Abstract
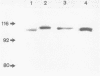
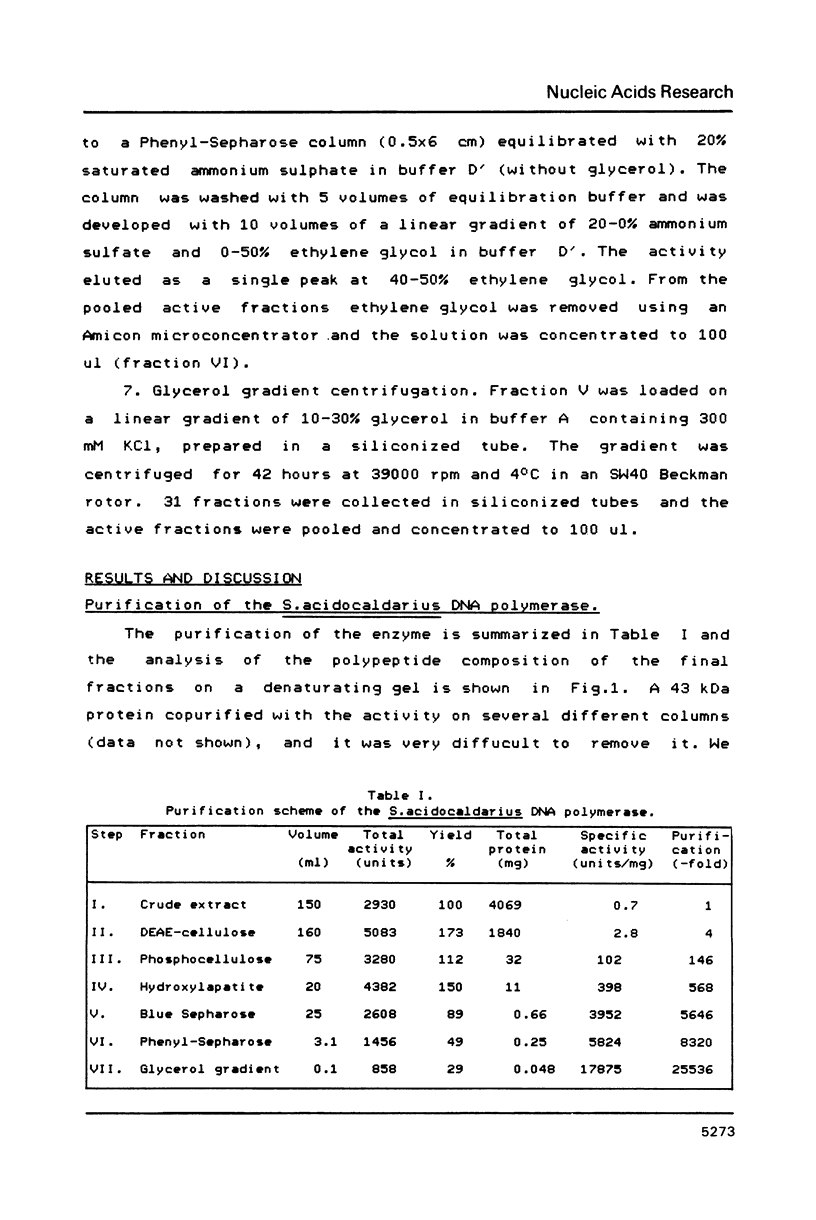
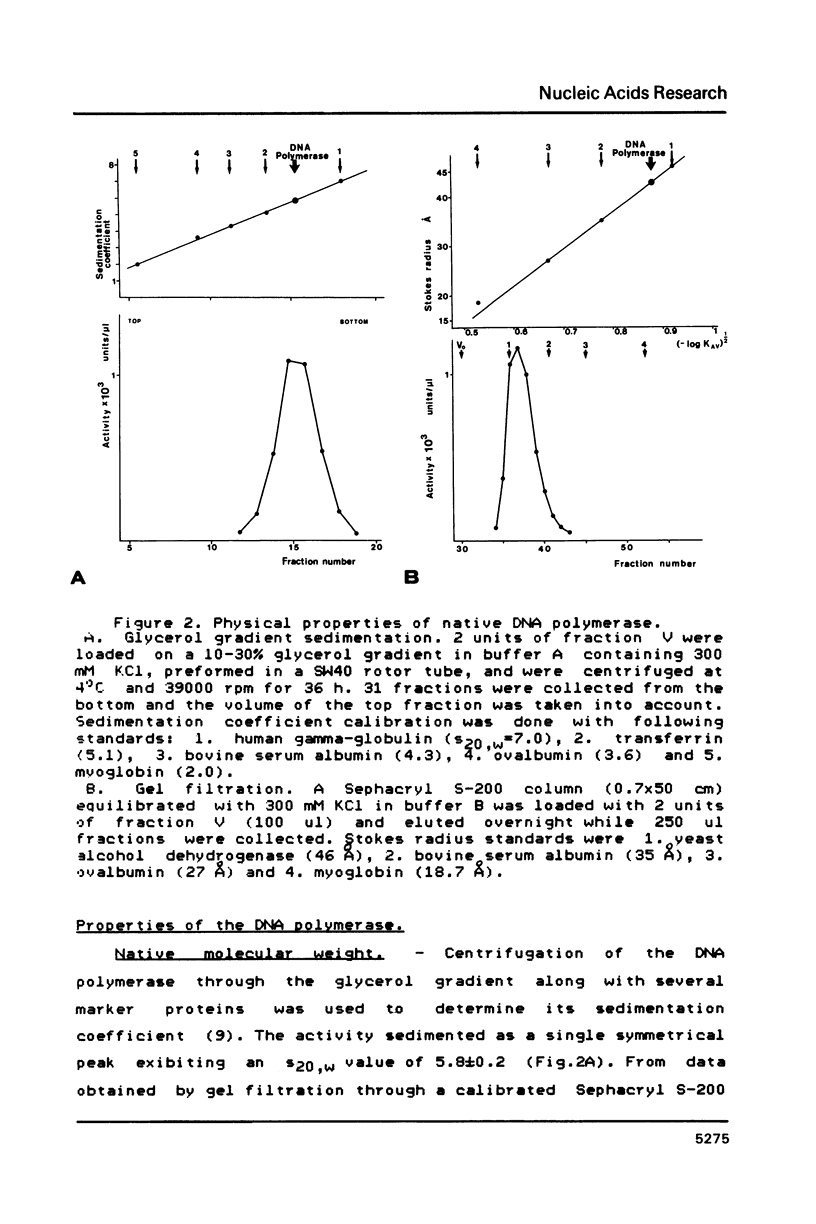
DNA polymerase has been purified about 25,000-fold from the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. On SDS-PAGE the enzyme was observed to have a molecular weight of 100 kDa and to be about 90% pure. The native molecular weight was 108 kDa indicating that the enzyme is composed of a single polypeptide. Activity gel analysis showed an active polypeptide of about 100 kDa. Under conditions promoting proteolysis this polypeptide was degraded to a slightly smaller form of 98 kDa. The enzyme has been characterized in respect to optimal assay conditions, template specificity, sensitivity to inhibitors and associated nuclease activities. The high temperature optimum of 65 degrees C should be emphasized. No substantial similarities have been found with other prokaryotic and eukaryotic DNA polymerases, although the enzyme bears certain resemblances to prokaryotic non-replicative polymerases.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Forterre P., Elie C., Kohiyama M. Aphidicolin inhibits growth and DNA synthesis in halophilic arachaebacteria. J Bacteriol. 1984 Aug;159(2):800–802. doi: 10.1128/jb.159.2.800-802.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Karawya E., Swack J. A., Wilson S. H. Improved conditions for activity gel analysis of DNA polymerase catalytic polypeptides. Anal Biochem. 1983 Dec;135(2):318–325. doi: 10.1016/0003-2697(83)90689-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McKown R. L., Tewari K. K. Purification and properties of a pea chloroplast DNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2354–2358. doi: 10.1073/pnas.81.8.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi M., Weissbach A. The isolation and characterization of DNA polymerase alpha from spinach. J Biol Chem. 1982 Mar 10;257(5):2323–2329. [PubMed] [Google Scholar]
- Pringle J. R. Methods for avoiding proteolytic artefacts in studies of enzymes and other proteins from yeasts. Methods Cell Biol. 1975;12:149–184. doi: 10.1016/s0091-679x(08)60956-5. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]