Abstract
Little is known about the presence and role of neutralizing antibodies (NtAbs) in perinatal hepatitis C virus (HCV) infection. Using HCV pseudoparticles, NtAbs were studied longitudinally in 12 HCV-infected children with or without evidence of acute hepatitis during the first year of life. Broadly reactive NtAbs of maternal origin did not prevent vertical HCV transmission or progression to chronicity. NtAbs against homologous genotype or subtype appeared during the chronic phase and were more abundant and sustained in children with acute hepatitis. Cross-reactive NtAbs were present in both groups of children, but their appearance did not correlate with better control of viremia or HCV clearance.
Studies on the natural history of perinatally acquired hepatitis C virus (HCV) infection have shown that the infection becomes chronic in 80% of the cases [1]. Chronic HCV infection in children is associated with minimal or mild liver disease and rarely with advanced liver damage. However, perinatal HCV infection is characterized by a wide range of alanine aminotransferase (ALT) levels in the first year of life, with some infants reaching values compatible with acute hepatitis, whereas others show normal or slightly elevated levels [2]. The mechanisms responsible for such variability are unknown. A recent study in perinatally infected children provided evidence that hepatitis is associated with a mono- or oligoclonal viral population, whereas mild or no liver damage correlated with the early emergence of a complex viral quasispecies temporally associated with the nascent humoral antibody response of the infant [3].
The lack of robust and reproducible HCV systems to propagate HCV in vitro has been a major hindrance for the study of HCV neutralizing antibodies (NtAbs). Their role in the clinical outcome of HCV infection remains largely unknown in adults [4], and no data are presently available in the setting of perinatal HCV infection. Access to the European Pediatric HCV Network provided us with a unique opportunity to investigate the role of NtAbs in a cohort of children prospectively followed up from birth for up to 15 years.
Methods
Study Subjects. We studied serial serum samples obtained from 12 children (3 boys and 9 girls) with perinatally acquired HCV infection enrolled in the European Pediatric HCV Network that were selected to represent 2 different ALT profiles during the first year of life [3]. The first group included 6 children with high ALT levels (geometric mean of maximum values, 263 IU/L; range, 161–1213). The second group included 6 children with no or minimal ALT values (geometric mean of maximum values, 58 IU/L; range, 36–72). Children were followed up for a median of 53 months (range, 24–180), and all were positive for HCV RNA at the last observation. None had coinfection with other hepatitis viruses or human immunodeficiency virus (HIV). For each patient, HCV NtAbs were measured in 1 or 2 samples before antibody seroconversion, 1 sample at the time of seroconversion, and then 1 sample every 6–12 months. The first available sample for measuring HCV NtAbs was obtained at a mean age (± standard error) of 3.15 ± 0.7 months in 9 patients and at 11, 16, and 19 months of age in 1 patient each, although in the last patient anti-HCV antibodies and viremia were first tested at age 9 months. The presence and levels of serum HCV RNA and anti-HCV throughout the follow-up period have been reported elsewhere [3]. Permission for this study was obtained from the Office of Human Subjects Research of the National Institutes of Health, granted on the condition that all samples be made anonymous.
The effect of HCV NtAbs on the titer of HCV RNA was evaluated by a nonparametric Spearman correlation test for correlation between the presence of HCV NtAbs and viral load, as well as by Mann–Whitney U test for correlation between the levels of HCV RNA before and after the detection of a NtAb response against the homologous genotype or subtype, using Statistica data analysis software Version 6 (StatSoft).
In Vitro Neutralization Test Using HCV Pseudoparticles. Retroviral pseudoparticles bearing the HCV E1 and E2 glycoproteins (HCVpp) representative of the 6 major genotypes were used to test NtAbs, as described elsewhere [5] (see supplementary data).
Results
In total, 5 of the 6 major HCV genotypes and 7 genetic subtypes were represented among the children. Maternally transmitted NtAbs were detected in 3 of the 9 children for whom early samples were available, 2 with acute hepatitis and 1 with minimally elevated ALT values. In children with acute hepatitis, NtAbs appeared in 4 of 6 cases between 19 and 67 months and then persisted throughout the follow-up period (Figure 1). Interestingly, NtAbs never developed in 1 child (patient 6) who had the most severe and prolonged acute hepatitis (ALT peak, 1213 IU/mL), as well as in a second child (patient 5), in whom only 2 samples were available for testing (11 and 13 months). In children with persistently normal or minimally elevated ALT levels, NtAbs were not detected in 2 (1 with maternal NtAbs at 9 days) and were present at low levels and transiently in 2 and only at the latest available time point in 1; the only exception was patient 11 who had sustained NtAbs during the observation period from 16 to 180 months (Figure 1). Thus, in children with no or minimal ALT elevations, NtAbs were detected only transiently and at low levels, whereas they were generally more abundant and sustained in those who developed acute hepatitis. Because the test was performed with a reference HCV strain for each homologous genotype, the presence of strain-specific NtAbs in both groups of children could not be ruled out. Interestingly, there was no significant correlation between the presence of NtAbs against the homologous HCV genotype or subtype and the levels of HCV viremia; likewise, when we compared the levels of HCV RNA before and after the detection of NtAbs against the homologous HCV genotype or subtype, no significant effect of the NtAb response was observed (data not shown).
Figure 1.
Neutralization of homologous genotype or subtype hepatitis C virus (HCV) pseudoparticles (HCVpp) by serum samples obtained from children with high (upper panel) or normal to low (lower panel) alanine aminotransferase (ALT) levels at the indicated months. Neutralizing antibodies (NtAbs) were detected using HCVpp bearing E1 and E2 glycoproteins of the homologous genotype or subtype as indicated. The percentage of neutralization was calculated by comparison with the mean results obtained with 3 normal anti-HCV negative human serum samples. Neutralization was defined as ≥50% reduction of green fluorescent protein–positive cells; 50% neutralization is indicated by horizontal line. Replicates for neutralization test in serial serum samples collected longitudinally from each patient were not performed because of the limited amount of available serum.
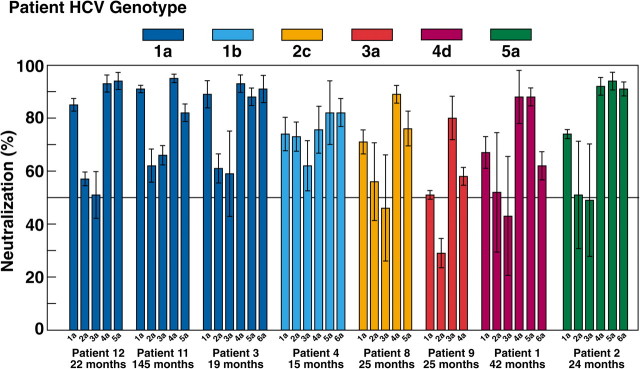
To investigate the breadth of activity of the NtAbs detected in the course of perinatally acquired HCV infection, selected chronic-phase serum samples that neutralized the homologous genotype or subtype were tested for the presence of cross-genotype neutralization using HCVpp representing the major HCV genotypes (1a, 2a, 3a, 4a, 5a, and 6a) (Figure 2). Serum samples from 4 children with acute hepatitis and 4 children with normal or minimally elevated ALT levels were tested. In both groups of children, chronic-phase serum samples strongly neutralized the HCVpp of genotypes 1a, 4a, 5a, and 6a, whereas limited neutralization was documented against the HCVpp of genotype 2a and 3a (Figure 2). It should be noted, however, that patient 9 (infected with genotype 3a) had the highest neutralization titers against the 3a HCVpp.
Figure 2.
Cross-neutralization of different hepatitis C virus pseudoparticle (HCVpp) genotypes with selected serum samples, positive for neutralizing antibodies against the homologous genotype or subtype, obtained from children with high (patients 1, 2, 3, and 4) or normal to low (patients 8, 9, 11, and 12) alanine aminotransferase (ALT) levels at indicated time points. HCVpp were from genotype 1a (H77), 2a (J6CF), 3a (S52), 4a (ED43), 5a (SA13), and 6a (HK6a). Colors of bars indicate patient genotypes. Neutralization was defined as ≥50% reduction of green fluorescent protein–positive cells; 50% neutralization is indicated by horizontal line. Average infectivity of HCVpp performed in triplicates was 3.3 % (range, 3.1%–3.6%) for 1a, 1.0% (range, 0.9%–1.2%) for 2a, 5.4% (range, 4.5%–6.3%) for 3a, 1.7% (range, 1.7%–1.8%) for 4a, 1.7% (range, 1.3%–2.0%) for 5a and 1.1% (range, 1.0%–1.2%) for 6a. Three replicates were performed for each HCVpp used in the neutralization test, and the results are expressed as the average percentages of neutralization (± standard deviation).
Serum was available from 3 mothers who transmitted HCV to their children (patients 1, 7, and 10). All 3 mothers had a significant NtAb response against HCVpp of homologous genotype or subtype. When HCVpp of heterologous genotypes were used, serum from the mother of patient 7 (infected with genotype 3a) strongly neutralized all HCVpp (1–6), serum from the mother of patient 10 (infected with genotype 4a) neutralized all genotypes except 3a, whereas serum from the mother of patient 1 (infected with genotype 4d) failed to neutralize genotypes 1a, 2a and 3a (see supplementary data).
Discussion
In this study, we investigated the role of NtAbs in children with perinatally acquired HCV infection with or without biochemical evidence of acute hepatitis during the first year of life, who were followed up prospectively for up to 15 years. As documented in chimpanzees and adults, in infants HCV starts to replicate early, rapidly reaching high titers of viremia [3] despite the initial presence of maternal antibodies and, subsequently, of infant antibodies. Using HCVpp-bearing envelope glycoproteins derived from the homologous genotype or subtype, we failed to show the presence of specific NtAb responses during the acute phase of HCV infection, in agreement with the data obtained elsewhere in adults and in chimpanzees [5–8]. Interestingly, maternal NtAbs were detected in 2 of 6 children who developed acute hepatitis and in 1 of 6 who did not, providing evidence that the initial presence of maternal antibodies does not influence the outcome of HCV infection since all children developed chronic HCV infection. Even more important, however, was the observation that NtAbs against both homologous and heterologous genotypes in 3 mothers for whom serum samples were available for testing did not prevent vertical transmission of HCV to their offspring. Thus, our data are in agreement with a recent study performed in HCV-HIV–coinfected mothers in whom the presence of maternal cross-reactive NtAbs did not prevent perinatal HCV transmission [9]. Whether the strains transmitted from the mother to the children represent neutralization escape variants, as recently documented for the HCV strains reinfecting the liver graft in the setting of liver transplantation, remains to be established [10].
As documented in adults, in the setting of perinatal HCV infection, NtAbs against the homologous genotype or subtype typically appeared during the chronic phase. Of note, we found that NtAbs were more frequently detected in children who developed acute hepatitis (4 of 6) than in those with persistently normal or slightly elevated ALT levels during the first year of life (2 of 6), although the HCV genotype distribution differed in the 2 groups. After their appearance, NtAbs persisted throughout the follow-up period, for up to 15 years. Strikingly, 1 child who developed an unusual acute hepatitis, with very high and persistent ALT values (peak, 1213 IU/L) never developed NtAbs during a follow-up period of 5 years. As observed elsewhere in adults [5–7], our longitudinal study confirmed that chronic HCV infection in children is associated with the presence of cross-genotype NtAbs. Interestingly, NtAbs appearing during the chronic phase strongly cross-neutralized HCVpp from genotypes 1a, 4a, 5a, or 6a but showed only a limited activity against genotypes 2a or 3a. In adults, chronic hepatitis C is characterized by high levels of viremia with little or no fluctuation over time even in the presence of NtAbs [5]. A similar pattern of viremia can be observed in children even at the time of appearance of NtAbs, suggesting that the appearance and persistence of NtAbs did not influence the levels of viremia (data not shown).
Our collection of samples provided us with the opportunity to study the spectrum of activity of NtAbs in 3 mothers who transmitted HCV to their offspring. All 3 mothers showed the presence of broadly reactive NtAbs, but whereas a mother with genotype 3a showed cross-reactive NtAbs against all HCV genotypes (from 1a to 6a), serum from the other 2 mothers infected with genotypes 4a or 4d showed no or very limited activity against HCVpp 2a or 3a. Thus, the results obtained in both adults and children suggest that genotypes 1, 4, 5, and 6 are more closely related to one another, with a more distant serologic relationship to genotypes 2 and 3 [5]. However, it is becoming apparent that the neutralization susceptibility varies greatly for HCVpp strains of the same genotype or subtype [11]. Thus, the limited neutralization of 2a and 3a HCVpp used here might not necessarily be representative of HCVpp of other genotype 2 and 3 strains.
A limitation of our study, as well as of several published studies on HCV NtAbs, is that these studies were performed using HCVpp representing the same HCV genotype or subtype derived from prototype laboratory strains, differing from the strains harbored by the children enrolled in the study. More recent studies using autologous HCVpp representing the infecting HCV strains demonstrated that HCV does elicit NtAbs during the acute phase of HCV infection, but these antibodies are strain specific [12–14], as previously documented elsewhere in the chimpanzee model [15].
In conclusion, our data provide evidence that the presence of broadly reactive NtAbs in the mother does not prevent vertical HCV transmission or progression to chronicity in children with perinatal infection. Moreover, the appearance of cross-reactive NtAbs during the chronic phase does not correlate with better control of viremia or with the clearance of HCV. Whether the lack of detectable NtAbs during the acute phase of perinatal HCV infection was due to the lack of a system able to detect strain-specific NtAbs remains to be determined. To address this question, NtAbs must be measured against autologous HCVpp rather than against reference HCVpp of the homologous genotype or subtype. However, this task is complicated by the complexity of the HCV quasispecies, with the simultaneous circulation of different HCV strains within the same subject. As a consequence, although understanding the role of strain-specific NtAbs in the course of HCV infection may have major implications for the development of an HCV vaccine, the study of the contribution of these NtAbs during the acute phase of HCV infection remains a major challenge.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Tovo PA, Pembrey LJ, Newell ML. Persistence rate and progression of vertically acquired hepatitis C infection. European Paediatric Hepatitis C Virus Infection. J Infect Dis. 2000;181:419–24. doi: 10.1086/315264. [DOI] [PubMed] [Google Scholar]
- 2.Resti M, Jara P, Hierro L, et al. Clinical features and progression of perinatally acquired hepatitis C virus infection. J Med Virol. 2003;70:373–7. doi: 10.1002/jmv.10405. [DOI] [PubMed] [Google Scholar]
- 3.Farci P, Quinti I, Farci S, et al. Evolution of hepatitis C viral quasispecies and hepatic injury in perinatally infected children followed prospectively. Proc Natl Acad Sci U S A. 2006;103:8475–80. doi: 10.1073/pnas.0602546103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeisel MB, Fafi-Kremer S, Fofana I, et al. Neutralizing antibodies in hepatitis C virus infection. World J Gastroenterol. 2007;13:4824–30. doi: 10.3748/wjg.v13.i36.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meunier JC, Engle RE, Faulk K, et al. Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1. Proc Natl Acad Sci U S A. 2005;102:4560–5. doi: 10.1073/pnas.0501275102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartosch B, Bukh J, Meunier JC, et al. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc Natl Acad Sci U S A. 2003;100:14199–04. doi: 10.1073/pnas.2335981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logvinoff C, Major ME, Oldach D, et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A. 2004;101:10149–54. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinmann D, Barth H, Gissler B, et al. Inhibition of hepatitis C virus-like particle binding to target cells by antiviral antibodies in acute and chronic hepatitis C. J Virol. 2004;78:9030–40. doi: 10.1128/JVI.78.17.9030-9040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowd KA, Hershow RC, Yawetz S, et al. Maternal neutralizing antibody and transmission of hepatitis C virus to infants. J Infect Dis. 2008;198:1651–5. doi: 10.1086/593067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fafi-Kremer S, Fofana I, Soulier E, et al. Viral entry and escape from antibody-mediated neutralization influence hepatitis C virus reinfection in liver transplantation. J Exp Med. 2010;207:2019–31. doi: 10.1084/jem.20090766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarr AW, Urbanowicz RA, Hamed MR, et al. Hepatitis C virus (HCV) patient-derived glycoproteins exhibit marked differences in their susceptibility to serum neutralizing antibodies: genetic subtype defines antigenic but not neutralization serotype. J Virol. 2011;85:4246–57. doi: 10.1128/JVI.01332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Hahn T, Yoon JC, Alter H, et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–78. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Pestka JM, Zeisel MB, Blaser E, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A. 2007;104:6025–30. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowd KA, Netski DM, Wang X-H, Cox AL, Ray SC. Selection pressure from neutralizing antibodies drive sequence evolution during acute infection with hepatitis C virus. Gastroenterology. 2009;136:2377–86. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farci P, Alter HJ, Wong DC, et al. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci U S A. 1994;91:7792–6. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.