:575–585. doi: 10.1016/0012-1606(92)90195-M. [DOI] [PubMed] [Google Scholar]
- 95.Otto JJ, Kane RE, Bryan J. Redistribution of actin and fascin in sea urchin eggs after fertilization. Cell Motil. 1980;1:31–40. doi: 10.1002/cm.970010104. [DOI] [PubMed] [Google Scholar]
- 96.Otto JJ, Kane RE, Bryan J. Formation of filopodia in coelomocytes: localization of fascin, a 58,000 dalton actin cross-linking protein. Cell. 1979;17:285–293. doi: 10.1016/0092-8674(79)90154-5. [DOI] [PubMed] [Google Scholar]
- 97.Friederich E, Huet C, Arpin M, Louvard D. Villin induces microvilli growth and actin redistribution in transfected fibroblasts. Cell. 1989;59:461–475. doi: 10.1016/0092-8674(89)90030-5. [DOI] [PubMed] [Google Scholar]
- 98.Tomar A, George S, Kansal P, Wang Y, Khurana S. Interaction of phospholipase C-gamma1 with villin regulates epithelial cell migration. J Biol Chem. 2006;281:31972–31986. doi: 10.1074/jbc.M604323200. [DOI] [PubMed] [Google Scholar]
- 99.Khurana S, Tomar A, George SP, Wang Y, Siddiqui MR, Guo H, et al. Autotaxin and lysophosphatidic acid stimulate intestinal cell motility by redistribution of the actin modifying protein villin to the developing lamellipodia. Exp Cell Res. 2008;314:530–542. doi: 10.1016/j.yexcr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Loomis PA, Zheng L, Sekerkova G, Changyaleket B, Mugnaini E, Bartles JR. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J Cell Biol. 2003;163:1045–1055. doi: 10.1083/jcb.200309093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li H, Liu H, Balt S, Mann S, Corrales CE, Heller S. Correlation of expression of the actin filament-bundling protein espin with stereociliary bundle formation in the developing inner ear. J Comp Neurol. 2004;468:125–134. doi: 10.1002/cne.10944. [DOI] [PubMed] [Google Scholar]
- 102.Cohan CS, Welnhofer EA, Zhao L, Matsumura F, Yamashiro S. Role of the actin bundling protein fascin in growth cone morphogenesis: localization in filopodia and lamellipodia. Cell Motil Cytoskeleton. 2001;48:109–120. doi: 10.1002/1097-0169(200102)48:2<109::AIDCM1002>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 103.Tao YS, Edwards RA, Tubb B, Wang S, Bryan J, McCrea PD. beta-Catenin associates with the actin-bundling protein fascin in a noncadherin complex. J Cell Biol. 1996;134:1271–1281. doi: 10.1083/jcb.134.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wong V, Ching D, McCrea PD, Firestone GL. Glucocorticoid downregulation of fascin protein expression is required for the steroid-induced formation of tight junctions and cell-cell interactions in rat mammary epithelial tumor cells. J Biol Chem. 1999;274:5443–5453. doi: 10.1074/jbc.274.9.5443. [DOI] [PubMed] [Google Scholar]
- 105.Tyska MJ, Mooseker MS. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys J. 2002;82:1869–1883. doi: 10.1016/S0006-3495(02)75537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferrary E, Cohen-Tannoudji M, Pehau-Arnaudet G, Lapillonne A, Athman R, Ruiz T, et al. In vivo, villin is required for Ca(2+)-dependent F-actin disruption in intestinal brush borders. J Cell Biol. 1999;146:819–830. doi: 10.1083/jcb.146.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Misch DW, Giebel PE, Faust RG. Intestinal microvilli: responses to feeding and fasting. Eur J Cell Biol. 1980;21:269–279. [PubMed] [Google Scholar]
- 108.Lecount TS, Grey RD. Transient shortening of microvilli induced by cycloheximide in the duodenal epithelium of the chicken. J Cell Biol. 1972;53:601–605. doi: 10.1083/jcb.53.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weinman MD, Allan CH, Trier JS, Hagen SJ. Repair of microvilli in the rat small intestine after damage with lectins contained in the red kidney bean. Gastroenterology. 1989;97:1193–1204. doi: 10.1016/0016-5085(89)91690-9. [DOI] [PubMed] [Google Scholar]
- 110.Mooseker MS, Bonder EM, Grimwade BG, Howe CL, Keller TC, 3rd, Wasserman RH, et al. Regulation of contractility, cytoskeletal structure and filament assembly in the brush border of intestinal epithelial cells. Cold Spring Harb Symp Quant Biol. 1982;46:855–870. doi: 10.1101/sqb.1982.046.01.080. [DOI] [PubMed] [Google Scholar]
- 111.Halbleib JM, Saaf AM, Brown PO, Nelson WJ. Transcriptional modulation of genes encoding structural characteristics of differentiating enterocytes during development of a polarized epithelium in vitro. Mol Biol Cell. 2007;18:4261–4278. doi: 10.1091/mbc.E07-04-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Steffen A, Faix J, Resch GP, Linkner J, Wehland J, Small JV, et al. Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. Mol Biol Cell. 2006;17:2581–2591. doi: 10.1091/mbc.E05-11-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tilney LG, Connelly PS, Vranich KA, Shaw MK, Guild GM. Why are two different cross-linkers necessary for actin bundle formation in vivo and what does each cross-link contribute? J Cell Biol. 1998;143:121–133. doi: 10.1083/jcb.143.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bartles JR, Zheng L, Li A, Wierda A, Chen B. Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J Cell Biol. 1998;143:107–119. doi: 10.1083/jcb.143.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cant K, Knowles BA, Mooseker MS, Cooley L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J Cell Biol. 1994;125:369–380. doi: 10.1083/jcb.125.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cant K, Cooley L. Single amino acid mutations in Drosophila fascin disrupt actin bundling function in vivo. Genetics. 1996;143:249–258. doi: 10.1093/genetics/143.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mahajan-Miklos S, Cooley L. The villin-like protein encoded by the Drosophila quail gene is required for actin bundle assembly during oogenesis. Cell. 1994;78:291–301. doi: 10.1016/0092-8674(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 119.Cant K, Knowles BA, Mahajan-Miklos S, Heintzelman M, Cooley L. Drosophila fascin mutants are rescued by overexpression of the villin-like protein, quail. J Cell Sci. 1998;111:213–221. doi: 10.1242/jcs.111.2.213. [DOI] [PubMed] [Google Scholar]
- 120.Wulfkuhle JD, Petersen NS, Otto JJ. Changes in the F-actin cytoskeleton during neurosensory bristle development in Drosophila: the role of singed and forked proteins. Cell Motil Cytoskeleton. 1998;40:119–132. doi: 10.1002/(SICI)1097-0169(1998)40:2<119::AIDCM2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 121.Roulier EM, Fyrberg C, Fyrberg E. Perturbations of Drosophila alpha-actinin cause muscle paralysis, weakness and atrophy but do not confer obvious nonmuscle phenotypes. J Cell Biol. 1992;116:911–922. doi: 10.1083/jcb.116.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robinson DN, Smith-Leiker TA, Sokol NS, Hudson AM, Cooley L. Formation of the Drosophila ovarian ring canal inner rim depends on cheerio. Genetics. 1997;145:1063–1072. doi: 10.1093/genetics/145.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li MG, Serr M, Edwards K, Ludmann S, Yamamoto D, Tilney LG, et al. Filamin is required for ring canal assembly and actin organization during Drosophila oogenesis. J Cell Biol. 1999;146:1061–1074. doi: 10.1083/jcb.146.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Noguchi T, Lenartowska M, Rogat AD, Frank DJ, Miller KG. Proper cellular reorganization during Drosophila spermatid individualization depends on actin structures composed of two domains, bundles and meshwork, that are differentially regulated and have different functions. Mol Biol Cell. 2008;19:2363–2372. doi: 10.1091/mbc.E07-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sobue K, Kanda K. Alpha-actinins, calspectin (brain spectrin or fodrin), and actin participate in adhesion and movement of growth cones. Neuron. 1989;3:311–319. doi: 10.1016/0896-6273(89)90255-9. [DOI] [PubMed] [Google Scholar]
- 126.Bretscher A, Weber K. Fimbrin, a new microfilamentassociated protein present in microvilli and other cell surface structures. J Cell Biol. 1980;86:335–340. doi: 10.1083/jcb.86.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555–562. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ohta Y, Suzuki N, Nakamura S, Hartwig JH, Stossel TP. The small GTPase RalA targets filamin to induce filopodia. Proc Natl Acad Sci USA. 1999;96:2122–2128. doi: 10.1073/pnas.96.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xue F, Janzen DM, Knecht DA. Contribution of filopodia to cell migration: a mechanical link between protrusion and contraction. Int J Cell Biol. 2010;2010:507821. doi: 10.1155/2010/507821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamakita Y, Matsumura F, Yamashiro S. Fascin1 is dispensable for mouse development but is favorable for neonatal survival. Cell Motil Cytoskeleton. 2009;66:524–534. doi: 10.1002/cm.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tilney LG, DeRosier DJ. Actin filaments, stereocilia and hair cells of the bird cochlea. IV. How the actin filaments become organized in developing stereocilia and in the cuticular plate. Dev Biol. 1986;116:119–129. doi: 10.1016/0012-1606(86)90048-5. [DOI] [PubMed] [Google Scholar]
- 132.Shibayama T, Carboni JM, Mooseker MS. Assembly of the intestinal brush border: appearance and redistribution of microvillar core proteins in developing chick enterocytes. J Cell Biol. 1987;105:335–344. doi: 10.1083/jcb.105.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chambers C, Grey RD. Development of the structural components of the brush border in absorptive cells of the chick intestine. Cell Tissue Res. 1979;204:387–405. doi: 10.1007/BF00233651. [DOI] [PubMed] [Google Scholar]
- 134.Tilney LG, Connelly P, Smith S, Guild GM. F-actin bundles in Drosophila bristles are assembled from modules composed of short filaments. J Cell Biol. 1996;135:1291–1308. doi: 10.1083/jcb.135.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nakagawa H, Terasaki AG, Suzuki H, Ohashi K, Miyamoto S. Short-term retention of actin filament binding proteins on lamellipodial actin bundles. FEBS Lett. 2006;580:3223–3228. doi: 10.1016/j.febslet.2006.04.082. [DOI] [PubMed] [Google Scholar]
- 136.Athman R, Louvard D, Robine S. Villin enhances hepatocyte growth factor-induced actin cytoskeleton remodeling in epithelial cells. Mol Biol Cell. 2003;14:4641–4653. doi: 10.1091/mbc.E03-02-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khurana S. Aspects of the Cytoskeleton. Vol. 5. Elsevier; 2006. Structure and function of villin. Advances in Molecular and Cell Biology, 37; pp. 89–117. Series Editor: Bittar EE. Volume Editor: Khurana S, [Google Scholar]
- 138.Khurana S, George SP. Regulation of cell structure and function by actin-binding proteins: Villin's perspective. FEBS Lett. 2008;582:2128–2139. doi: 10.1016/j.febslet.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Revenu C, Courtois M, Michelot A, Sykes C, Louvard D, Robine S. Villin severing activity enhances actin-based motility in vivo. Mol Biol Cell. 2007;18:827–838. doi: 10.1091/mbc.E06-05-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Athman R, Fernandez MI, Gounon P, Sansonetti P, Louvard D, Philpott D, et al. Shigella flexneri infection is dependent on villin in the mouse intestine and in primary cultures of intestinal epithelial cells. Cell Microbiol. 2005;7:1109–1116. doi: 10.1111/j.1462-5822.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 141.Tomar A, Wang Y, Kumar N, George S, Ceacareanu B, Hassid A, et al. Regulation of cell motility by tyrosine phosphorylated villin. Mol Biol Cell. 2004;15:4807–4817. doi: 10.1091/mbc.E04-05-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y, Tomar A, George SP, Khurana S. Obligatory role for phospholipase C-gamma(1) in villin-induced epithelial cell migration. Am J Physiol Cell Physiol. 2007;292:1775–1786. doi: 10.1152/ajpcell.00420.2006. [DOI] [PubMed] [Google Scholar]
- 143.Kumar N, Zhao P, Tomar A, Galea CA, Khurana S. Association of villin with phosphatidylinositol-4,5-bisphosphate regulates the actin cytoskeleton. J Biol Chem. 2004;279:3096–3110. doi: 10.1074/jbc.M308878200. [DOI] [PubMed] [Google Scholar]
- 144.Panebra A, Ma SX, Zhai LW, Wang XT, Rhee SG, Khurana S. Regulation of phospholipase C-gamma(1) by the actin-regulatory protein villin. Am J Physiol Cell Physiol. 2001;281:1046–1058. doi: 10.1152/ajpcell.2001.281.3.C1046. [DOI] [PubMed] [Google Scholar]
- 145.Duh FM, Latif F, Weng Y, Geil L, Modi W, Stackhouse T, et al. cDNA cloning and expression of the human homolog of the sea urchin fascin and Drosophila singed genes which encodes an actin-bundling protein. DNA Cell Biol. 1994;13:821–827. doi: 10.1089/dna.1994.13.821. [DOI] [PubMed] [Google Scholar]
- 146.Mosialos G, Yamashiro S, Baughman RW, Matsudaira P, Vara L, Matsumura F, et al. Epstein-Barr virus infection induces expression in B lymphocytes of a novel gene encoding an evolutionarily conserved 55-kilodalton actin-bundling protein. J Virol. 1994;68:7320–7328. doi: 10.1128/jvi.68.11.7320-7328.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pinkus GS, Pinkus JL, Langhoff E, Matsumura F, Yamashiro S, Mosialos G, et al. Fascin, a sensitive new marker for Reed-Sternberg cells of hodgkin's disease. Evidence for a dendritic or B cell derivation? Am J Pathol. 1997;150:543–562. [PMC free article] [PubMed] [Google Scholar]
- 148.Adams JC. Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol. 2004;16:590–596. doi: 10.1016/j.ceb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 149.Zhang FR, Tao LH, Shen ZY, Lv Z, Xu LY, Li EM. Fascin expression in human embryonic, fetal and normal adult tissue. J Histochem Cytochem. 2008;56:193–199. doi: 10.1369/jhc.7A7353.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.De Arcangelis A, Georges-Labouesse E, Adams JC. Expression of fascin-1, the gene encoding the actin-bundling protein fascin-1, during mouse embryogenesis. Gene Expr Patterns. 2004;4:637–643. doi: 10.1016/j.modgep.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 151.Grothey A, Hashizume R, Ji H, Tubb BE, Patrick CW Jr, Yu D, et al. C-erbB-2/HER-2 upregulates fascin, an actin-bundling protein associated with cell motility, in human breast cancer cell lines. Oncogene. 2000;19:4864–4875. doi: 10.1038/sj.onc.1203838. [DOI] [PubMed] [Google Scholar]
- 152.Jawhari AU, Buda A, Jenkins M, Shehzad K, Sarraf C, Noda M, et al. Fascin, an actin-bundling protein, modulates colonic epithelial cell invasiveness and differentiation in vitro. Am J Pathol. 2003;162:69–80. doi: 10.1016/S0002-9440(10)63799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hashimoto Y, Ito T, Inoue H, Okumura T, Tanaka E, Tsunoda S, et al. Prognostic significance of fascin overexpression in human esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:2597–2605. doi: 10.1158/1078-0432.CCR-04-1378. [DOI] [PubMed] [Google Scholar]
- 154.Cao D, Ji H, Ronnett BM. Expression of mesothelin, fascin and prostate stem cell antigen in primary ovarian mucinous tumors and their utility in differentiating primary ovarian mucinous tumors from metastatic pancreatic mucinous carcinomas in the ovary. Int J Gynecol Pathol. 2005;24:67–72. [PubMed] [Google Scholar]
- 155.Guild GM, Connelly PS, Ruggiero L, Vranich KA, Tilney LG. Actin filament bundles in Drosophila wing hairs: hairs and bristles use different strategies for assembly. Mol Biol Cell. 2005;16:3620–3631. doi: 10.1091/mbc.E05-03-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tseng Y, Fedorov E, McCaffery JM, Almo SC, Wirtz D. Micromechanics and ultrastructure of actin filament networks crosslinked by human fascin: a comparison with alpha-actinin. J Mol Biol. 2001;310:351–366. doi: 10.1006/jmbi.2001.4716. [DOI] [PubMed] [Google Scholar]
- 157.Honda M, Takiguchi K, Ishikawa S, Hotani H. Morphogenesis of liposomes encapsulating actin depends on the type of actin-crosslinking. J Mol Biol. 1999;287:293–300. doi: 10.1006/jmbi.1999.2592. [DOI] [PubMed] [Google Scholar]
- 158.Brown JW, McKnight CJ. Molecular model of the microvillar cytoskeleton and organization of the brush border. PLoS ONE. 2010;5:9406. doi: 10.1371/journal.pone.0009406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Matsudaira P, Mandelkow E, Renner W, Hesterberg LK, Weber K. Role of fimbrin and villin in determining the interfilament distances of actin bundles. Nature. 1983;301:209–214. doi: 10.1038/301209a0. [DOI] [PubMed] [Google Scholar]
- 160.Matsudaira PT. Structural and functional relationship between the membrane and the cytoskeleton in brush border microvilli. Ciba Found Symp. 1983;95:233–252. doi: 10.1002/9780470720769.ch14. [DOI] [PubMed] [Google Scholar]
- 161.Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Lae M, et al. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67:6844–6853. doi: 10.1158/0008-5472.CAN-07-0929. [DOI] [PubMed] [Google Scholar]
- 162.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 163.Yamashiro S, Yamakita Y, Ono S, Matsumura F. Fascin, an actin-bundling protein, induces membrane protrusions and increases cell motility of epithelial cells. Mol Biol Cell. 1998;9:993–1006. doi: 10.1091/mbc.9.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tao YS, Edwards R, Bryan J, McCrea PD. The actin-bundling protein fascin associates with beta-catenin. [Abstract] Mol Biol Cell. 1995;6:300. doi: 10.1083/jcb.134.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE. 2007;2007:5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- 166.Peng J, Wallar BJ, Flanders A, Swiatek PJ, Alberts AS. Disruption of the Diaphanous-related formin Drf1 gene encoding mDia1 reveals a role for Drf3 as an effector for Cdc42. Curr Biol. 2003;13:534–545. doi: 10.1016/S0960-9822(03)00170-2. [DOI] [PubMed] [Google Scholar]
- 167.Pellegrin S, Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol. 2005;15:129–133. doi: 10.1016/j.cub.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 168.Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol. 2005;7:619–625. doi: 10.1038/ncb1266. [DOI] [PubMed] [Google Scholar]
- 169.Schirenbeck A, Arasada R, Bretschneider T, Stradal TE, Schleicher M, Faix J. The bundling activity of vasodilator-stimulated phosphoprotein is required for filopodium formation. Proc Natl Acad Sci USA. 2006;103:7694–7699. doi: 10.1073/pnas.0511243103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/S0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 171.Faix J, Rottner K. The making of filopodia. Curr Opin Cell Biol. 2006;18:18–25. doi: 10.1016/j.ceb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 172.Craig SW, Powell LD. Regulation of actin polymerization by villin, a 95,000 dalton cytoskeletal component of intestinal brush borders. Cell. 1980;22:739–746. doi: 10.1016/0092-8674(80)90550-4. [DOI] [PubMed] [Google Scholar]
- 173.Coluccio LM, Bretscher A. Reassociation of microvillar core proteins: making a microvillar core in vitro. J Cell Biol. 1989;108:495–502. doi: 10.1083/jcb.108.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Matsudaira P, Janmey P. Pieces in the actin-severing protein puzzle. Cell. 1988;54:139–140. doi: 10.1016/0092-8674(88)90542-9. [DOI] [PubMed] [Google Scholar]
- 175.Schatten G. Motility during fertilization. Int Rev Cytol. 1982;79:59–163. doi: 10.1016/S0074-7696(08)61673-3. [DOI] [PubMed] [Google Scholar]
- 176.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 177.Withee J, Galligan B, Hawkins N, Garriga G. Caenorhabditis elegans WASP and Ena/VASP proteins play compensatory roles in morphogenesis and neuronal cell migration. Genetics. 2004;167:1165–1176. doi: 10.1534/genetics.103.025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shakir MA, Gill JS, Lundquist EA. Interactions of UNC-34 Enabled with Rac GTPases and the NIK kinase MIG-15 in Caenorhabditis elegans axon pathfinding and neuronal migration. Genetics. 2006;172:893–913. doi: 10.1534/genetics.105.046359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell. 2004;118:363–373. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 180.Sigal YJ, Quintero OA, Cheney RE, Morris AJ. Cdc42 and ARP2/3-independent regulation of filopodia by an integral membrane lipid-phosphatase-related protein. J Cell Sci. 2007;120:340–352. doi: 10.1242/jcs.03335. [DOI] [PubMed] [Google Scholar]
- 181.Mattila PK, Pykalainen A, Saarikangas J, Paavilainen VO, Vihinen H, Jokitalo E, et al. Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J Cell Biol. 2007;176:953–964. doi: 10.1083/jcb.200609176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta. 2006;1761:897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 183.Saarikangas J, Zhao H, Pykalainen A, Laurinmaki P, Mattila PK, Kinnunen PK, et al. Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr Biol. 2009;19:95–107. doi: 10.1016/j.cub.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 184.Nakagawa H, Miki H, Nozumi M, Takenawa T, Miyamoto S, Wehland J, et al. IRSp53 is colocalised with WAVE2 at the tips of protruding lamellipodia and filopodia independently of Mena. J Cell Sci. 2003;116:2577–2583. doi: 10.1242/jcs.00462. [DOI] [PubMed] [Google Scholar]
- 185.Yamagishi A, Masuda M, Ohki T, Onishi H, Mochizuki N. A novel actin bundling/filopodium-forming domain conserved in insulin receptor tyrosine kinase substrate p53 and missing in metastasis protein. J Biol Chem. 2004;279:14929–14936. doi: 10.1074/jbc.M309408200. [DOI] [PubMed] [Google Scholar]
- 186.Krugmann S, Jordens I, Gevaert K, Driessens M, Vandekerckhove J, Hall A. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr Biol. 2001;11:1645–1655. doi: 10.1016/S0960-9822(01)00506-1. [DOI] [PubMed] [Google Scholar]
- 187.Bockmann J, Kreutz MR, Gundelfinger ED, Bockers TM. ProSAP/Shank postsynaptic density proteins interact with insulin receptor tyrosine kinase substrate IRSp53. J Neurochem. 2002;83:1013–1017. doi: 10.1046/j.1471-4159.2002.01204.x. [DOI] [PubMed] [Google Scholar]
- 188.Soltau M, Richter D, Kreienkamp HJ. The insulin receptor substrate IRSp53 links postsynaptic shank1 to the small G-protein cdc42. Mol Cell Neurosci. 2002;21:575–583. doi: 10.1006/mcne.2002.1201. [DOI] [PubMed] [Google Scholar]
- 189.Govind S, Kozma R, Monfries C, Lim L, Ahmed S. Cdc42Hs facilitates cytoskeletal reorganization and neurite outgrowth by localizing the 58-kD insulin receptor substrate to filamentous actin. J Cell Biol. 2001;152:579–594. doi: 10.1083/jcb.152.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Choi J, Ko J, Racz B, Burette A, Lee JR, Kim S, et al. Regulation of dendritic spine morphogenesis by insulin receptor substrate 53, a downstream effector of Rac1 and Cdc42 small GTPases. J Neurosci. 2005;25:869–879. doi: 10.1523/JNEUROSCI.3212-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Disanza A, Mantoani S, Hertzog M, Gerboth S, Frittoli E, Steffen A, et al. Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex. Nat Cell Biol. 2006;8:1337–1347. doi: 10.1038/ncb1502. [DOI] [PubMed] [Google Scholar]
- 192.Bettache N, Baisamy L, Baghdiguian S, Payrastre B, Mangeat P, Bienvenue A. Mechanical constraint imposed on plasma membrane through transverse phospholipid imbalance induces reversible actin polymerization via phosphoinositide-3-kinase activation. J Cell Sci. 2003;116:2277–2284. doi: 10.1242/jcs.00424. [DOI] [PubMed] [Google Scholar]
- 193.Mogilner A, Oster G. Polymer motors: pushing out the front and pulling up the back. Curr Biol. 2003;13:721–733. doi: 10.1016/j.cub.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 194.Laurent V, Loisel TP, Harbeck B, Wehman A, Grobe L, Jockusch BM, et al. Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes. J Cell Biol. 1999;144:1245–1258. doi: 10.1083/jcb.144.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dickinson RB, Caro L, Purich DL. Force generation by cytoskeletal filament end-tracking proteins. Biophys J. 2004;87:2838–2854. doi: 10.1529/biophysj.104.045211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sekerková G, Zheng L, Loomis PA, Changyaleket B, Whitlon DS, Mugnaini E, et al. Espins are multifunctional actin cytoskeletal regulatory proteins in the microvilli of chemosensory and mechanosensory cells. J Neurosci. 2004;24:5445–5456. doi: 10.1523/JNEUROSCI.1279-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Corgan AM, Singleton C, Santoso CB, Greenwood JA. Phosphoinositides differentially regulate alpha-actinin flexibility and function. Biochem J. 2004;378:1067–1072. doi: 10.1042/BJ20031124. [DOI] [PMC free article] [PubMed] [Google Scholar]