Abstract
Introduction. LC16m8 is an attenuated cell culture–adapted Lister vaccinia smallpox vaccine missing the B5R protein and licensed for use in Japan.
Methods. We conducted a phase I/II clinical trial that compared the safety and immunogenicity of LC16m8 with Dryvax in vaccinia-naive participants. Adverse events were assessed, as were electrocardiography and laboratory testing for cardiotoxicity and viral culturing of the vaccination sites. Neutralization titers to vaccinia, monkeypox, and variola major were assessed and cell-mediated immune responses were measured by interferon (IFN)–γ enzyme-linked immunosorbent spot and lymphoproliferation assays.
Results. Local and systemic reactions after vaccination with LC16m8 were similar to those reported after Dryvax. No clinically significant abnormalities consistent with cardiac toxicity were seen for either vaccine. Both vaccines achieved antivaccinia, antivariola, and antimonkeypox neutralizing antibody titers >1:40, although the mean plaque reduction neutralization titer of LC16m8 at day 30 after vaccination was significantly lower than Dryvax for anti-NYCBH vaccinia (P < .01), antimonkeypox (P < .001), and antivariola (P < .001). LC16m8 produced robust cellular immune responses that trended higher than Dryvax for lymphoproliferation (P = .06), but lower for IFN-γ ELISPOT (P = .02).
Conclusions. LC16m8 generates neutralizing antibody titers to multiple poxviruses, including vaccinia, monkeypox, and variola major, and broad T-cell responses, indicating that LC16m8 may have efficacy in protecting individuals from smallpox.
Clinical Trials Registration. NCT00103584.
The local and systemic reactions reported after vaccination with Dryvax, a standard vaccinia strain used to prevent smallpox, highlight the need to develop attenuated smallpox vaccines that would be effective against variola but with fewer complications [1, 2] in case of mass vaccination due to the spread of smallpox from a bioterrorism attack. One candidate vaccine is LC16m8, an attenuated Lister strain that has a deletion mutation in the B5R viral protein. The strain was originally developed in 1975 by the Chiba Serum Institute (Kaketsuken). In animal studies LC16m8 did not produce neurotoxicity, but elicited neutralizing antibody titers comparable to the Lister strain [3]. Recently LC16m8 was compared with Dryvax in both in vitro and mouse models. Sera from mice vaccinated with either vaccine similarly neutralized both extracellular and intracellular forms of vaccinia, provoked comparable cellular immune responses, and demonstrated similar levels of protection against Western Reserve vaccinia strain infection [4]. LC16m8 was extensively evaluated in clinical trials in Japan by the Ministry of Health [5, 6] with 90 000 doses administered in Japan between 1974 and 1975 without any reports of serious adverse events (AEs) attributed to the vaccine [3]. LC16m8 immunization has been used for vaccination of select personnel in the Japanese Self-Defense Forces and was both safe and immunogenic in vaccinia-naive and vaccinia-experienced participants [7].
The objective of this phase I/II trial in the United States was to compare the safety and immunogenicity of LC16m8 with Dryvax in a cohort of healthy vaccinia-naive volunteers between the ages of 18 and 34 years. Human immune responses to several vaccinia strains, monkeypox, and variola were measured; cellular immune responses to a Dryvax-derived vaccinia strain were also measured using standardized enzyme-linked immunosorbent spot (ELISPOT) and lymphoproliferation assays.
METHODS
Study Design, Vaccine Dosing, and Volunteer Enrollment
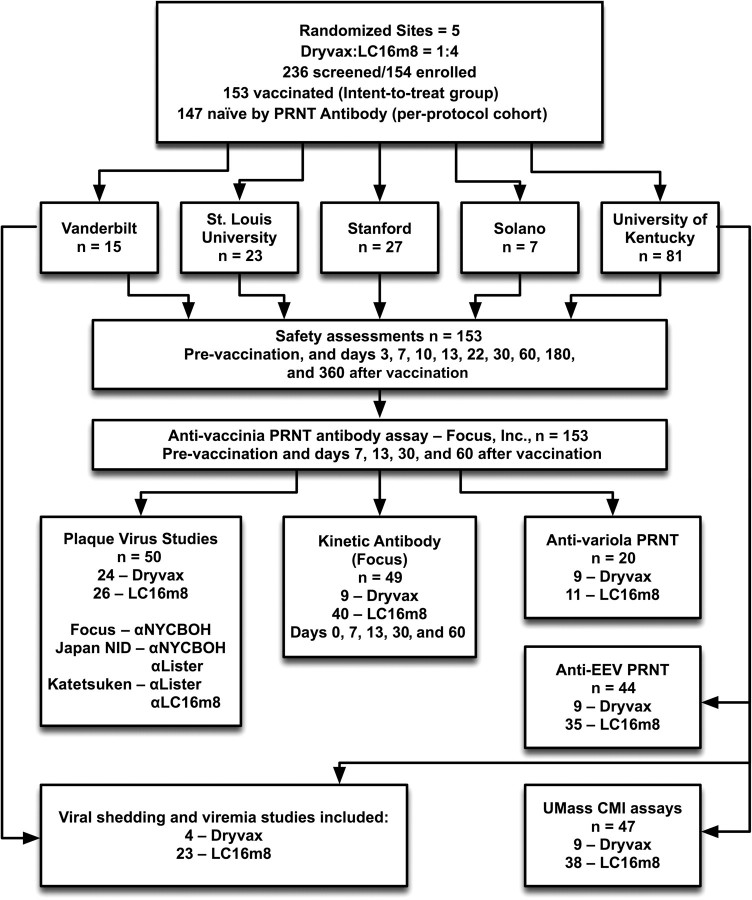
This study was a randomized, multicenter, double-blind comparative study of the LC16m8 and Dryvax smallpox vaccines conducted in healthy vaccinia-naive adult volunteers at 5 sites in the United States (Figure 1). The primary endpoints were the neutralizing antibody titer to intracellular mature virus as measured by plaque reduction neutralization titer (PRNT) 30 days after vaccination and the rates of AEs attributed to the vaccines. The secondary or exploratory endpoints included vaccine take rates, size of the lesion at the vaccination site, extracellular enveloped virus (EEV) neutralizing antibody determination, cell-mediated immune (CMI) responses, viral persistence, and viremia after vaccination. All assays were blinded as to vaccine group.
Figure 1.
Study flow diagram. CMI, cell-mediated immune; NYCBH, New York City Board of Health; PRNT, plaque reduction neutralization titer.
The protocol was approved by the institutional review boards and institutional biosafety committees at each study site. The trial began 20 October 2004 and the last vaccination was given 30 June 2005. There was a 3-member safety monitoring committee. A total of 154 vaccinia-naive adults were randomized in a 4:1 ratio to receive a single dose of 108 plaque-forming units/mL of either LC16m8 vaccine or Dryvax vaccine (Figure 1; Table 1). Both vaccines were delivered in a volume of 0.02 μL via a 15-puncture intraepidermal inoculation using a bifurcated needle in the deltoid area of the nondominant arm.
Table 1.
Baseline Demographics and Inclusion/Exclusion Criteriaa
| Dryvax (n = 29) | LC16m8 (n = 125) | All Groups (n = 154) | P value | |
| Gender | ||||
| Female | 13 (45%) | 44 (35%) | 57 (37%) | .39 |
| Male | 16 (55%) | 81 (65%) | 97 (97%) | |
| Ethnicity | ||||
| Hispanic | 2 (7%) | 3 (2%) | 5 (3%) | .24 |
| Non-Hispanic | 27 (93%) | 122 (98%) | 149 (97%) | |
| Race | ||||
| Native American | 0 | 2 (2%) | 2 (1%) | .84 |
| Asian | 2 (7%) | 7 (6%) | 9 (6%) | |
| African American | 1 (3%) | 2 (2%) | 3 (2%) | |
| Pacific Islander | 0 | 1 (1%) | 1 (1%) | |
| Caucasian | 26 (90%) | 110 (88%) | 136 (88%) | |
| Multiracial | 0 | 3 (2%) | 3 (2%) | |
| Age, y | ||||
| Mean (SD) | 22.7 (3.5) | 23.4 (4.0) | 23.3 (3.9) | .56 |
| Median | 21.6 | 22.4 | 22.2 | |
| Range | 18.8–32.6 | 18.1–34.0 | 18.1–34.0 |
Percentages are calculated from the number of volunteers in each vaccine group.
Inclusion criteria: Participants were 18–34-year-old individuals (year of birth, 1971–1987) with no history of smallpox vaccination, and in good health as ascertained by medical history, clinical assessment, and baseline blood chemistries including hepatic function tests; were willing to refrain from any blood donations until vaccination scab was gone; and had negative serology for hepatitis B and C and human immunodeficiency virus, negative urine glucose, and a normal ECG. Exclusion critera included active or past history of atopic dermatitis, eczema, or immunosuppression; known cardiac disease or 3 or more cardiac risk factors; known allergies to study materials and vaccine components; females who were pregnant or not willing to use birth control or were breastfeeding; frequent contact or sharing of linen with someone who was pregnant or breastfeeding or had atopic dermatitis or eczema; Darier disease or contact with someone with Darier disease; frequent contact with an infant < 1 year of age; history of exuberant keloid formation; issues that would preclude compliance with protocol or would be exacerbated by the protocol such as psychiatric illness; recent vaccinations either before or after smallpox vaccination (live within 30 days, killed within 2 weeks); military service prior to 1989; receipt of blood products within 60 days prior to screening; or participation in another experimental drug protocol.
Safety Assessments and Primary Reactions
Following vaccination, clinical and laboratory responses to vaccination were evaluated in volunteers on days 3, 7, 10, 13, 22, 30, 60, 180, and 360 after vaccination (Table 2). The presence of a primary reaction (take) was assessed from day 6 to day 12 after vaccination and date of first appearance recorded. Local and systemic reactions, including fever, were assessed twice daily by the participant. Solicited events were recorded each day on a diary card maintained by the participant for 21 days after vaccination or until the scab over the vaccine site separated. Interviews based on the diaries were conducted at each visit in order to ascertain AEs. Enrollment numbers were not large enough to be powered to identify differences for any particular AE. A sample of 12 500 total participants randomized 4-to-1 LC16m8:Dryvax would have been required to have 81% power to detect a decrease from an expected 1% AE rate in the Dryvax arm to a 0.5% AE rate in LC16m8 arm at the .05 level of significance. Safety laboratory tests for metabolic, renal, and hepatic function were performed on days 0, 7, 13, and 30. Monitoring for potential cardiac toxicity consisted of electrocardiographic (ECG) testing and assessment of troponin T, creatine phosphokinase-MB (CPK-MB), and C-reactive protein on days 7, 13, and 30. At 2 sites, lesions were swabbed and a blood sample was taken for quantitative viral culture assessment at each clinic visit until resolution of the skin lesion.
Table 2.
Percentage of Volunteers With Local Reactogenicity at Any Clinic Visit (Intent-to-Treat Cohort)
| Dryvax | LC16m8 | ||
| (n = 28) | (n = 125) | P value | |
| Local reactogenicity | 68% (19/28) | 82% (102/125) | .12 |
| Warmth | 39% (11/28) | 36% (45/125) | .83 |
| Tenderness at vaccination site | 46% (13/28) | 42% (52/125) | .68 |
| Limited arm motion | 18% (5/28) | 12% (15/125) | .37 |
| Axillary lymph node(s) swollen | 46% (13/28) | 37% (46/125) | .39 |
| Axillary lymph node(s) tender | 50% (14/28) | 48% (60/125) | 1.00 |
| Rash | 4% (1/28) | 2% (3/125) | .56 |
| Satellite lesion | 0% (0/28) | 2% (3/125) | 1.00 |
Volunteer is counted once at the highest level of severity for each reactogenicity category.
Anti-Dryvax–Derived Vaccinia Virus PRNT (Focus) Method
The principal method used to assess immunogenicity was a PRNT that used vaccinia virus derived from Dryvax as the plaquing virus [8]. The assay was performed at Focus, Inc (Cypress, California). The reciprocal of the serum dilution resulting in a 50% reduction in plaque numbers compared with the negative control was defined as the resultant titer. An individual volunteer was considered to have seroconverted if, within 30 days of vaccination, a PRNT titer was at least 4 times greater than the preimmunization titer. To determine the impact of the source of plaque virus on PRNT, a subset of samples from 49 participants (40 LC16m8, 9 Dryvax) was assessed using a panel of plaque viruses (Table 3).
Table 3.
Comparison of PRNT Titers Using Different Plaquing Viruses at Day 30 After Vaccination in a Subset of Dryvax and LC16m8 Volunteers Tested at All Laboratories
| PRNT assay virus | Dryvax | LC16m8 | Dryvax/LC16m8 ratio | P value |
| Number of titers | 23 | 26 | … | |
| Anti-Dryvax (Focus) | ||||
| Geometric mean | 919 | 329 | 2.8 | <.001 |
| 95% CI | 565–1493 | 228–474 | 1.6–5.0 | |
| Anti-LC16m8 (KKT) | ||||
| Geometric mean | 471 | 733 | 0.6 | .24 |
| 95% CI | 301–739 | 400–1343 | .3–1.4 | |
| Anti-Lister (KKT) | ||||
| Geometric mean | 7686 | 17 523 | 0.4 | .09 |
| 95% CI | 3687–16 026 | 9104–33 725 | .2–1.1 | |
| Anti-monkeypox (JNIID) | ||||
| Geometric mean | 368 | 112 | 3.3 | <.001 |
| 95% CI | 225–597 | 82–307 | 1.7–6.3 | |
| Anti-NYCBH (JNIID) | ||||
| Geometric mean | 482 | 158 | 3.0 | .01 |
| 95% CI | 287–810 | 82–307 | 1.3–7.0 | |
| Anti-Lister (JNIID) | ||||
| Geometric mean | 298 | 207 | 1.4 | .29 |
| 95% CI | 176–504 | 129–332 | .7–2.9 |
Per protocol cohort excludes 4 volunteers who had anti-Dryvax PRNT (Focus) titers ≥40 at baseline and 2 volunteers who had missing anti-Dryvax PRNT (Focus) titer data at day 30. Plaque viruses included Dryvax (NYCBH–vaccine-derived virus), NYCBH (Japan vaccine–derived seed), Lister, LC16m8, and monkeypox. PRNT tests were performed at 3 different laboratories: anti-Dryvax assay at Focus, Inc; anti-NYCBH at JNIID (Tokyo, Japan); anti-Lister vaccinia at both JNIID and KKT (Kumamoto, Japan); anti-LC16m8 vaccinia at KKT; and the antimonkeypox at JNIID. These assays were performed on sera obtained on day 30 after vaccination. P values were calculated using Wilcoxon rank-sum test.
Abbreviations: CI, confidence interval; JNIID, Japanese National Institute of Infectious Diseases; KKT, Kaketsuken; NYCBH, New York City Board of Health; PRNT, plaque reduction neutralization titers.
Anti-EEV PRNT Assay
Virus was grown as previously described [9, 10]. A subset of 45 vaccinated volunteers resulted in a subset of 36 LC16m8 volunteers and 9 Dryvax volunteers (1 Dryvax-vaccine recipient was excluded due to a positive baseline PRNT). Serum samples from both day 0 and day 30 were assayed using 2 different thresholds for positivity, the first requiring a 50% reduction in EEV plaque count and the second requiring a 30% reduction.
Anti-Variola PRNT Assay
Fifty percent PRNT testing with variola as the plaquing virus was performed at the Centers for Disease Control Biosafety Level 4 laboratory after World Health Organization approval to perform the testing on day 0 and day 30 sera from a subset of 20 volunteers (Figure 1) using a previously described method [11].
CMI Assays
CMI measurements including interferon (IFN)–γ ELISPOT and lymphoproliferation assays were conducted on cryopreserved peripheral blood mononuclear cells (PBMCs) from the first 48 volunteers. One participant was excluded from analysis for having a positive PRNT response at baseline (Figure 1). The proliferation index (SI) and frequencies of vaccinia-specific IFN-γ–positive PBMCs were determined by methods previously described [12].
Statistical Methods
The sample size was chosen to satisfy the primary endpoints of the study with the assumption that vaccine take would be 95%, with < 1% chance that the observed take rate would be lower than 90% (power of 60%). P values were calculated using Wilcoxon rank-sum test for age, and Fisher exact test for gender, ethnicity, race, and safety comparisons. A logistic model was used to investigate the effects of age, gender, and race on local reactogenicity. The likelihood ratio was used to assess significance. Viral shedding results were compared using the Wilcoxon rank-sum test. The difference in antibody response rate and 95% confidence intervals (CIs) were computed. PRNT was summarized by the geometric mean titer (GMT) and its CI. CIs were not adjusted for multiple comparisons to make the estimates more comparable to historical data, and the Wilcoxon rank-sum test was used to calculate P values. In addition, PRNT antibody response against Dryvax and LC16m8 virus in a subset of volunteers was measured at days 0, 7, 13, 30, and 60. For this subset, the mean log-transformed response at each time point and its CI was estimated for each vaccine group. These calculations were performed using the 2-group t test of equivalence in means (unequal n’s) in nQuery Advisor (version 5.0) software and checked via simulation in S-Plus software (version 7.0). The significance level α was .05. Comparisons for IFN-γ ELISPOT and lymphoproliferation responses were made using the Wilcoxon test of equality and Student t test with significance level α of .05.
RESULTS
Demographics
Overall, 236 volunteers were screened and 154 were enrolled. As shown in Table 1 the randomization process yielded a balance of demographic characteristics. One hundred fifty-three of the 154 volunteers received their scheduled vaccination and are included in the intent-to-treat cohort (ITT). Of those in the ITT group, 147 (27 Dryvax, 120 LC16m8) had undetectable preimmunization anti-Dryvax PRNT titers and completed the day 30 visit. They comprise the per-protocol (PP) group.
Vaccine Skin Take
All 125 participants vaccinated with LC16m8 developed a primary lesion (pustule with induration) at the vaccination site between 6 and 12 days following vaccination. Significantly fewer Dryvax volunteers, 24 of 28 (86%), developed a take (P < .001). Results were similar when restricting the comparison to the PP group. Take rates were 100% and 85% for the LC16m8 and Dryvax groups, respectively. The 4 Dryvax volunteers who did not develop a take also did not seroconvert. All 4 were vaccinated at the same study site on the same day and were the only ones to receive Dryvax on that day. Dryvax had been reconstituted and kept at 4°C 56 days prior to vaccination. None of that reconstituted vaccine was used on additional participants and none remains for characterization. We believe this was an issue of vaccine viral potency.
Inoculation skin lesions developed at similar times in both vaccine groups with a median of 5 days between vaccination and the appearance of a pustule in each group. Eighty-three percent of Dryvax and 82% of LC16m8 volunteers exhibited pustules at the day 7 clinic visit and by day 10 reached 96% and 88%, respectively. The median number of days to scab separation for Dryvax volunteers was 31 days compared with 28 days for LC16m8 volunteers (P = .08). Lesions were, on average, larger within the Dryvax group than the LC16m8 group; specifically, the median maximal lesion size was 1.4 cm for Dryvax volunteers compared with 1.1 cm for LC16m8 volunteers (P = .04). The median maximal extent of erythema and swelling was also significantly greater among Dryvax volunteers (5.6 cm) than among LC16m8 volunteers (3.5 cm; P = .002).
Viral Shedding and Viremia
A viral shedding cohort, chosen from only 2 sites, included 27 volunteers (4 Dryvax and 23 LC16m8 volunteers). Viral shedding was detected on day 3 after vaccination in 2 of the 4 Dryvax volunteers but in none of the 23 LC16m8 volunteers. By day 7, all Dryvax volunteers and 87% of LC16m8 volunteers had detectable virus. Shedding lasted for a median of 16.5 days among the Dryvax volunteers and 14 days within the LC16m8 group (P = .60). Blood samples from 52 volunteers (10 Dryvax and 42 LC16m8), collected at day 0, prior to vaccination, and at days 3, 7, 13, and 22 after vaccination, were assessed for viremia by polymerase chain reaction and no viremia was detected at any of the timepoints.
Safety Assessments
During clinic assessments, 68% of Dryvax volunteers and 82% of LC16m8 volunteers exhibited at least 1 sign or symptom of local reactogenicity at 1 or more visits; the difference between the vaccine groups was not statistically significant (Table 2). At least 1 instance of systemic reactogenicity was reported by 75% of Dryvax volunteers and 74% of LC16m8 volunteers (Table 2). This study did record more participants who received LC16m8 with swollen axillary lymph(s) (37%) and rash (2%) than was reported in a larger trial that included 491 Japanese vaccinia-naive participants (16% and 1%, respectively). Possibly accounting for the differences in the 2 trials were 10 fewer skin punctures during vaccination, different vaccine lots, and a larger number of observers with potentially different levels of training and experience in that trial [7]. Among the subcategories of systemic reactogenicity, there was 1 significant difference between groups: Dryvax volunteers (6 of 28 [21%]) reported a median of 4 days of joint pain compared with a median of 1 day among LC16m8 volunteers (21 of 125 [17%]; P < .01). No meaningful differences were noted in laboratory assessments between the groups. There were no serious AEs related to vaccination. One volunteer in each vaccine group developed fever (38.4–39°C) between days 7 and 20 after vaccination. There were 3 severe AEs in LC16m8 volunteers but none were considered due to vaccine: vertigo 26 days after vaccination and diagnosed as motion sickness by an allergy specialist, a tibia fracture from a car accident (the only severe AE in the study), and tendonitis and myalgia reported 10 months after vaccination. One Dryvax volunteer had a generalized skin rash associated with face swelling and eyelid edema that started 16 days after vaccination and lasted for a total of 3 days (not biopsied, moderate severity). There were 2 cases of rhabdomyolysis after vaccination with LC16m8, both considered unrelated to vaccination, one 10 and the other 14 days after LC16m8 vaccination, assessed as moderate and mild severity, respectively. There were no cases of generalized vaccinia, progressive vaccinia, or eczema vaccinatum. One participant in the Dryvax group and 12 volunteers in the LC16m8 vaccine group reported symptoms or clinical signs that were categorized as being of potential cardiac origin. These events were confirmed by the site principal investigator to have noncardiac origins (eg, reflux disease, alcohol abuse, preexisting pleuritic chest pain, lymph node pain) and none were accompanied by ECG or laboratory findings suggesting myocardial disease. Three participants underwent cardiac assessment by a cardiologist based on a protocol algorithm. None had myocarditis or pericarditis [13], but 1 participant presented with a previously undiagnosed rare congenital QT syndrome.
Immunologic Responses
Seroconversion by Anti-Dryvax (Focus) PRNT Assay.
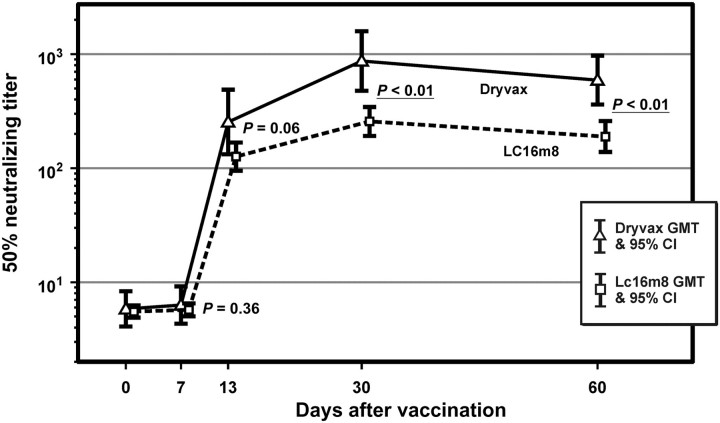
All 125 volunteers vaccinated with LC16m8 seroconverted. For those vaccinated with Dryvax, 24 of 28 (86%) seroconverted. At 30 days after vaccination, Dryvax volunteers who seroconverted exhibited a significantly higher geometric mean PRNT titer than LC16m8 volunteers who seroconverted, 919 (CI, 565–1493) vs 279 (CI, 233–333), respectively. Samples from a subset comprising 40 LC16m8 and 9 Dryvax volunteers (Figure 1) were assayed at days 0, 7, 13, 30, and 60 (Figure 2) to provide detail of the kinetics of the antibody response in both vaccine groups. The group-specific GMTs are shown in Figure 2 with the Dryvax group experiencing significantly higher PRNT50 titers at days 30 and 60.
Figure 2.
Anti-Dryvax (Focus) plaque reduction neutralization titer (PRNT) response by day and vaccine. The first 50 volunteers were enrolled (41 LC16m8 and 9 Dryvax vacinees); a subset of 4 Dryvax nonseroconverters was not included. P values at timepoints are noted to reflect level of significance for differences between groups. Abbreviations: CI, confidence interval; GMT, geometric mean titer.
Effect of Different Vaccinia Plaque Viruses on the PRNT Assay.
The comparison among different PRNT methods was based on day 30 samples in 24 Dryvax volunteers who had seroconverted and 26 LC16m8 volunteers with day 30 samples chosen in a random manner (Table 3). Dryvax volunteers exhibited a significantly higher GMT than LC16m8 volunteers when Dryvax, Japan-NYCBH, or monkeypox strains were used as the plaque viruses.
Anti-EEV PRNT Titers.
Results from the anti-EEV assay were complicated by the requirement that a 50% reduction in plaque count be achieved. This proved to be too restrictive to obtain a meaningful measurement of neutralization; in particular, only 3 of 44 samples yielded titers above the detection limit of the assay (PRNT = 4). The results based on 30% reduction in plaque count in the anti-Dryvax PRNT assay at day 30 were significantly higher in the Dryvax group than in the LC16m8 group (24 vs 4, respectively). Moreover, across this subset of 44 samples 8 of the 9 Dryvax samples exceeded the assay’s lower limit, while only 3 of the 35 LC16m8 samples exceeded the assay’s lower limit.
Anti-Variola PRNT.
At day 30 Dryvax volunteers exhibited a significantly higher antivariola PRNT response than LC16m8 volunteers; GMTs were 274 and 75, respectively (P = .02, Table 4). Although the use of crystal violet staining methodology limited the quantitative capabilities of the assay, the ratio of Dryvax GMT to LC16m8 was 3.7 (95% CI, 1.3–10.2). This was similar to the ratios of 2.8, 3.0, and 3.3 observed in the anti-Dryvax, anti-Japan-NYCBH and antimonkeypox PRNT assays, respectively.
Table 4.
Anti–Variola Virus PRNT Titers
| Dryvax | LC16m8 | Dryvax/LC16m8 ratio | ||
| (n = 9) | (n = 11) | P value | ||
| Day 0 | ||||
| GMT | 20 | 20 | 1.0 | 1.00 |
| 95% CI | … | … | … | |
| Day 30 | ||||
| GMT | 274 | 75 | 3.7 | .02 |
| 95% CI | 110–684 | 38–147 | 1.3–10.2 |
The subset was chosen from the first 50 volunteers vaccinated and was intended to have roughly equal numbers of Dryvax and LC16m8 volunteers. In order to have comparable numbers of Dryvax and LC16m8 vaccinees, all 9 Dryvax volunteers among the first 50 vaccinees were included and a randomly selected subset of 11 LC16m8 volunteers was chosen. Titers of the variola strain (Solaimen) were determined by plaque assay. Vaccinia-immune globulin was used as the positive control; serial dilutions of serum plus virus were incubated over BSC40 cell monolayers (African green monkey kidney cells, American Type Culture Collection) and allowed to develop plaques over 72 hours. Plaques were counted following crystal violet staining of cell monolayers. P value is calculated using t test on log-transformed data.
Abbreviations: CI, confidence interval; GMT, geometric mean titer.
Cellular Immune Responses
Gamma Interferon Production.
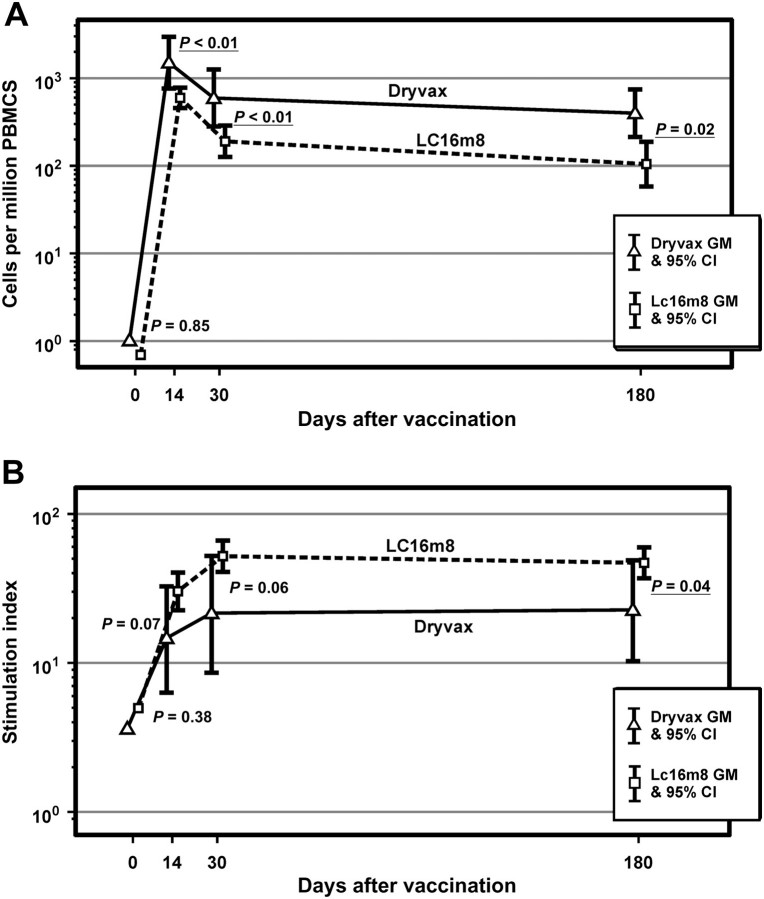
In 47 participants tested, all 9 volunteers in the Dryvax group exhibited a positive IFN-γ response at postvaccination days 13, 30, and 180 (Figure 3). Similarly, all 38 LC16m8 volunteers exhibited a positive response at day 13 and all but 1 at day 30. The proportion of positive responders within the LC16m8 group fell off slightly to 33 of 37 at day 180. At each time point, the number of IFN-γ–producing cells was approximately 2-fold higher in the Dryvax group than in the LC16m8 group. These differences were not significantly different.
Figure 3.
Comparison of interferon (IFN)–γ enzyme-linked immunosorbent spot and lymphoproliferation assay responses following vaccination with Dryvax versus LC16m8. A, Geometric mean number of IFN-γ–producing cells by vaccine and time from vaccination. B, Lymphocyte proliferation: geometric mean stimulation index by vaccine and time from vaccination. Abbreviations: CI, confidence interval; GM, geometric mean; IFN, interferon; PBMC, peripheral blood mononuclear cell.
Lymphocyte Proliferation.
Similarly, 47 participants were tested for lymphoproliferation. At day 13 a response was found in 95% of the LC16m8 group to Dryvax-derived vaccinia virus compared with 67% in the Dryvax group (P = .04). At days 30 and 180 these results were not significantly different. Specifically, 95% of LC16m8 volunteers had a positive response at day 30 compared with 78% of Dryvax volunteers; the percentages were 100% and 89%, respectively, at day 180. LC16m8 volunteers also exhibited higher SI response at each of the postvaccination time points (Figure 3).
DISCUSSION
Next-generation smallpox vaccines like LC16m8, which have never been tested in the setting of natural variola, rely on surrogate markers for evaluation of effectiveness [14]. The LC16m8 virus has a 1-base deletion within the B5R gene that results in a premature stop codon that truncates this protein and encodes for only 29% of the residue protein. B5R is a vaccinia gene product with a highly related homologue in variola virus. Deletion of the B5R results in decreased production of EEV [15], a target for EEV neutralizing antibodies [16, 17]. Loss of B5R would predict attenuation of the virus. However, the success of vaccinia-based smallpox vaccines in protecting humans against variola major lies in the ability of the vaccinia to induce strong neutralizing antibody responses across multiple viral proteins with immunological homology to variola proteins, resulting in a “safety net” of highly redundant neutralizing antibody responses [18, 19].
This phase I/II trial assessed the safety and immunogenicity of LC16m8 compared with Dryvax in vaccinia-naive adult volunteers. There were no vaccine-related serious AEs or AEs graded greater than mild associated with either vaccine; other AEs (local or systemic) were generally equivalent in both groups. LC16m8 vaccine lesions were significantly smaller with less swelling or erythema than Dryvax lesions. In comparison to recently published data from a larger study in which 1529 nonnaive and 1692 vaccinia-naive volunteers received LC16m8, the extent of AEs, both systemic and local, were quite similar. Cardiac toxicity was not apparent in our study. US military and historical studies suggest a cardiac toxicity rate of between 0.1% and 0.3% of vaccine recipients [1, 20, 21].
LC16m8 was immunogenic, although with lower humoral and cellular immunity kinetic responses than with Dryvax. The virus strain used in the PRNT assays had a significant impact on the results. In PRNT assays conducted using variola or monkeypox, Dryvax produced higher titers compared with LC16m8. Our data suggest that deletion of individual proteins, particularly B5R, results in different patterns of humoral immunity and that proteins targeted by the immune system may be different depending on the strain of vaccinia used. Our data suggest that vaccine prototypes that alter EEV production will have some attenuation of antivariola neutralization.
While the GMTs achieved by LC16m8 in the Dryvax-based PRNT assay were less than that of Dryvax at all measured time points, the kinetic profile was similar, suggesting that single-dose vaccination with LC16m8 will afford immunity in a manner paralleling that of the less attenuated vaccines. More important than absolute antibody titer is the specificity of antibody required for protection. Although the absolute immunological correlates of protection have yet to be defined, consensus has been that neutralizing antibody titers >1:40 against vaccinia are protective [22, 23]. In addition, the antivariola titers achieved with LC16m8, although semiquantitative due to variability in control samples, elicited neutralizing antibody titers that are associated with protection. However, duration of effective titer response after vaccination needs to be evaluated.
CMI was assessed in a subset of 47 participants. At day 13, 95% of LC16m8 and 67% of Dryvax vaccinees had lymphocyte proliferative responses, increasing to 97% and 89%, respectively, by day 180 (P = .04 and .34, respectively). All vaccinees produced IFN-γ peak response by day 13, although 1 LC16m8-vaccinated individual lost detectable IFN-γ response at day 30. Memory responses following vaccination with LC16m8 appear to be durable, even at 180 days. It is interesting to note the lower IFN-γ ELISPOT responses given the B5R deletion in LC16m8. The IFN-γ response difference may reflect contribution of epitopes from B5R in the overall antivaccinial T-cell repertoire after vaccination. Previous publications have shown this protein to be a source of dominant T-cell epitopes although whether these are essential for protective immunity is unclear [24, 25]. In addition, robust T-cell responses could be contributors to AEs, particularly myocarditis as shown with other viruses but not to date with vaccinia [26, 27, 28].
Our findings suggest that LC16m8 is well tolerated with similar reactogenicity compared with Dryvax. LC16m8, despite its deletion in B5R, elicited potent immune responses after single inoculation capable of neutralizing variola virus. Overall, the study demonstrated comparable safety between LC16m8 and Dryvax and immune responses that would support further development of LC16m8.
Notes
Acknowledgments.
The site clinical research teams included Beth Plummer, Ester Cook, Dana Hargis, Connie Geradeau, Nancy Johnson, Heather Vaughn, Steven Siztler, Susan Philips, Debbie Plummer, Stacy Plummer, Malissia Van Hook, and David Rudy (University of Kentucky); Sharon Irby-Moore and the staff of the Saint Louis University Center for Vaccine Development; Deborah Hunter (Vanderbilt University); Peter T. Rogge, MD (Solano Clinical Research); and Jose Montoya, Dora Ho, Eileen Cordoba Tongson, Ameth Aguirre, and Nancy Bouvier (Stanford University). J. S. K. performed the cellular immune assays with the assistance of Frank Ennis, MD, Laura Orphin, and John Cruz at the University of Massachusetts Medical School, and the authors acknowledge their support and assistance with ELISPOT and lymphoproliferation assays. VaxGen, Inc was the sponsor and created the protocol, obtained sites, and was responsible for the Investigational New Drug application and all reporting to the Food and Drug Administration. Each site had available board-certified cardiologists as well as other board-certified specialists to assist with any ocular, cutaneous, or neurological AEs; the authors appreciate their assistance.
Financial support.
This work was supported by VaxGen, Inc.
Potential conflicts of interest.
R. N. G. was a principal site investigator for clinical trials sponsored by DynPort Vaccine Company LLC, Acambis PLC, VaxGen, Inc, and Bavarian-Nordic A/S. J. S. K. received funding from VaxGen, Inc and the National Institutes of Health (NIH; AI057319) for this study. K. M. E. received research funding from the NIH, CDC, and Novartis. C. L. S. received research funding from the NIH, CDC, and Sanofi Pasteur. At the time of the trial, M. G., J. K., C. E., and M. L. were employed by VaxGen, Inc. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Greenberg RN, Kennedy JS. ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine. Expert Opin Investig Drugs. 2008;17:555–64. doi: 10.1517/13543784.17.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy JS, Greenberg RN. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev Vaccines. 2009;8:13–24. doi: 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenner J, Cameron F, Empig C, Jobes DV, Gurwith M. LC16m8: an attenuated smallpox vaccine. Vaccine. 2006;24:7009–22. doi: 10.1016/j.vaccine.2006.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meseda CA, Mayer AE, Kumar A, et al. Comparative evaluation of the immune responses and protection engendered by LC16m8 and Dryvax smallpox vaccines in a mouse model. Clin Vaccine Immunol. 2009;16:1261–71. doi: 10.1128/CVI.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashizume S Chiba Serum Institute. Special edition future of vaccination: everything about attenuated vaccines. Basics of new attenuated vaccine strain LC16m8. Clin Virus. 1975;3:229–35. [Google Scholar]
- 6.Hirayama M. In search of attenuated vaccine. Clinical special edition: vaccination in future—everything about attenuated vaccine. Clin Virus. 1975;3:269–79. [Google Scholar]
- 7.Saito T, Fujii T, Kanatani Y, et al. Clinical and immunological response to attenuated tissue-cultured smallpox vaccine LC16m8. JAMA. 2009;301:1025–33. doi: 10.1001/jama.2009.289. [DOI] [PubMed] [Google Scholar]
- 8.Newman FK, Frey SE, Blevins TP, et al. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax) vaccine. J Clin Microbiol. 2003;41:3154–7. doi: 10.1128/JCM.41.7.3154-3157.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell E, Shamim M, Whitbeck JC, Sfyroera G, Lambris JD, Isaacs SN. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology. 2004;325:425–31. doi: 10.1016/j.virol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Viner KM, Isaacs SN. Activity of vaccinia virus-neutralizing antibody in the sera of smallpox vaccinees. Microbes Infect. 2005;7:579–83. doi: 10.1016/j.micinf.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Damon IK, Davidson WB, Hughes CM, et al. Evaluation of smallpox vaccines using variola neutralization. J Gen Virol. 2009;90:1962–6. doi: 10.1099/vir.0.010553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey SE, Couch RB, Tacket CO, et al. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med. 2002;346:1265–74. doi: 10.1056/NEJMoa020534. [DOI] [PubMed] [Google Scholar]
- 13.Military Vaccine Agency, Army Medical Command, U.S. Dept of Defense. National Immunization Program, CDC. Cardiac adverse events following smallpox vaccination–United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:248–50. [Google Scholar]
- 14.Artenstein AW, Grabenstein JD. Smallpox vaccines for biodefense: need and feasibility. Expert Rev Vaccines. 2008;7:1225–37. doi: 10.1586/14760584.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelstad M, Howard ST, Smith GL. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology. 1992;188:801–10. doi: 10.1016/0042-6822(92)90535-w. [DOI] [PubMed] [Google Scholar]
- 16.Empig C, Kenner JR, Perret-Gentil M, et al. Highly attenuated smallpox vaccine protects rabbits and mice against pathogenic orthopoxvirus challenge. Vaccine. 2006;24:3686–94. doi: 10.1016/j.vaccine.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Midgley CM, Putz MM, Weber JN, Smith GL. Vaccinia virus strain NYVAC induces substantially lower and qualitatively different human antibody responses compared with strains Lister and Dryvax. J Gen Virol. 2008;89:2992–7. doi: 10.1099/vir.0.2008/004440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benhnia MR, McCausland MM, Su HP, et al. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J Virol. 2008;82:3751–68. doi: 10.1128/JVI.02244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saijo M, Ami Y, Suzaki Y, et al. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J Virol. 2006;80:5179–88. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey CG, Iskander JK, Roper MH, et al. Adverse events associated with smallpox vaccination in the United States, January–October 2003. JAMA. 2005;294:2734–43. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- 21.Morgan J, Roper MH, Sperling L, et al. Myocarditis, pericarditis, and dilated cardiomyopathy after smallpox vaccination among civilians in the United States, January–October 2003. Clin Infect Dis. 2008;46(Suppl 3):S242–50. doi: 10.1086/524747. [DOI] [PubMed] [Google Scholar]
- 22.Mack TM, Noble J, Jr, Thomas DB. A prospective study of serum antibody and protection against smallpox. Am J Trop Med Hyg. 1972;21:214–8. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar JK, Mitra AC, Mukherjee MK. The minimum protective level of antibodies in smallpox. Bull World Health Organ. 1975;52:307–11. [PMC free article] [PubMed] [Google Scholar]
- 24.Sirven P, Castelli FA, Probst A, Szely N, Maillere B. In vitro human CD4+ T cell response to the vaccinia protective antigens B5R and A33R. Mol Immunol. 2009;46:1481–7. doi: 10.1016/j.molimm.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Tang J, Murtadha M, Schnell M, Eisenlohr LC, Hooper J, Flomenberg P. Human T-cell responses to vaccinia virus envelope proteins. J Virol. 2006;80:10010–20. doi: 10.1128/JVI.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon SN, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043–53. doi: 10.1093/infdis/jiq162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinzierl AO, Rudolf D, Maurer D, et al. Identification of HLA-A*01- and HLA-A*02-restricted CD8+ T-cell epitopes shared among group B enteroviruses. J Gen Virol. 2008;89:2090–7. doi: 10.1099/vir.0.2008/000711-0. [DOI] [PubMed] [Google Scholar]
- 28.Yue Y, Gui J, Xu W, Xiong S. Gene therapy with CCL2 (MCP-1) mutant protects CVB3-induced myocarditis by compromising Th1 polarization. Mol Immunol. 2011;48:706–13. doi: 10.1016/j.molimm.2010.11.018. [DOI] [PubMed] [Google Scholar]