Abstract
Plasma protein S (PS) levels are reportedly low in patients with venous thrombosis but high in coronary heart disease (CHD) patients. The authors examined the association between free PS concentration and CHD or stroke risk and assessed risk in combination with C-reactive protein (CRP) levels. Free PS concentration was determined in 6 annual visits among 3,052 middle-aged (49–64 years) United Kingdom men from the Second Northwick Park Heart Study, with 297 CHD events from 1989 to 2005. The highest (vs. first) quintile was associated with a significantly increased CHD risk after adjustment for all other risk factors and correction for regression dilution bias (hazard ratio = 1.85, 95% confidence interval: 1.08, 3.16; P = 0.024). Models that included all well-known risk factors plus PS quintiles improved prediction of CHD (net reclassification improvement (NRI) = 7.0% (P = 0.007), category-less NRI (>0) = 22.1% (P < 0.001)), and the likelihood ratio statistic increased significantly (P = 0.018). The increase in CHD risk was particularly strong when subjects also had high CRP levels. There was no association between free PS level and stroke risk. This study confirms the independent association of elevated free PS levels with future risk of CHD, although elevated PS levels added only modestly to prediction metrics. The novel finding of increased CHD risk, particularly when CRP and PS levels are high, requires further study.
Keywords: coronary disease, inflammation, protein S, risk factors, stroke
Protein S is a 69-Mr vitamin K-dependent protein with anticoagulant properties which acts as a nonenzymatic cofactor to activated protein C in the proteolytic degradation of Factor Va and Factor VIIIa (1–3). Two forms of protein S are present in plasma, with approximately 60% being bound to complement factor 4b binding protein (C4bBP), while the remaining 40% is free (2, 4). Free protein S has activated protein C cofactor activity (2, 5). Deficiency (antigen) or impaired function (activity) of protein S leads to decreased degradation of Factor Va and Factor VIIIa and an increased propensity toward venous thrombosis (6–8).
Families with heterozygous protein S deficiency are susceptible to venous thromboembolism. Protein S deficiency is found in 1.5%–7% of selected groups of patients with thrombophilia, often appearing before the age of 30 years (2, 9–11), but because of the low prevalence of protein S deficiency, within a large population-based study, patients with deep vein thrombosis did not show significantly lower levels of protein S than age- and sex-matched healthy controls (12). Lower levels of free protein S in patients who have had an acute myocardial infarction have been reported in some studies (13–16), while other studies have found significantly higher total protein S concentrations in patients with a history of angina pectoris or myocardial infarction (17, 18). In addition, case studies have found reduced protein S levels in patients following ischemic stroke (19) or no significant difference in protein S concentration between stroke cases and controls (20).
Estimates of the heritability of protein S from family studies differ, ranging from 11% to 34% of the variance being attributable to a genetic component (21, 22). There are 2 protein S genes, protein S α (PROS1) and protein S pseudogene (β) (PROS2), located on chromosome 3 at p11.1–q11.2. PROS1 is the active gene responsible for the expression of protein S, whereas PROS2 is a pseudogene (2, 23). Loss-of-function mutation of PROS1 leads to a deficiency of protein S and is an established inherited cause of venous thrombotic disease (2, 23). However, variation in the gene encoding protein S explains only a small part of the total estimated genetic variance of circulating protein S levels (21, 22).
The basis and pathogenic significance of an increased protein S level in men at high risk of CHD and stroke remains unclear, and in addition, the association between protein S and CHD or stroke is understudied in prospective cohort studies. Rudnicka et al. (18) previously reported a crude association between high protein S levels and CHD risk over a 7-year follow-up period in the Second Northwick Park Heart Study (NPHS-II), a prospective cohort study based at that time on only 168 CHD cases. The association was lost, however, after adjustment for traditional CHD risk factors (18). Our primary purpose in this study was to reassess the relation between free protein S levels and prospective risk of CHD and stroke in NPHS-II after a longer duration of follow-up (14 years, with 297 CHD events and 98 stroke events). The increased number of persons with CHD allowed analysis across quintiles, enabling us to detect any potential association of low protein S levels with CHD, in addition to the association with high levels of protein S. Additionally, in the current study, we have assessed the combined associations of protein S levels and inflammatory factors with CHD.
MATERIALS AND METHODS
Study subjects and data collection
The prospective NPHS-II commenced in 1989 and includes 3,052 middle-aged (49–64 years) men recruited from 9 general medical practices in the United Kingdom. Participants were free of unstable angina, myocardial infarction, evidence of silent infarcts, coronary surgery, use of anticoagulant drugs (including aspirin), cerebrovascular disease, malignancy, and any condition or disease preventing the attainment of written, informed consent or long-term follow-up. Persons receiving treatment for hypertension or hyperlidemia were not excluded. Information on lifestyle, height, weight, blood pressure, and a number of blood biomarkers was recorded at baseline and at subsequent prospective follow-up visits. Recruitment, measurement, follow-up, and disease definitions are described in detail elsewhere (24–26). Information regarding use of medication for treatment of hypertension or hyperlidemia was recorded at the recruitment interview.
At recruitment, free protein S levels were determined using the Asserachrom Free Protein S immunoassay (Diagnostica Stago, Paris, France), which utilizes a monoclonal antibody sandwich technique (27). A single lot of assay kits was used to perform the analysis of all samples. The detection limit of the method is 2% of plasma free protein S. The within-day coefficient of variation of the assay was 2.1%, and the between-day coefficient of variation was 2.4% (18). Lipid, total cholesterol, and triglyceride concentrations were measured with automated enzyme procedures. Level of alcohol consumption for each subject was ascertained on the basis of self-reported average alcohol consumption (in the United Kingdom, 1 unit of alcohol is equivalent to half a pint of beer, 1 glass of wine, or 1 standard measure of spirits). CHD events taken as endpoints were fatal (sudden or not) myocardial infarction and nonfatal myocardial infarction, based on World Health Organization criteria (28), plus coronary artery interventions and silent myocardial infarction on the follow-up electrocardiogram or sudden unexplained death. Clinical information for each event was assembled by inquiries made through the participating medical practices, hospitals visited, and, for fatal events, coroners’ offices. This information was collated and submitted to an independent assessor who assigned qualifying events to the appropriate category. Stroke was categorized according to the definitions of the International Classification of Diseases, Ninth Revision: cerebral artery occlusion (code 434.9), unspecific cerebrovascular accident (code 436.0), intracerebral hemorrhage (code 431.0), subarachnoid hemorrhage (code 430.0), and cerebral embolism (code 434.1) (28).
Statistical analysis
Primary analysis was carried out using the first available measurement of free protein S; this measurement was taken at baseline for 86.1% of the men, at the first annual visit for 11.2%, and at the year 2 visit for 2.6% (all measurements of free protein S levels were made when the participants were free from CHD). Subjects were divided into quintiles according to free protein S levels. Associations between free protein S quintiles and cardiovascular risk factors were assessed using analysis of variance for continuous variables and chi-square tests for categorical variables. Free protein S was modeled by means of restricted cubic splines, with 5 knots at the 5th, 27th, 50th, 73rd, and 95th percentiles, in a Cox proportional hazards model for estimation of hazard ratios and 95% confidence intervals. Analysis of the association between CHD and stroke across free protein S quintiles used the first quintile as the reference category. Models were evaluated using Akaike's Information Criterion (AIC) and the likelihood ratio chi-square test. The contribution of free protein S to determination of CHD cases was assessed using Harrell's c statistic (29) as a discriminatory test which extends receiver operating characteristic curve analysis to the case of right-censored survival data. The difference in Harrell's c parameters and the confidence interval for the difference between models were calculated by bootstrap sampling. Detection rates (or sensitivities) for a 5% false-positive rate (DR5) were calculated by interpolation. The Hosmer-Lemeshow test was used to test differences between the observed and expected rates.
Measurement error or within-person variability (regression dilution bias) in free protein S concentration and the other risk factors can lead to misestimation of risk (30). Repeated measures of free protein S were used to correct for regression dilution bias. We estimated regression dilution factors by dividing the difference in mean free protein S levels between quintiles computed from the first measurement by the corresponding difference in the mean of all available annual measurements taken for each individual during the 5-year follow-up period (31). In addition, a sensitivity analysis was carried out, using the mean of all of the available annual measurements for each subject during the 6-year follow-up period. Eighty-one percent of the subjects had 3 or more measurements of free protein S levels. To examine the incremental ability of free protein S to classify subjects into risk categories according to commonly used categories of 10-year CHD risk, we calculated the reclassification percentages. We calculated estimated 10-year risks for each cell of the reclassification to show calibration of reclassified observations with observed risk. To evaluate true improvement in classification by the addition of free protein S to the well-known risk factors model, we calculated the net reclassification improvement (NRI) (32, 33). In addition, we calculated the category-less NRI (34), which is not affected by age ranges or the strength of the baseline model. We assessed the relations between other measured factors and free protein S levels using a best-subsets regression model to identify the component of free protein S that maximized the fit of the model.
RESULTS
A total of 3,052 men were included in the analysis. The mean age was 56.1 years (standard deviation (SD), 3.48), and the median follow-up time was 13.7 years. Participants were divided into quintiles according to free protein S levels, and group characteristics across the quintiles are presented in Table 1. A higher free protein S level was associated with higher body mass index (weight (kg)/height (m)2), systolic blood pressure, diastolic blood pressure, alcohol consumption, total cholesterol, triglycerides, high density lipoprotein (HDL) cholesterol, low density lipoprotein cholesterol, Factor VII antigen, Factor VIIc, activated Factor XII, fibrinopeptide A, and C-reactive protein (CRP); age, smoking status, and levels of Factor VII-activated fibrinogen, Factor X activation peptide, Factor IX activation peptide, and prothrombin Factor 1 + 2 were similar across free protein S quintiles.
Table 1.
Clinical and Laboratory Characteristics of Participants According to Quintile of Free Protein S Level, Second Northwick Park Heart Study, United Kingdom, 1989–2005a
| Clinical Characteristic or Laboratory Measurement | Quintile of Free Protein S, % standard |
P Value | |||||||||
| 1 (≤95) (n = 606; Referent) |
2 (96–106) (n = 590) |
3 (107–117) (n = 634) |
4 (118–130) (n = 569) |
5 (131–248) (n = 562) | |||||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | ||
| Clinical and Behavioral Characteristics | |||||||||||
| Age, years | 56.1 (3.40) | 56.2 (3.47) | 56.1 (3.50) | 56.0 (3.56) | 56.0 (3.55) | 0.367 | |||||
| Body mass indexb | 25.8 (3.44) | 26.1 (3.68) | 26.5 (3.46)** | 26.7 (3.43)** | 27.1 (3.49)** | <0.001 | |||||
| Systolic blood pressure, mm Hg | 134 (19) | 136 (19) | 139 (20)** | 139 (20)** | 140 (19)** | <0.001 | |||||
| Diastolic blood pressure, mm Hg | 82 (11) | 83 (11)* | 85 (11)** | 85 (12)** | 85 (11)** | <0.001 | |||||
| Diabetes | 2.2 | 2.2 | 3.0 | 3.2 | 2.0 | 0.579 | |||||
| Current smoker | 31.5 | 25.8 | 27.6 | 29.5 | 28.3 | 0.625 | |||||
| Alcohol consumption, units/weekc | 9.8 (13.7) | 9.8 (13.2) | 10.7 (14.4) | 13.0 (16.8)** | 14.7 (18.8)** | <0.001 | |||||
| Use of lipid-lowering medications | 2.0 | 2.4 | 1.3 | 1.1 | 2.9 | 0.135 | |||||
| Use of blood-pressure-lowering medications | 7.4 | 7.3 | 10.6 | 8.1 | 10.5 | 0.093 | |||||
| Lipid Levels | |||||||||||
| Total cholesterol, mmol/L | 5.42 (0.94) | 5.59 (0.96)** | 5.69 (0.98)** | 5.74 (1.01)** | 6.09 (1.05)** | <0.001 | |||||
| Triglycerides, mmol/L | 1.72 (0.97) | 1.89 (1.09)** | 2.08 (1.29)** | 2.14 (1.24)** | 2.44 (1.55)** | <0.001 | |||||
| High density lipoprotein cholesterol, mmol/L | 1.76 (0.61) | 1.75 (0.57) | 1.69 (0.57) | 1.71 (0.56) | 1.68 (0.61)* | 0.020 | |||||
| Low density lipoprotein cholesterol, mmol/L | 2.93 (0.97) | 2.99 (0.96) | 3.09 (0.99)** | 3.10 (1.04)** | 3.30 (1.03)** | <0.001 | |||||
| Hemostatic and Inflammation Factors | |||||||||||
| Fibrinogen, g/L | 2.79 (0.56) | 2.75 (0.56) | 2.75 (0.51) | 2.82 (0.61) | 2.77 (0.51) | 0.634 | |||||
| Activated Factor VII, ng/mL | 2.20 (1.03) | 2.33 (1.35) | 2.34 (1.25) | 2.47 (1.71) | 2.24 (1.66) | 0.890 | |||||
| Factor VII antigen, % standard | 129 (33) | 133 (35) | 132 (33) | 133 (44) | 137 (40)** | 0.005 | |||||
| Factor VIIc, % standard | 106 (27) | 109 (27) | 112 (30)** | 112 (31)** | 112 (30)** | <0.001 | |||||
| Activated Factor XII, ng/mL | 1.79 (0.90) | 1.93 (0.96)* | 1.90 (1.06) | 2.06 (1.15)** | 2.18 (1.11)** | <0.001 | |||||
| Factor X activation peptide, pmol/L | 84 (30) | 86 (29) | 87 (31) | 87 (38) | 86 (38) | 0.690 | |||||
| Factor IX activation peptide, pmol/L | 216 (83) | 220 (109) | 205 (90) | 217 (89) | 218 (81) | 0.845 | |||||
| Prothrombin Factor 1 + 2, μmol/L | 0.76 (0.39) | 0.79 (0.36) | 0.80 (0.46) | 0.82 (0.80) | 0.80 (0.83) | 0.337 | |||||
| Fibrinopeptide A, μmol/L | 1.93 (6.68) | 2.24 (9.33) | 2.00 (5.22) | 2.67 (8.28)* | 3.08 (15.50)* | 0.004 | |||||
| C-reactive protein, mg/L | 5.35 (7.17) | 5.29 (6.82) | 5.96 (8.78)* | 5.76 (7.01)* | 6.47 (7.81)** | <0.001 | |||||
Abbreviation: SD, standard deviation.
* P < 0.05; **P < 0.01 (vs. first quintile).
Data on some characteristics were missing for 91 subjects.
Weight (kg)/height (m)2.
In the United Kingdom, 1 unit of alcohol is equivalent to half a pint of beer, 1 glass of wine, or 1 standard measure of spirits.
The strongest associations were with total cholesterol (r = 0.21, P < 0.001) and triglyceride (r = 0.18, P < 0.001) concentrations (see Web Table 1, which appears on the Journal’s Web site (http://aje.oxfordjournals.org/)). The mean level of free protein S in smokers compared with nonsmokers was not significantly different (113 percent standard (SD, 23) vs. 112 percent standard (SD, 24); P = 0.316).
Association of free protein S levels with CHD and stroke
During the follow-up period (1989–2005; 36,564 person-years in total), 297 subjects (9.7%) experienced a CHD event and 98 subjects (3.2%) experienced a stroke event. The men who went on to develop CHD during follow-up had approximately 4.5% higher plasma free protein S levels than those who remained CHD-free (113 percent standard (SD, 23) vs. 118 percent standard (SD, 25); P < 0.001). There was no difference in free protein S levels between men who developed stroke during follow-up and those who remained stroke-free (114 percent standard (SD, 22) vs. 113 percent standard (SD, 24); P = 0.573).
The association between free protein S and CHD was modeled using restricted cubic splines with 5 knots (79, 100, 111, 123, and 153) at the 5th, 27th, 50th, 73rd, and 95th percentiles in a Cox proportional hazards model (Table 2). The Wald test of the model was not significant (P = 0.366), indicating that a linear relation was the best fit for the data. Compared with the first knot (median, 79), the second (hazard ratio (HR) = 1.71, 95% confidence interval (CI): 1.12, 2.62; P = 0.013), third (HR = 1.89, 95% CI: 1.21, 2.94; P = 0.005), fourth (HR = 2.14, 95% CI: 1.41, 3.24; P < 0.001), and fifth (HR = 2.54, 95% CI: 1.63, 3.96; P < 0.001) knots were associated with significantly increased risks of CHD. Multivariate adjustment for age, clinic, body mass index, diabetes, smoking, HDL cholesterol, total cholesterol, systolic blood pressure, and use of lipid- or blood-pressure-lowering medications did not materially change these estimates (HR = 1.65 (95% CI: 1.04, 2.64; P = 0.035) for the second knot, HR = 1.50 (95% CI: 0.91, 2.46; P = 0.110) for the third knot, HR = 1.58 (95% CI: 0.99, 2.52; P = 0.055) for the fourth knot, and HR = 1.84 (95% CI: 1.11, 3.06; P = 0.018) for the fifth knot).
Table 2.
Hazard Ratios for Coronary Heart Disease and Stroke According to Quintile of Free Protein S Level (Modeled Using Restricted Cubic Splines), Second Northwick Park Heart Study, United Kingdom, 1989–2005a
| Outcome and Quintile of Free Protein S, % standard | Median Value | Casesb |
Unadjusted Model |
Adjusted Modelc |
|||||
| No. | % | HR | 95% CI | P Value | HR | 95% CI | P Value | ||
| Coronary heart disease | |||||||||
| ≤95 | 79 | 36 | 12.1 | 1 | Reference | 1 | Reference | ||
| 96–106 | 100 | 59 | 19.9 | 1.71 | 1.12, 2.62 | 0.013 | 1.65 | 1.04, 2.64 | 0.035 |
| 107–117 | 111 | 62 | 20.9 | 1.89 | 1.21, 2.94 | 0.005 | 1.50 | 0.91, 2.46 | 0.110 |
| 118–130 | 123 | 69 | 23.2 | 2.14 | 1.41, 3.24 | <0.001 | 1.58 | 0.99, 2.52 | 0.055 |
| 131–248 | 153 | 71 | 23.9 | 2.54 | 1.63, 3.96 | <0.001 | 1.84 | 1.11, 3.06 | 0.018 |
| P for trend | <0.001 | ||||||||
| Stroke | |||||||||
| ≤95 | 79 | 16 | 16.7 | 1 | Reference | 1 | Reference | ||
| 96–106 | 100 | 22 | 22.9 | 1.99 | 0.88, 4.50 | 0.098 | 1.49 | 0.63, 3.52 | 0.364 |
| 107–117 | 111 | 23 | 24.0 | 2.08 | 0.93, 4.62 | 0.073 | 1.53 | 0.64, 3.64 | 0.338 |
| 118–130 | 123 | 19 | 19.8 | 1.58 | 0.73, 3.44 | 0.247 | 1.11 | 0.47, 2.60 | 0.811 |
| 131–248 | 153 | 16 | 16.7 | 1.55 | 0.66, 3.62 | 0.313 | 0.91 | 0.35, 2.34 | 0.846 |
| P for trend | 0.804 | ||||||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Free protein S was modeled by means of restricted cubic splines with 5 knots (79, 100, 111, 123, and 153) at the 5th, 27th, 50th, 73rd, and 95th percentiles in a Cox proportional hazards model. The value of 79% standard was used as the referent for estimation of all hazard ratios.
Data were missing for 2 stroke cases.
Results were adjusted for age, clinic, body mass index, diabetes, smoking, high density lipoprotein cholesterol, total cholesterol, systolic blood pressure, and use of lipid-/blood-pressure-lowering medications.
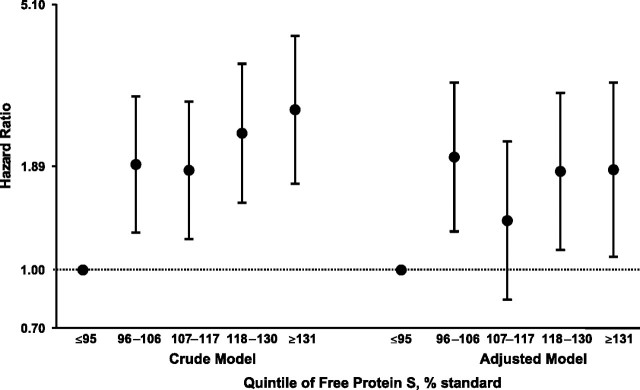
Figure 1 shows the results obtained for the relation between free protein S quintiles and CHD in Cox regression analysis. Compared with persons in the first quintile, those in the fifth (highest) quintile (HR = 2.68, 95% CI: 1.70, 4.21; P < 0.001) and the fourth quintile (HR = 2.32, 95% CI: 1.51, 3.55; P < 0.001) had significantly increased risks of CHD. This association was essentially unchanged after further adjustment for age, clinic, body mass index, diabetes, smoking, HDL cholesterol, total cholesterol, systolic blood pressure, and use of lipid- or blood-pressure-lowering medications and correction for regression dilution bias (compared with the first quintile, HR = 1.85 (95% CI: 1.08, 3.16; P = 0.024) for the fifth quintile and HR = 1.83 (95% CI: 1.13, 2.97; P = 0.014) for the fourth quintile). No association between free protein S level and stroke risk was identified.
Figure 1.
Crude and adjusted (adjusted for age, clinic, body mass index, diabetes, smoking, high density lipoprotein cholesterol, total cholesterol, systolic blood pressure, and use of lipid-/blood-pressure-lowering medications) hazard ratios for coronary heart disease according to quintile of free protein S level (corrected for regression dilution bias), Second Northwick Park Heart Study, United Kingdom, 1989–2005. The hazard ratio of 1 (dashed line) represents the reference group. Bars, 95% confidence interval.
Contribution of free protein S quintiles to CHD risk models
The potential contribution of free protein S quintiles to CHD risk models was examined by means of the AIC, the likelihood ratio test, and Harrell's c statistic (Table 3). A model that included the classical risk factors of age, body mass index, diabetes, smoking, HDL cholesterol, total cholesterol, systolic blood pressure, and use of lipid-/blood-pressure-lowering medications was improved significantly by the addition of the free protein S quintiles; Harrell's c increased from 68.2% to 69.4% (P = 0.072) and was close to statistical significance, with DR5 being 15.2%. In this model with all risk factors plus the free protein S quintiles, the AIC value decreased, and the likelihood ratio statistic increased significantly (P = 0.018). The AIC and the likelihood ratio test take into account the increase in the number of predictors used in the model. The nonsignificant P values for the Hosmer-Lemeshow statistic suggested good calibration with all of the models, and the increase in the P values as terms were added indicated that predicted risks corresponded better to observed risks.
Table 3.
Independent Contribution of Free Protein S Quintiles to the Risk of Coronary Heart Disease in a Cox Proportion Hazards Model, Second Northwick Park Heart Study, United Kingdom, 1989–2005
| Model | Independent Variable(s)a | Akaike's Information Criterion | Likelihood Ratio Test P Valueb | Harrell's c | 95% CI | Difference |
||
| Harrell's cc | 95% CI | P Value | ||||||
| Known risk factors | ||||||||
| Model 1 | All risk factorsd | 3,440.192 | 0.682 | 0.646, 0.717 | ||||
| Model 2 | All risk factors + protein S quintiles | 3,436.328 | 0.018 | 0.694 | 0.660, 0.727 | 0.012 | −0.001, 0.025 | 0.072 |
| Framingham risk score | ||||||||
| Model 1 | Framingham risk score | 3,442.540 | 0.649 | 0.613, 0.686 | ||||
| Model 2 | Framingham risk score + protein S quintiles | 3,437.131 | 0.009 | 0.663 | 0.628, 0.698 | 0.013 | −0.002, 0.029 | 0.089 |
Abbreviation: CI, confidence interval.
The dependent variable was coronary heart disease.
P for comparison of model 2 with model 1.
Bootstrap estimation for 1,000 replications.
Age, clinic, body mass index, diabetes, smoking, high density lipoprotein cholesterol, total cholesterol, systolic blood pressure, and use of lipid-/blood-pressure-lowering medications.
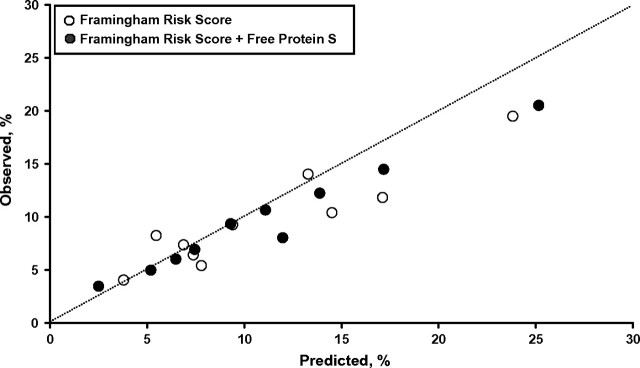
The same pattern was seen in models based on Framingham risk score (35), with and without the addition of free protein S measures (Table 3). When free protein S level was added to the Framingham risk score model, Harrell's c statistic improved from 64.9% to 66.3% (P = 0.089) with a DR5 of 13.1%, the AIC value decreased, and the likelihood ratio statistic increased significantly (P = 0.009). Figure 2 shows calibration plots for both models, with the majority of points being closer to the predicted line for the model including protein S levels.
Figure 2.
Observed risk of a coronary heart disease event versus risk predicted by the Framingham risk score model and the Framingham risk score model plus quintiles of free protein S level (% standard), Second Northwick Park Heart Study, United Kingdom, 1989–2005. Observed risk is plotted against predicted risk, with the line of identity (dashed line) indicating perfect calibration. Calibration refers to the accuracy of the model in predicting the probability of an event.
We examined risk reclassification for free protein S quintiles by using models that included the classical risk factors plus free protein S quintiles as compared with a model without free protein S quintiles (Table 4). Ten-year CHD risk categories were set for 3 strata: <6%, 6%–<20%, and ≥20%. Of the men who went on to develop CHD, 25 (10.5%) correctly moved up a risk category and 10 (4.1%) incorrectly moved down when we added free protein S quintiles to the model, which resulted in a relative improvement for case subjects of 6.4%. For control subjects, 206 (7.3%) correctly moved down, whereas 190 (6.8%) incorrectly moved up, yielding an overall change of 0.6% and an NRI of 7.0% (95% CI: 1.9, 12.0; P = 0.007). The category-less NRI (>0), which is not affected by age range or the strength of the baseline model, was 22.1% (95% CI: 11.8, 32.4; P < 0.001).
Table 4.
Comparisons of 10-Year Coronary Heart Disease Risk Strata in Models of Coronary Heart Disease Risk Factors With and Without Inclusion of Free Protein S Quintiles, Second Northwick Park Heart Study, United Kingdom, 1989–2005a
| Model and 10-Year Risk Category |
Reclassification Into a New Risk Category, % | ||||||||||
| Model Without Free Protein S | Model With Free Protein S |
Total |
|||||||||
| <6% |
6%–<20% |
≥20% |
No. | % | Lower Category | Higher Category | Total | ||||
| No. | Row % | No. | Row % | No. | Row % | ||||||
| <6% | |||||||||||
| Persons included | 684 | 79.4 | 177 | 20.6 | 0.0 | 0.0 | 861 | 28.2 | 0.0 | 20.6 | 20.6 |
| Case patients | 21.8 | 61.6 | 13.6 | 38.4 | 0.0 | 0.0 | 35.4 | 15.0 | 0.0 | 38.4 | 38.4 |
| Control participants | 662.2 | 80.2 | 163.4 | 19.8 | 0.0 | 0.0 | 825.6 | 29.3 | 0.0 | 19.8 | 19.8 |
| Observed risk | 0.032 | 0.077 | 0.000 | ||||||||
| 6%–<20% | |||||||||||
| Persons included | 197 | 13.5 | 1,224 | 83.9 | 38 | 2.6 | 1,459 | 47.8 | 13.5 | 2.6 | 16.1 |
| Case patients | 6.5 | 5.1 | 109.4 | 86.1 | 11.1 | 8.7 | 127.0 | 53.9 | 5.1 | 8.7 | 13.8 |
| Control participants | 190.5 | 14.3 | 1,114.6 | 83.7 | 26.9 | 2.0 | 1,332.0 | 47.3 | 14.3 | 2.0 | 16.3 |
| Observed risk | 0.033 | 0.089 | 0.292 | ||||||||
| ≥20% | |||||||||||
| Persons included | 0.0 | 0.0 | 19 | 2.6 | 713 | 97.4 | 732 | 24.0 | 2.6 | 0.0 | 2.6 |
| Case patients | 0.0 | 0.0 | 3.2 | 4.4 | 69.9 | 95.6 | 73.1 | 31.0 | 4.3 | 0.0 | 4.3 |
| Control participants | 0.0 | 0.0 | 15.8 | 2.4 | 643.1 | 97.6 | 659.0 | 23.4 | 2.4 | 0.0 | 2.4 |
| Observed risk | 0.000 | 0.167 | 0.098 | ||||||||
| Total | |||||||||||
| Persons included | 881 | 28.9 | 1,420 | 46.5 | 751 | 24.6 | 3,052 | 100.0 | |||
| Case patients | 28.3 | 12.0 | 126.2 | 53.6 | 81.0 | 34.4 | 235.5 | 100.0 | |||
| Control participants | 852.7 | 30.3 | 1,293.8 | 45.9 | 670.0 | 23.8 | 2,816.5 | 100.0 | |||
| Observed risk | 0.032 | 0.089 | 0.107 | ||||||||
Reclassification improved by 6.4% in case patients, whereas classification improved in control participants by 0.6%, leading to a net reclassification improvement of 7.0% (95% confidence interval: 1.9, 12.0; P = 0.007). Category-less net reclassification improvement (>0) was 22.1% (95% confidence interval: 11.8, 32.4; P < 0.001).
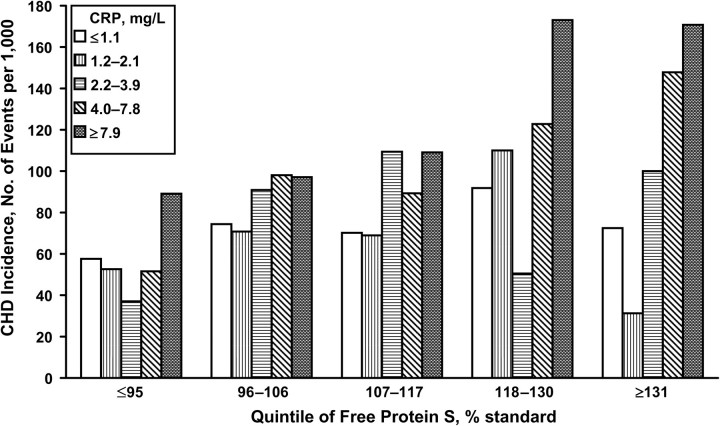
Hingorani et al. (36) previously reported a strong association between levels of the inflammatory marker CRP and CHD in the NPHS-II subjects, with the hazard ratio for subjects in the top tertile being 2.61 (95% CI: 1.78, 3.82; P = 0.0001) compared with those in the lowest tertile. This association was attenuated in the current study when we examined quintiles; no association was identified for CRP quintiles and CHD risk after adjustment for all other risk factors. Persons in the fifth (highest) quintiles of both free protein S and CRP exhibited a significantly higher risk of CHD (HR = 3.34, 95% CI: 1.48, 7.55; P = 0.004) than did persons in the first (reference) quintiles of free protein S and CRP. This estimate was reduced after adjustment for age, clinic, body mass index, diabetes, smoking, HDL cholesterol, total cholesterol, systolic blood pressure, and lipid-/blood-pressure-lowering medications (HR = 2.25, 95% CI: 0.79, 6.41; P = 0.130). Interestingly, there was no suggestion of increased frequency of CHD for subjects in the highest quintile of CRP when protein S levels were within the lowest quintile. Similarly, in the highest quintile of protein S but the lowest quintile of CRP, the frequency of CHD was very low (Figure 3). However, there was no significant evidence of interaction (P = 0.349) for increased frequency of CHD as CRP and protein S levels increased from the lowest quintiles of each variable to the highest.
Figure 3.
Incidence of coronary heart disease (CHD) according to quintiles of free protein S and C-reactive protein (CRP) levels, Second Northwick Park Heart Study, United Kingdom, 1989–2005. P for interaction = 0.35.
Multiple regression analysis of factors influencing free protein S concentration
Clinical characteristics, lipids, and hemostatic and inflammation factors shown to be significantly correlated with protein S in the above analysis were analyzed further by multiple regression to determine the independent association with free protein S levels. We used the best-subsets regression model to identify the component of free protein S that maximized the fit of the model. As Table 5 shows, 6 variables (out of a total of 20 used for correlation analysis (Web Table 1)) had a significant association with free protein S levels. Alcohol consumption and plasma levels of total cholesterol and triglycerides had highly significant independent associations, as did body mass index, the clotting factor Factor VIIc, and systolic blood pressure.
Table 5.
Relation Between Other Study Variables and Free Protein S Levels in Multiple Regression Analysis, Second Northwick Park Heart Study, United Kingdom, 1989–2005
| Independent Variablea | Effect Size (Slope) |
Test Statistic | P Value | Variance Explained (R2), % | |
| β | SE | ||||
| Total cholesterol, mmol/L | 3.47 | 0.42 | 7.86 | <0.001 | 3.99 |
| Alcohol consumption, units/week | 0.20 | 0.03 | 7.81 | <0.001 | 1.79 |
| Body mass indexb | 0.52 | 0.12 | 4.36 | <0.001 | 1.18 |
| Triglycerides, mmol/L | 1.43 | 0.35 | 4.13 | <0.001 | 0.60 |
| Factor VIIc, % standard | 0.04 | 0.01 | 2.75 | 0.006 | 0.12 |
| Systolic blood pressure, mm Hg | 0.04 | 0.02 | 2.18 | 0.030 | 0.21 |
| Constant | 61.20 | 4.42 | −1.2 | 0.234 | |
Abbreviation: SE, standard error.
Total variance explained (including clinic): R2 = 17.30.
Weight (kg)/height (m)2.
DISCUSSION
The most commonly described function for protein S is that it acts as a cofactor for activated protein C in the down-regulation of thrombin generation. An inability to regulate thrombin generation has been suggested to make an important contribution to risk of CHD, as shown by meta-analysis of 2 low-frequency variants that lead to increased thrombin generation: Factor V Leiden and prothrombin G20210A, both of which are associated with increased risk of CHD (37–40). If the activated protein C cofactor activity of protein S contributes to a protective association with CHD risk, then low levels might be expected to contribute to CHD risk. In this study, we wanted to assess the possibility that both low and high protein S levels might be associated with CHD, but examining CHD risk across tertiles or using the standard deviation of protein S would mask any association of increased risk with the lowest levels. In the current study, analyses of restricted cubic splines and quintiles showed no tendency toward a “J-shaped” risk relation, with a linear association between free protein S and higher CHD risk fitting the data best. Conclusions in the current study can only be drawn at the cohort level, and the results do not rule out the possibility that protein S mutation leading to protein S deficiency may play a role in arteriothrombosis, as identified by case studies (15, 41).
This 14-year follow-up study of healthy middle-aged men confirms earlier reports from the NPHS-II (18) that overall, high free protein S levels are associated with CHD risk. To our knowledge, this is the first prospective cohort study that has examined the association between free protein S levels and risks of CHD and stroke. Although the study had limited statistical power to detect an association with stroke, there was no evidence of higher stroke risk in subjects with high protein S levels. The CHD risk estimates associated with free protein S quintiles in these United Kingdom men are stronger in magnitude than those reported previously in this sample of men on the basis of fewer than 168 events (18). The increasing risk seen with higher free protein S quintiles, even after adjustment for other classical risk factors, demonstrates that the risk association is independent of these intermediate traits. Furthermore, free protein S level exhibited a modest improvement in risk prediction when it was added to the Framingham risk score (35) and a modest improvement in risk prediction when the Harrell's c index was used (1.35%, P = 0.089).
These risk prediction metrics have been considered too conservative once key classical risk factors have been added to the model (32, 34), and the NRI has been suggested as a simple intuitive method for examining the improvement offered by a new biomarker (32, 34). Further, a category-less or continuous NRI has been shown to be the most objective and versatile measure of improvement in risk prediction (32, 34). In the current study, the NRI increased by 7.0% (P = 0.007) and the category-less NRI (>0) increased by 22.1% (P < 0.001). Sensitivity analyses with the mean of all available annual measurements of protein S for each individual in the 6-year follow-up period strengthened the results (Web Table 2). When we used multiple regression analysis including all well-known risk factors as well as CRP and/or alcohol consumption, free protein S levels were still significantly associated with CHD, confirming that the association with free protein S was acting independently of all classical CHD risk factors. Because men with any evidence of prior CHD were not recruited into the study, the subjects here could be considered a low-risk cohort, and we cannot exclude the possibility that elevated levels of protein S may not add significantly to prediction metrics, such as the area under the receiver operating characteristic curve, in cohorts with other baseline risk characteristics. However, reclassification improved in the intermediate risk group (6%–20% 10-year risk), which comprised 48% of the subjects.
When the frequency of CHD was analyzed across both quintiles of protein S and quintiles of CRP, a high frequency of CHD was present only in subjects with both high protein S and high CRP levels, and an approximately 3.5-fold elevation in risk was observed when the highest quintiles of CRP and protein S were compared with the lowest quintiles. Since the interaction term was not statistically significant, these findings may have been due to chance and need to be confirmed in future studies. The suggestion that persons in the top quintiles of both free protein S and CRP have the highest CHD risk suggests that these 2 risk factors are acting in an additive and mechanistically independent manner.
The mechanism of the association between protein S and CHD risk is unclear. Atherosclerosis is an inflammatory disease of arterial endothelium and intima arising in part from persistent exposure to oxidatively modified low density lipoproteins (42). Endothelial activation induces expression of vasoactive substances, adhesion molecules, procoagulant factors, cytokines, and growth factors. The proinflammatory cytokines, including interleukin-1α, interleukin-1β, interleukin-6, and tumor necrosis factor α, are expressed by many cell types involved in the inflammatory process, including endothelial cells, macrophages, monocytes, neutrophils, and fibroblasts (18), and a heightened inflammatory response has been shown to have a major role in destabilization of athermanous plaques (43). The inflammatory cytokines are remarkably pleiotropic, but among their actions are associations with free protein S concentration. Tumor necrosis factor α has been reported to down-regulate endothelial cell production of protein S but not hepatocyte expression (44).
While most models have suggested that protein S is down-regulated by inflammation, in another inflammatory condition, systemic lupus erythematosus (45), free protein S has been positively correlated with complement component 3 and complement component 4. Protein S has been shown to be a ligand for TAM kinases, which are key regulators of innate immunity and are important in phagocytosis of apoptotic cells. It is interesting to speculate that protein S levels may be raised because of a heightened, perhaps inappropriate, immune response. The reason stroke and CHD do not exhibit similar risk profiles with regard to protein S is probably multifactorial. While CHD and ischemic stroke are both associated with inflammation, ischemic events causing stroke are often due to embolization of a clot, either from the heart (including atrial fibrillation, valvular heart disease, and atrial septal aneurysm) or from the carotid artery, while most CHD events are due to atherosclerosis at the site of the event (coronary arteries). There were insufficient numbers of subjects in this study to analyze stroke by subgroup (just thrombotic stroke), but the frequency of bleeding events was low and is unlikely to have biased any potentially positive finding regarding thrombotic events. In addition, the incidence of stroke in NPHS-II was less than 3.3%, resulting in limited statistical power to detect a modest association.
Approximately 40% of protein S circulates in a free form, while the remaining 60% circulates in complex with C4bBP. It used to be thought that only the circulating free protein S was active in the context of the protein C pathway. However, recently studies (46) have suggested that the C4bBP-protein S complex also directly participates in Factor Va and Factor VIIIa inactivation, and further, that C4bBP can inhibit activated protein C-catalyzed Factor Va inactivation in the absence of protein S. In addition, in this context, in a recent genomewide analysis, Buil et al. (46) identified single nucleotide polymorphisms within the complement component 4 binding protein β (C4BPB)/complement component 4 binding protein α (C4BPA) gene cluster at chromosome 1q32.2 as being contributory to thrombophilia but unrelated to protein S levels. The C4bBP β-chain is specifically required for binding to protein S, and this isoform is considered a surrogate marker of free protein S levels. It would be expected, therefore, that the associations that have been identified for free protein S in the current study might show the same associations, but be negatively correlated, for the C4bBP β-chain-containing isoform. Note also that the C4bBP α-chain binds CRP, and perhaps the complex CHD risk profile observed between protein S and CRP may reflect the relative expression of the various forms of C4bBP; this requires further study.
In conclusion, we have confirmed and extended earlier observations (18) of an independent association between elevated levels of protein S and future risk of CHD. Since the increase in NRI was modest, the addition of free protein S measurement may not be of great clinical utility in identifying subjects at high future risk of CHD, but if this relation is confirmed and extended in further studies, it may help identify novel pathways in CHD risk and open up new avenues for potential risk reduction strategies. The novel finding of the highest CHD risk in the highest quintiles of both CRP and protein S requires additional analysis in further prospective studies. Whether protein S is acting as a marker of CHD or lies within a causal pathway of CHD could not be addressed directly in the current study but will be the focus of future work.
Supplementary Material
Acknowledgments
Author affiliations: Centre for Cardiovascular Genetics, BHF Laboratories, Department of Medicine, Royal Free and University College Medical School, University of London, London, United Kingdom (Gie Ken-Dror, Jackie A. Cooper, Steve E. Humphries, Fotios Drenos, Helen A. Ireland); and Northwick Park Institute for Medical Research, Harrow, Middlesex, United Kingdom (Helen A. Ireland).
The Second Northwick Park Heart Study (NPHS-II) was supported by the Medical Research Council, the US National Institutes of Health (National Heart, Lung, and Blood Institute grant 33014), and Du Pont Pharma (Wilmington, Delaware). Drs. Fotios Drenos and Steve E. Humphries were supported by the British Heart Foundation (grant RG2008/008). Dr. Helen A. Ireland was supported by the Coronary Thrombosis Trust, the Northwick Park Institute for Medical Research, and the UCLH/UCL Comprehensive Biomedical Research Centre, a partnership between the University College London Hospitals NHS Foundation Trust (UCLH), University College London (UCL), and the National Institute for Health Research.
The authors thank Sarah Leigh for providing bioinformatics support. They also thank the medical staff who contributed to the NPHS-II and the Office for National Statistics Central Registry for provision of mortality data.
Conflict of interest: none declared.
Glossary
Abbreviations
- AIC
Akaike's Information Criterion
- C4bBP
complement factor 4b binding protein
- CHD
coronary heart disease
- CI
confidence interval
- CRP
C-reactive protein
- DR5
5% false-positive detection rate
- HDL
high density lipoprotein
- HR
hazard ratio
- NRI
net reclassification improvement
- NPHS-II
Second Northwick Park Heart Study
- SD
standard deviation
References
- 1.Esmon CT. The regulation of natural anticoagulant pathways. Science. 1987;235(4794):1348–1352. doi: 10.1126/science.3029867. [DOI] [PubMed] [Google Scholar]
- 2.Makris M, Leach M, Beauchamp NJ, et al. Genetic analysis, phenotypic diagnosis, and risk of venous thrombosis in families with inherited deficiencies of protein S. Blood. 2000;95(6):1935–1941. [PubMed] [Google Scholar]
- 3.Miller GJ, Ireland HA, Cooper JA, et al. Relationship between markers of activated coagulation, their correlation with inflammation, and association with coronary heart disease (NPHSII) J Thromb Haemost. 2008;6(2):259–267. doi: 10.1111/j.1538-7836.2008.02819.x. [DOI] [PubMed] [Google Scholar]
- 4.Dahlbäck B, Stenflo J. High molecular weight complex in human plasma between vitamin K-dependent protein S and complement component C4b-binding protein. Proc Natl Acad Sci U S A. 1981;78(4):2512–2516. doi: 10.1073/pnas.78.4.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlbäck B. Inhibition of protein Ca cofactor function of human and bovine protein S by C4b-binding protein. J Biol Chem. 1986;261(26):12022–12027. [PubMed] [Google Scholar]
- 6.Comp PC, Nixon RR, Cooper MR, et al. Familial protein S deficiency is associated with recurrent thrombosis. J Clin Invest. 1984;74(6):2082–2088. doi: 10.1172/JCI111632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comp PC, Esmon CT. Recurrent venous thromboembolism in patients with a partial deficiency of protein S. N Engl J Med. 1984;311(24):1525–1528. doi: 10.1056/NEJM198412133112401. [DOI] [PubMed] [Google Scholar]
- 8.Broekmans AW, Bertina RM, Reinalda-Poot J, et al. Hereditary protein S deficiency and venous thrombo-embolism. A study in three Dutch families. Thromb Haemost. 1985;53(2):273–277. [PubMed] [Google Scholar]
- 9.Lane DA, Mannucci PM, Bauer KA, et al. Inherited thrombophilia: part 1. Thromb Haemost. 1996;76(5):651–662. [PubMed] [Google Scholar]
- 10.Simmonds RE, Ireland H, Lane DA, et al. Clarification of the risk for venous thrombosis associated with hereditary protein S deficiency by investigation of a large kindred with a characterized gene defect. Ann Intern Med. 1998;128(1):8–14. doi: 10.7326/0003-4819-128-1-199801010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Engesser L, Broekmans AW, Briët E, et al. Hereditary protein S deficiency: clinical manifestations. Ann Intern Med. 1987;106(5):677–682. doi: 10.7326/0003-4819-106-5-677. [DOI] [PubMed] [Google Scholar]
- 12.van der Meer FJ, Koster T, Vandenbroucke JP, et al. The Leiden Thrombophilia Study (LETS) Thromb Haemost. 1997;78(1):631–635. [PubMed] [Google Scholar]
- 13.Zangrillo A, Valentini G, Casati A, et al. Myocardial infarction and death after caesarean section in a woman with protein S deficiency and undiagnosed phaeochromocytoma. Eur J Anaesthesiol. 1999;16(4):268–270. doi: 10.1046/j.1365-2346.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 14.Zöller B, García de Frutos P, Dahlbäck B. A common 4G allele in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene as a risk factor for pulmonary embolism and arterial thrombosis in hereditary protein S deficiency. Thromb Haemost. 1998;79(4):802–807. [PubMed] [Google Scholar]
- 15.Manzar KJ, Padder FA, Conrad AR, et al. Acute myocardial infarction with normal coronary artery: a case report and review of literature. Am J Med Sci. 1997;314(5):342–345. doi: 10.1097/00000441-199711000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Beattie S, Norton M, Doll D. Coronary thrombosis associated with inherited protein S deficiency: a case report. Heart Lung. 1997;26(1):76–79. doi: 10.1016/s0147-9563(97)90012-1. [DOI] [PubMed] [Google Scholar]
- 17.Dart AM, Cooper B, Kay SB, et al. Relationships between protein C, protein S, von Willebrand factor and euglobulin lysis time and cardiovascular risk factors in subjects with and without coronary heart disease. Atherosclerosis. 1998;140(1):55–64. doi: 10.1016/s0021-9150(98)00109-9. [DOI] [PubMed] [Google Scholar]
- 18.Rudnicka AR, Miller GJ, Nelson T, et al. An association between plasma free protein S concentration and risk of coronary heart disease in middle-aged men. Thromb Res. 2001;101(2):1–11. doi: 10.1016/s0049-3848(00)00379-0. [DOI] [PubMed] [Google Scholar]
- 19.Majer RV, Chisholm M, Hickton MC. Replacement therapy for protein C deficiency using fresh frozen plasma [letter] Br J Haematol. 1989;72(3):475. doi: 10.1111/j.1365-2141.1989.tb07738.x. [DOI] [PubMed] [Google Scholar]
- 20.Mayer SA, Sacco RL, Hurlet-Jensen A, et al. Free protein S deficiency in acute ischemic stroke. A case-control study. Stroke. 1993;24(2):224–227. doi: 10.1161/01.str.24.2.224. [DOI] [PubMed] [Google Scholar]
- 21.Vossen CY, Hasstedt SJ, Rosendaal FR, et al. Heritability of plasma concentrations of clotting factors and measures of a prethrombotic state in a protein C-deficient family. J Thromb Haemost. 2004;2(2):242–247. doi: 10.1111/j.1538-7933.2003.00592.x. [DOI] [PubMed] [Google Scholar]
- 22.Warren DM, Soria JM, Souto JC, et al. Heritability of hemostasis phenotypes and their correlation with type 2 diabetes status in Mexican Americans. Hum Biol. 2005;77(1):1–15. doi: 10.1353/hub.2005.0034. [DOI] [PubMed] [Google Scholar]
- 23.Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369–384. doi: 10.1007/s004390100593. [DOI] [PubMed] [Google Scholar]
- 24.Cooper JA, Miller GJ, Bauer KA, et al. Comparison of novel hemostatic factors and conventional risk factors for prediction of coronary heart disease. Circulation. 2000;102(23):2816–2822. doi: 10.1161/01.cir.102.23.2816. [DOI] [PubMed] [Google Scholar]
- 25.Miller GJ, Bauer KA, Barzegar S, et al. The effects of quality and timing of venepuncture on markers of blood coagulation in healthy middle-aged men. Thromb Haemost. 1995;73(1):82–86. [PubMed] [Google Scholar]
- 26.Miller GJ, Bauer KA, Barzegar S, et al. Increased activation of the haemostatic system in men at high risk of fatal coronary heart disease. Thromb Haemost. 1996;75(5):767–771. [PubMed] [Google Scholar]
- 27.Amiral J, Grosley B, Boyer-Neumann C, et al. New direct assay of free protein S antigen using two distinct monoclonal antibodies specific for the free form. Blood Coagul Fibrinolysis. 1994;5(2):179–186. doi: 10.1097/00001721-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Shearman AM, Cooper JA, Kotwinski PJ, et al. Estrogen receptor alpha gene variation and the risk of stroke. Stroke. 2005;36(10):2281–2282. doi: 10.1161/01.STR.0000181088.76518.ec. [DOI] [PubMed] [Google Scholar]
- 29.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 30.Fibrinogen Studies Collaboration. Correcting for multivariate measurement error by regression calibration in meta-analyses of epidemiological studies. Stat Med. 2009;28(7):1067–1092. doi: 10.1002/sim.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692):765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 32.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 33.Steyerberg EW, Pencina MJ. Reclassification calculations for persons with incomplete follow-up. Ann Intern Med. 2010;152(3):195–196. doi: 10.7326/0003-4819-152-3-201002020-00019. [DOI] [PubMed] [Google Scholar]
- 34.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 36.Hingorani AD, Shah T, Casas JP, et al. C-reactive protein and coronary heart disease: predictive test or therapeutic target? Clin Chem. 2009;55(2):239–255. doi: 10.1373/clinchem.2008.115923. [DOI] [PubMed] [Google Scholar]
- 37.Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146(6):948–957. doi: 10.1016/S0002-8703(03)00519-2. [DOI] [PubMed] [Google Scholar]
- 38.Dowaidar M, Settin A. Risk of myocardial infarction related to factor V Leiden mutation: a meta-analysis. Genet Test Mol Biomarkers. 2010;14(4):493–498. doi: 10.1089/gtmb.2010.0017. [DOI] [PubMed] [Google Scholar]
- 39.Mannucci PM, Asselta R, Duga S, et al. The association of factor V Leiden with myocardial infarction is replicated in 1880 patients with premature disease. J Thromb Haemost. 2010;8(10):2116–2121. doi: 10.1111/j.1538-7836.2010.03982.x. [DOI] [PubMed] [Google Scholar]
- 40.Casas JP, Hingorani AD, Bautista LE, et al. Meta-analysis of genetic studies in ischemic stroke: thirty-two genes involving approximately 18,000 cases and 58,000 controls. Arch Neurol. 2004;61(11):1652–1661. doi: 10.1001/archneur.61.11.1652. [DOI] [PubMed] [Google Scholar]
- 41.Castellino FJ, Ploplis VA. The protein C pathway and pathologic processes. J Thromb Haemost. 2009;7(suppl 1):140–145. doi: 10.1111/j.1538-7836.2009.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 43.Davies MJ. The contribution of thrombosis to the clinical expression of coronary atherosclerosis. Thromb Res. 1996;82(1):1–32. doi: 10.1016/0049-3848(96)00035-7. [DOI] [PubMed] [Google Scholar]
- 44.Hooper WC, Phillips DJ, Ribeiro MJ, et al. Tumor necrosis factor-alpha downregulates protein S secretion in human microvascular and umbilical vein endothelial cells but not in the HepG-2 hepatoma cell line. Blood. 1994;84(2):483–489. [PubMed] [Google Scholar]
- 45.Suh CH, Hilliard B, Li S, et al. TAM receptor ligands in lupus: protein S but not Gas6 levels reflect disease activity in systemic lupus erythematosus. Arthritis Res Ther. 2010;12(4):R146. doi: 10.1186/ar3088. (doi:10.1186/ar3088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buil A, Trégouët DA, Souto JC, et al. C4BPB/ C4BPA is a new susceptibility locus for venous thrombosis with unknown protein S-independent mechanism: results from genome-wide association and gene expression analyses followed by case-control studies. Blood. 2010;115(23):4644–4650. doi: 10.1182/blood-2010-01-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.