Abstract
Background. The baboon (Papio hamadryas anubis) can be transcervically instrumented, facilitating studies of intrauterine contraception and reproductive tract infection. We sought to determine if the baboon could become infected with a single cervical inoculation of Chlamydia trachomatis.
Methods. Ten female baboons were randomized and inoculated cervically with C. trachomatis serovar E (or buffer alone). Animals underwent weekly clinical and laparoscopic evaluations for four weeks and at post-inoculation week 8, to monitor upper tract infection. Cervical culture and nucleic acid amplification testing (NAAT) were completed weekly throughout the study. Animals were euthanized at week 16 and the reproductive tracts were examined histologically.
Results. All inoculated animals developed cervical infection. The average duration of positive NAAT results was 6.8 weeks (range 2–16). Two of eight (25%) animals tested positive from fallopian tube samples. Infected animals showed histological findings consistent with chlamydial infection, such as germinal centers. Five of ten animals seroconverted to C. trachomatis.
Conclusions. Baboons cervically inoculated once with C. trachomatis develop infection similar to humans, with a low incidence of upper tract infection. This novel model of Chlamydia infection closely resembles human disease and opens new avenues for studying the pathogenesis of sexually transmitted infections and contraceptive safety.
Pelvic inflammatory disease (PID) describes an infectious disease of the upper female reproductive tract that affects nearly 800000 women annually in the United States [1]. It is an important cause of infertility, ectopic pregnancy, chronic pelvic pain, and tuboovarian abscess [1]. Chlamydia trachomatis is the most common sexually transmitted infection (STI) in the United States and is one of the most common pathogens associated with PID [2]. A better understanding of the mechanisms whereby bacterial pathogens cause PID and its sequelae is necessary to develop novel preventive and therapeutic measures.
Concerns regarding an increased risk for PID have been associated with intrauterine contraception (IUC), limiting the application of this form of contraception in the United States [3]. Investigating the association between IUC and PID in humans is difficult due to an inability to control for confounders, difficulty in defining PID, lack of (or inappropriate) comparison groups, and the inability to follow infection prospectively [3–5]. The use of an animal model might allow a systematic evaluation of PID and IUC use. Baboons have been previously used in IUC trials [6, 7]. However, while the male baboon is susceptible to infection with C. trachomatis [8], this has not been studied in the female reproductive tract.
The objective of this study was to establish a baboon (Papio hamadryas anubis) PID model using C. trachomatis. Other than the great apes, the baboon is most similar to Homo sapiens in reproductive tract anatomy, physiology, and biochemistry, including hormonal fluctuations and cycling, and has a rectilinear cervical canal that allows for ease of transcervical procedures [9, 10].
Establishing the infective dose, clinical features, and pathological effects of C. trachomatis are key first steps in establishing the use of this animal model for future use in the study of PID. Herein, we tested the hypothesis that baboons undergoing a single cervical inoculation of C. trachomatis will develop reproductive tract infection analogous to that observed in humans.
METHODS
Animals and Study Site
In vivo studies took place at the Institute of Primate Research (IPR) in Nairobi, Kenya. Ten wild-caught sexually mature female olive baboons (P. hamadryas anubis) were used. Animals underwent a 3-month quarantine and conditioning period entailing physical examination, treatment for ecto- and endoparasitic infection, and screening for significant zoonotic infections, specifically Mycobacterium tuberculosis and simian immunodeficiency virus. Additionally, animals were grossly and laparoscopically screened for evidence of reproductive tract pathology (ie, endometriosis). Animals completing quarantine and lacking evidence of reproductive disease were used.
Animals were individually housed to avoid cross-contamination. Visual contact with conspecifics and food enrichment items were provided. Behavior, food and water intake, and physical well-being were visually monitored and recorded daily. A clinical pain scale was developed by the study veterinarians (I. L. B., D. C.) comprising features including inappetence, lethargy, and abnormal postures and was incorporated into daily observations. Cyclic changes in perivulvar/perianal skin tumescence (“sex skin”) and menses were recorded, but infection was not synchronized across animals with respect to menstrual cycle. This study was granted an off-site exemption by the University of Michigan Committee on Use and Care of Animals (UCUCA) and was approved by the Institutional Review Committee (IRC) at IPR. The IRC at IPR adheres to humane animal use principles as guided by the Primate Vaccine Evaluation Network (PVEN), the Council for the International Organizations of Medical Sciences (a World Health Organization working group), and the National Institutes of Health Public Health Service policies.
Study Design and PID Criteria
Ten wild-caught olive baboons (P. hamadryas anubis), 8 experimental and 2 control (uninfected), underwent the 16-week study. All animals underwent baseline laparoscopy and collection of serum and cervical swabs for C. trachomatis culture and nucleic acid amplification tests (NAAT). The experimental animals received a single cervical inoculation of C. trachomatis. Laparoscopy was repeated weekly for the first 4 weeks post-inoculation and then again at 8 weeks post-inoculation. Cervical swabs for culture and NAAT were obtained weekly for the 16-week study duration. At 16 weeks post-inoculation, animals were euthanized and fresh and fixed tissues obtained for culture, NAAT, and histology. Human diagnostic criteria for PID [1] were adapted to the baboon (Table 1). These criteria included gross or histological evidence of inflammation in the upper reproductive tract and organism detection by culture or polymerase chain reaction (PCR) in the upper or lower reproductive tract.
Table 1.
Clinical Symptoms and Diagnostic Criteria for PID
| Minor criteria |
| Positive cervical culture for C. trachomatis on 2 or more successive samples (weekly intervals) |
| Positive cervical NAAT for C. trachomatis on 2 or more successive samples (weekly intervals) |
| Major criteria |
| Positive NAAT for C. trachomatis from fallopian tube |
| Histopathologically evident salpingitis |
| Laparoscopic or necropsy evidence of thickened, fluid filled fallopian tubes or gross inflammation/adhesions |
| Definitions |
| PID—2 major criteria from above |
| Suggestive PID—1 major criterion and 2 minor criteria. |
| Lower tract infection—1 or 2 minor criteria |
Abbreviations: NAAT, nucleic acid amplification tests; PID, pelvic inflammatory disease.
C. trachomatis Inoculum
Genital strain of C. trachomatis serovar E, a clinical isolate obtained from a patient with PID, was prepared at the University of Washington Chlamydia Laboratory in a McCoy cell culture and then purified. The inocula, containing 107 inclusion forming units/milliliter (IFU/mL), were then aliquoted in sucrose-phosphate-glutamate (SPG) buffer and frozen at −70°C. The prepared aliquots were shipped on dry ice to IPR.
Cervical Inoculation
Two animals were randomly assigned to the control group, and 8 were assigned to the experimental inoculation group. Baseline cervical examination, laparoscopic evaluation, cervical cultures, and collection of NAAT samples were performed on all 10 animals. After baseline evaluation and sample collection, the experimental animals were inoculated with 1 × 107 IFU/mL of serovar E C. trachomatis administered by pipette directly onto the ectocervix during speculum exam. Control animals were inoculated in the same way with an equal volume of SPG solution.
Monitoring: Cervical Evaluation and Laparoscopy
Cervical examination was performed weekly throughout the 16 weeks of the study. The presence and appearance of vaginal discharge or erythema were recorded. Laparoscopy was performed at baseline and repeated weekly for 4 weeks after inoculation and again at 8 weeks post-inoculation. Each animal was sedated with an intramuscular mixture of 10% xylazine (Rompun®, Bayer) and 10% ketamine (Rotex medica GMBH Tritau-Germany) in the ratio of 10 cc of ketamine to 0.5 cc of xylazine. The mixture was then given at 0.1 cc/kg body weight. Following intubation, anesthesia was maintained on inhalent anesthesia (Halothane) using a semiclosed rebreathing circuit, and surgery was performed with the animal in dorsal recumbency. As previously described, 1 umbilical port and 2 accessory ports were inserted during laparoscopy [11]. Using a blunt probe, scored at 1-mm increments, the diameters of the fallopian tubes were measured. The accessory ports were also used to allow passage of the NAAT swab so that the right and left fimbria could be swabbed. At the completion of the surgery, the animals were extubated and returned to their cages after postoperative observation to ensure smooth recovery from anesthesia.
Specimen Collections
Cervical swabs for culture and NAAT were obtained weekly throughout the 16 weeks of the study. Additionally, at laparoscopy (weeks 1, 2, 3, 4, 8), swabs were collected from the fimbrial os for NAAT. At the study endpoint, the animals were sedated with 10 mg/kg intramuscular injection of ketamine (Rotex medica, GMBH Tritau) followed by humane euthanasia via intravenous injection of 60-mg/kg-sodium pentobarbital (Euthanaze, CENTAUR LABSa). Control animals were examined first, followed by experimental animals to avoid cross-contamination of equipment or working surfaces. The reproductive tract was removed en bloc and representative sections of vagina, ectocervix, endocervix, uterus, tubes, and ovaries were collected and fixed in 10% neutral buffered formalin. Remaining visceral organs were examined grossly and representative samples of any gross lesion were also collected and fixed. After 48 hours of fixation, tissues were processed to paraffin blocks by standard histological methods.
Shipping and Permits
A Kenyan export permit (CITES) and permits for import of fixed and unfixed baboon tissues were obtained from the US Department of Agriculture and Centers for Disease Control. A commercial carrier was used to transport the samples from IPR to the University of Michigan. Nonfixed, frozen samples (ie, sera, NAATs, cultures) were shipped on dry ice with constant monitoring and resupplying dry ice as needed. Cold chain was maintained from collection until receipt at the University of Michigan, at which point samples were stored at −70°C.
Histological Evaluation
Paraffin blocks were sectioned at 5-μm thickness, and stained with hematoxylin and eosin for light microscopic evaluation. Histological evaluation was performed by a board-certified veterinary pathologist (I. L. B.) blinded to the experimental groups. Sections of vagina, ectocervix, endocervix, uterus, left and right fallopian tube, and left and right ovary were evaluated. The cervix and fallopian tubes were scored with respect to inflammation using the following criteria: 0: no inflammation, 1: mild; few small scattered inflammatory aggregates without discrete or organized nodules, 2: moderate; multifocal discrete inflammatory aggregates evident at low magnification, 3: severe; large multifocal to coalescing inflammatory aggregates, often with lymphofollicular organization, 4: marked; same features as category 3 but with coalescing to diffuse distribution. Inflammatory aggregates were descriptively characterized as to cell type including lymphocytes, plasma cells, neutrophils, and macrophages.
C. trachomatis Detection: Nucleic Acid Amplification
Cervical swab specimens were collected with the APTIMA Swab Specimen Collection Kit, stored, and transported to the UW laboratory for processing. Specimens were tested by the GenProbe APTIMA Combo 2 (GenProbe) [12] according to the manufacturer’s instructions. The APTIMA assay employs transcription-mediated amplification, in which the RNA target molecule from C. trachomatis (23S rRNA) is isolated, and specific regions are amplified by using a separate capture oligomer and a unique set of primers for the target [13].
C. trachomatis Detection: Culture
Cervical swabs were collected and placed into a viral transport media (M4RT; Remel Products) and then maintained at −70°C. Chlamydia culture was performed in dram vials containing Buffalo Green Monkey Kidney Cells (BGMK) (Diagnostic Hybrids) following a modification of Krech [14, 15]. Briefly, after aspiration of maintenance medium, duplicate BGMK vials were inoculated with 200 μL of vortexed specimen. The vials were then centrifuged at 1500 × g for 60 minutes at 35°C. After centrifugation, the vials were fed with chlamydial isolation medium containing cycloheximide. The vials were incubated for 48–72 hours at 35°C. After incubation, the cells were fixed and stained with fluorescein isothiocyanate-labeled monoclonal antibody (MicroTrak; Trinity Biotek) and read using a fluorescence microscope at 400× magnification.
Serology: Determination of IgG/IgA Antibody
Qualitative detection of C. trachomatis-specific immunoglobulin G (IgG)/immunoglobulin A (IgA) was determined from serum samples using a commercially available enzyme-linked immunosorbent assay (ELISA) (C. trachomatis-pELISA, Medac). This assay uses a synthetic peptide from the immunodominant region of the major outer membrane protein. The test was performed according to manufacturer’s instructions. Optical density (OD) values were read at 450 nm (reference 620–650 nm) and cutoff values determined according to manufacturer’s specifications. A confirmatory micro-immunoflourescent (microIF) assay was performed according to a previously published protocol [16] to validate the ELISA results.
RESULTS
Clinical, Gross, and Laparoscopic Observations
Appetite, behavior, and postures remained normal throughout the observation period. Yellow or malodorous vaginal discharge was noted in 4 of 8 inoculated animals 3 weeks post-inoculation and continued for a mean of 4.5 weeks (range 1–12). Vaginal erythema was measured as mild, moderate, severe, and/or ulcerated. All inoculated animals demonstrated some degree of erythema. In total, 7 (87.5%) of 8 animals demonstrated moderate to severe erythema and 2 (25%) of 8 demonstrated ulcerations of the vaginal epithelium. Laparoscopic evidence of infection was defined by tubal dilatation, erythema, and the presence and degree of fibrous adhesions; 4 (50%) of 8 inoculated animals had tubal enlargement (> 2 mm of change from baseline) and erythema. No animals had adhesions. Neither control animal had upper or lower tract abnormal gross or laparoscopic findings.
Evidence of C. trachomatis Infection
No animals showed gross, microbiological, nucleic acid, or serological evidence of chlamydial infection prior to inoculation. All inoculated animals had evidence of chlamydial infection by at least one of these parameters at some time point during the study (Table 2).
Table 2.
Nucleic Acid, Culture, and Serology Results
| Testing type and animal number | Week of observation |
|||||||||||||||||
| 0a | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Cervical NAAT/tubal NAAT | S1 | −/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| S2 | −/− | +/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | +/− | −/− | −/− | +/− | −/− | −/− | |
| S3 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | +/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | +/− | −/− | |
| S4 | −/− | −/−− | +/− | −/− | −/− | +/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| S5 | −/− | +/− | +/+ | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | −/− | −/− | −/− | −/− | −/+ | |
| S6 | −/− | +/− | −/− | +/− | +/− | −/− | −/− | −/− | −/− | −/− | +/− | +/− | −/− | −/− | −/− | −/− | −/− | |
| S7 | −/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | +/+ | |
| S8 | −/− | +/− | +/− | −/− | +/− | +/− | +/− | +/− | −/− | −/− | −/− | −/− | +/− | −/− | −/− | +/− | −/− | |
| Cervical cultureb | S1 | −− | − | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − |
| S5 | − | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | |
| S7 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | |
| S8 | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| IgG/IgAc | S1 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | +/− | −/− | +/− | +/− | +/− | +/− |
| S3 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | +/− | |
| S5 | −/− | −/− | −/− | −/− | −/− | +/− | +/− | +/− | +/− | +/− | +/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| S7 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | +/− | +/− | +/− | +/− | +/− | +/− | +/− | |
| S8 | −/− | −/− | −/− | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | −/− | −/− | −/− | −/− | −/− | −/− | |
Animals S9 and S10 were control animals and had negative results.
Abbreviations: IgG/IgA, immunoglobulin G/A; NAAT, nucleic acid amplification tests.
Represents testing done prior to first inoculation.
Animals S2–S4 and S6 did not have a positive cervical culture result.
Animals S2, S4, and S6 did not seroconvert.
Laboratory Tests
Nucleic Acid Amplification.
Cervical samples from each of the inoculated animals were positive for C. trachomatis by NAAT assay on at least one time point during the study. In sum, 6 (75%) of 8 animals were cervical positive one week after inoculation (Table 2). The average duration of positive NAAT detection was 6.8 weeks (range 2–16). Two of eight (25%) animals (study numbers S5 and S7) tested positive from the fallopian tubes, one (S5) at weeks 2 and 16 and the other (S7) at week 16. Each control animal had one cervical NAAT swab test positive for C. trachomatis at weeks 2 and 5, respectively. However, when these samples were tested with an alternate PCR based assay, Abbott RealTime CT/NG (Abbott) and with standard chlamydia culture, neither tested positive. Thus, the unexpected occurrence of NAAT tube positivity was attributed to nucleic acid contamination (a recognized complication of C. trachomatis NAAT testing) [17, 18], and it was concluded that the controls were negative for C. trachomatis.
C. trachomatis Culture.
Of the 8 inoculated animals, 4 (50%) tested positive for chlamydial infection by cervical culture. For these 4 animals, the average duration of infection was 7 weeks (range 1–15). Neither of the control animals were culture positive for C. trachomatis at any time point.
Serology.
Serum anti-C. trachomatis IgG were positive in 5 (62.5%) of 8 inoculated animals. However, serological conversion did not correlate with either gross lesions or histological severity of disease and were present in both the most severely infected animals (S5 and S7) and in animals that lacked tubal inflammation (S1 and S8). Control animals did not seroconvert. MicroIF results confirmed the results seen in all animals with the exception of animal S3. This animal had a positive ELISA result but negative microIF result.
Evidence for Pelvic Inflammatory Disease
All inoculated animals were positive for C. trachomatis by 1 or more criterion at 1 or more time point (Table 2). Among the 8 animals receiving a single cervical inoculation with C. trachomatis, 1 animal (S5) met the diagnostic criteria (Table 1) defined a priori for PID (Table 3). One animal (S7) was suggestive for PID with positive tubal NAAT and a positive cervical culture and NAAT but lacked gross or histological evidence of disease. The remaining 6 animals were considered infected, without evidence of PID. This included animal S2 having only mild tubal histological changes and 1 minor criterion for PID (positive cervical NAAT).
Table 3.
Classification of Chlamydial Infections
| Minor criteria |
Major criteria |
||||
| Cervical NAAT | Culture | Tubal NAAT | Tubal enlargementa | Histology | |
| PID | |||||
| S5 | + | + | + | + | + |
| Suggestive PID | |||||
| S7 | + | + | + | − | − |
| Lower tract infection | |||||
| S2 | + | − | − | − | + |
| S3 | + | − | − | − | − |
| S4 | + | − | − | + | − |
| S1 | + | + | − | − | − |
| S8 | + | + | − | − | − |
| S6 | + | − | − | − | − |
Controls (S9, S10) demonstrated no evidence of C. trachomatis infection.
Abbreviations: NAAT, nucleic acid amplification tests; PID, pelvic inflammatory disease.
Tubal enlargement was defined as ≥2 mm of change from baseline laparoscopic measurements.
Fallopian Tube Histology
Of the 8 inoculated animals, 1 (S5) had high inflammation (scores 3 and 4) bilaterally in the fallopian tubes. One animal (S2) had very mild focal inflammation (score 1) unilaterally in the left fallopian tube. The remaining 6 inoculated animals and the 2 controls lacked histologically evident fallopian tube inflammation (score 0).
In the more severely affected animal (S5), tubal inflammation consisted of densely packed, mature mononuclear cells expanding the tubal propria (Figure 1) without significant neutrophils. These cells morphologically appeared as lymphocytes with fewer macrophages. This animal had regionally extensive distribution in both tubes, while the less affected animal (S2) had less dense, focal mononuclear aggregates in the propria of only 1 tube (Figure 1). This animal also had scant lymphocytic inflammation within the tubal wall (Figure 1). The fallopian tube histological findings of animal S5 correlated well with laparoscopic observations of tubal dilatation. However, the mild histological changes noted in animal S2 did not correlate with laparoscopy. Additionally, S3 and S4 had dilated tubes noted by laparoscopy but no histological evidence of inflammation.
Figure 1.
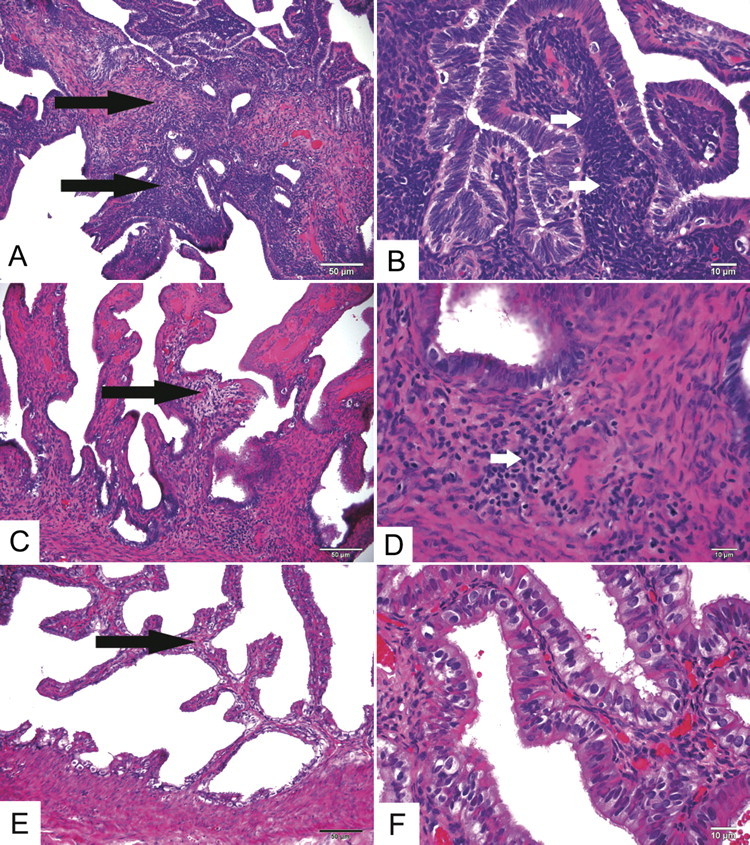
Representative fallopian tube C. trachomatis-associated histological findings at low (A, C, E) and high (B, D, E) magnification. In animal S5 (A and B), which met disease criteria for PID, the tubal propria (interstitium) was significantly and extensively thickened (long arrows) by numerous mononuclear cells, predominantly lymphocytes (short arrows). In animal S2 (C and D), which had 2 minor criteria consistent with infection but was not classified as PID, there was mild, localized expansion of the propria (long arrows) and scant lymphocytic inflammation in the propria and tubal wall (short arrow). In control animal S9 (E and F), the tubal propria was thin (long arrow) and lacked significant infiltrating inflammatory cells. Hematoxylin and eosin. Original magnifications panels A, C, E: 200×. Panels B, D, F: 600×.
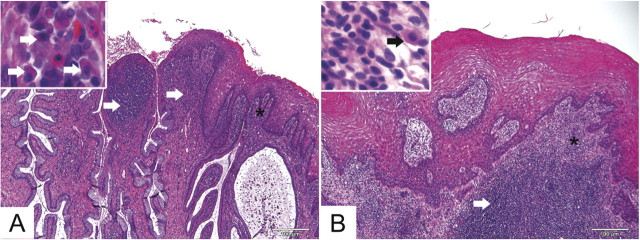
In contrast to the tubal findings, vaginal and/or cervical inflammation were not reflective of chlamydial infection status or of laparoscopic findings. All animals, including the 2 controls, had mild (score 1) to marked (score 4) ectocervical and/or vaginal inflammation. Inflammation consisted of aggregates of lymphocytes with fewer plasma cells within the vaginal and cervical submucosa. In animals with the most severe inflammation, there was frequent formation of submucosal lymphofollicular structures with germinal centers (Figure 2). One control and one inoculated animal demonstrated cervical epithelial dysplasia near the squamocolumnar junction. Basal cell hyperplasia, consisting of thickening and irregularity of the basal epithelial layer was present in both controls and in 5 (62.5%) of 8 inoculated animals.
Figure 2.
Representative cervical and vaginal histological findings in C. trachomatis colonized (animal S7; A) and uninfected control (animal S10; B). Animal S7 (A), which had positive NAAT findings for 16 weeks, had robust lymphocytic and plasmacytic submucosal inflammation at the cervical squamocolumnar junction (long arrows). Inset shows plasma cells (short arrows) from area with asterisk. There was also extensive lymphocytic and plasmacytic submucosal inflammation in a control animal (S10; B) in the proximal vagina adjacent to the cervix (arrow). Inset shows plasma cell (short arrow) in area with asterisk. Hematoxylin and eosin. Original magnifications 100×.
DISCUSSION
This study established that the female baboon was highly susceptible to cervical infection with C. trachomatis after a single cervical inoculation and demonstrated that clinical disease was similar to that seen in women [1, 19]. Gross and histological findings were similar to those described in previous nonhuman primate models and demonstrated that in a minority of animals challenged in the cervix, the organism was able to ascend to the upper tract [20, 21].
The results of a single cervical inoculation were diverse with respect to disease occurrence in the baboon model, with some animals developing moderate-to-severe disease and others showing mild infection. This is consistent with human infection, where exposure to STI-causing pathogens leads to different outcomes. Duration of infection, as determined by PCR or culture, was variable among animals. Culture positivity was noted from the cervix for up to 15 weeks in 4 of the experimental animals (mean duration of 7 weeks). This was shorter than the mean duration of 9.3 weeks of culture positivity observed in the pigtailed macaque model [22]. However, in that study, no positive tubal NAAT for C. trachomatis or evidence of upper tract infection were documented. Two animals in our study (S5 and S7) had a positive tubal NAAT despite a shorter duration of infection as determined by cervical NAAT. One of these animals (S5) had clinical evidence, both laboratory and histological, that correlated with a diagnosis of PID.
The findings in this small study are encouraging for our disease model, but several limitations deserve attention. First, all of the animals enrolled were wild caught and although each had undergone a 3-month quarantine period, it is possible that some animals had subclinical infections (not due to C. trachomatis) that were not detected during the quarantine. Second, conducting the study in Kenya required that the samples be frozen and shipped to the University of Michigan. Logistical considerations to maintain the cold chain both in Kenya (preshipment) and in transit were daunting yet surmountable. Freezing the C. trachomatis culture samples may have reduced the sensitivity of routine cultures. To minimize this potential, samples were placed in an ice bath immediately after being obtained and then placed in a −70°C freezer until transport. Furthermore, the culture was performed with the first freeze-thaw cycle. Third, we were surprised that not all of the animals seroconverted after inoculation and were concerned that it could have been associated with ELISA kit we used and its use in baboons. However, we were encouraged that the ELISA results corresponded with the microIF results.
Lastly, the histological findings were likely impacted by the wild source of animals. The baboons in this study, including those with histological changes and confirmed infection, showed no inappetence, abnormal postures, or other clinical signs suggestive of abdominal discomfort. This is not inconsistent, however with C. trachomatis infection in women, which can vary widely in its clinical manifestations [1]. Fertility was not evaluated in this study. Fallopian tube blockage was not histologically or grossly evident in animal S5 with the most significant upper tract inflammation. This too, is consistent with C. trachomatis infection in humans, which can range from inapparent or minimally symptomatic to complete blockage of the fallopian tube by fibrosis or inflammation [23]. The lack of inflammation in the animal with possible PID (S7) might be individual animal variation or may reflect sampling limitations, as histological lesions in mild disease may be focal. The relatively short duration of infection evaluated in this study and the use of single rather than multiple inoculations may have played a role in the relatively mild disease manifestations.
Although upper tract histological findings were consistent with serial laparoscopic observations in the single severely affected animal, the lower tract was more difficult to evaluate. The occurrence of cervicovaginal inflammation in both control and experimental baboons was a confounding variable in this study. Cervical lymphocytic and plasmacytic inflammation, particularly with germinal center formation has been considered characteristic of C. trachomatis infection in humans [24]. In nonhuman primates, this association may not be as robust, since spontaneous lymphoplasmacytic inflammation with or without germinal centers has been reported as a background finding in captive macaques [25]. Since epithelial dysplasia and koilocytes were observed in some animals, we screened for papillomaviruses by PCR and were able to identify novel papillomavirus types by PCR in cervical tissue from 6 animals (manuscript in preparation). Cervical samples were also screened by PCR for Papaiine herpesvirus 2 (Herpesvirus papio 2), a baboon-specific alphaherpesvirus associated with genital inflammation and fibrosis [26]. P. herpesvirus 2 was not identified in any animal, nor were herpesviral inclusions evident histologically. Since papillomavirus affects the lower genital tract, the unexpected finding of this concurrent disease does not negate our conclusion of C. trachomatis-induced upper tract disease in 2 of the 8 animals. Future studies in the baboon will examine the impact of repeated cervical inoculations on the development of PID in the baboon to enhance the usefulness of this model to evaluate intrauterine contraception and STIs.
In summary, these results establish the use of the baboon model for C. trachomatis-induced PID. Due to the low incidence of upper tract disease using the present inoculation protocol, it is possible that multiple inoculations will be necessary to establish upper tract infection with predictability. Multiple inoculations have been previously used in macaque models of PID [21]. Future studies in the baboon will examine the impact of repeated cervical inoculations on the development of PID in the baboon to enhance the usefulness of this model to evaluate intrauterine contraception and STIs.
Notes
Acknowledgments.
We would like to thank the University of Washington Chlamydia Laboratory for their knowledge and expertise, the University of Michigan Pathology Cores for Animal Research for histological assistance, and the laboratory and animal care staff at IPR for their expertise in baboon research. Thank you to Courtland Kouassiaman, Fatima Jibril, and Paige Fairchild for their assistance with data management.
Financial support.
This work was supported by the Fellowship in Family Planning. The first author (J. B.) is funded by the National Institutes of Health (K12 HD065257), and D. M. A. was supported by a National Institutes of Health grant (HD057176).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Soper DE. Pelvic inflammatory disease. Obstet Gynecol. 2010;116:419–28. doi: 10.1097/AOG.0b013e3181e92c54. [DOI] [PubMed] [Google Scholar]
- 2. CDC. Sexually Transmitted Diseases (STDs); Chlamydia CDC Fact Sheet, 2011. Atlanta, GA: Centers for Disease Control and Prevention, 2011.
- 3.Meirik O. Intrauterine devices—upper and lower genital tract infections. Contraception. 2007;75:S41–7. doi: 10.1016/j.contraception.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet. 2000;356:1013–9. doi: 10.1016/S0140-6736(00)02699-4. [DOI] [PubMed] [Google Scholar]
- 5.Mohllajee AP, Curtis KM, Peterson HB. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception. 2006;73:145–53. doi: 10.1016/j.contraception.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Breed WG, Butt WR, Eckstein P, Stephens JM, Peplow PV. Effect of an intrauterine device on menstrual cyclicity and luteal function in baboon. J Reprod Fertil. 1972;28:249–57. doi: 10.1530/jrf.0.0280249. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler AG, Hurst PR, Poyser NL, Eckstein P. Uterine histology and prostaglandin concentrations and utero-ovarian venous steroid and prostaglandin concentrations during the luteal phase of the menstrual cycle in baboons (Papio spp.) with or without and IUD. J Reprod Fertil. 1983;67:35–46. doi: 10.1530/jrf.0.0670035. [DOI] [PubMed] [Google Scholar]
- 8.Digiacomo RF, Gale JL, Wang SP, Kiviat MD. Chlamydial infection of the male baboon urethra. Br J Vener Dis. 1975;51:310–3. doi: 10.1136/sti.51.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Hooghe T, Kyama C, Chai DC, et al. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16:152–61. doi: 10.1177/1933719108322430. [DOI] [PubMed] [Google Scholar]
- 10.Chai D, Cuneo S, Falconer H, Mwenda JM, D'Hooghe T. Olive baboon (Papio anubis anubis) as a model for intrauterine research. J Med Primatol. 2007;36:365–9. doi: 10.1111/j.1600-0684.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 11.D'Hooghe TM, Bambra CS, Cornillie FJ, Isahakia M, Koninckz PR. Prevalence and laparascopic appearance of spantaneous endometriosis in the baboon (Papio anubis, Paio cynocephalus) Biol Reprod. 1991;45:411–6. doi: 10.1095/biolreprod45.3.411. [DOI] [PubMed] [Google Scholar]
- 12.Gaydos CA, Quinn TC, Willis D, et al. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J Clin Microbiol. 2003;41:304–9. doi: 10.1128/JCM.41.1.304-309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patton DL, Cosgrove Sweeney YT, Paul KJ. A summary of preclinical topical microbicide vaginal safety and chlamydial efficacy evaluations in a pigtailed macaque model. Sex Transm Dis. 2008;35:889–97. doi: 10.1097/OLQ.0b013e31817dfdb8. [DOI] [PubMed] [Google Scholar]
- 14.Johnston SL, Siegel C. Comparison of Buffalo green monkey kidney cells and McCoy cells for the isolation of Chlamydia trachomatis in shell vial centrifugation culture. Diagn Microbiol Infect Dis. 1992;15:355–7. doi: 10.1016/0732-8893(92)90023-m. [DOI] [PubMed] [Google Scholar]
- 15.Krech T, Bleckmann M, Paatz R. Comparison of buffalo green monkey cells and McCoy cells for isolation of Chlamydia trachomatis in a microtiter system. J Clin Microbiol. 1989;27:2364–5. doi: 10.1128/jcm.27.10.2364-2365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patton DL, Askienazy-Elbhar M, Henry-Suchet J, et al. Detection of Chlamydia trachomatis in fallopian tube tissue in women with postinfectious tubal infertility. Am J Obstet Gynecol. 1994;171:95–101. doi: 10.1016/s0002-9378(94)70084-2. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro DS. Quality control in nucleic acid amplification methods: use of elementary probability theory. J Clin Microbiol. 1999;37:848–51. doi: 10.1128/jcm.37.3.848-851.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward M. Molecular diagnosis of chlamydial infections—Nucleic acid amplification based tests. http://www.chlamydiae.com/restricted/docs/labtests/diag_nucleicacidamplification.asp. Accessed 28 January 2011.
- 19.US Department of Health and Human Services. Sexually transmitted disease surveillance, 1999. Atlanta, GA: Centers for Disease Control and Prevention; 2000. [Google Scholar]
- 20.Moller B, Mardh PA. Experimental salpingitis in grivet monkeys by Chlamydia trachomatis spread of infection to the fallopian tubes. Acta Pathol Microbiol Scand B. 1980;88:107–14. [PubMed] [Google Scholar]
- 21.Patton D, Kuo C-C. Histopathology of Chlamydia trachomatis salpingitis after primary and repeated reinfections in the monkey subcutaneous pocket model. J Reprod Fertil. 1989;85:647–56. doi: 10.1530/jrf.0.0850647. [DOI] [PubMed] [Google Scholar]
- 22.Wolner-Hanssen P, Patton DL, Holmes KK. Protective immunity in pig-tailed macaques after cervical infection with Chlamydia trachomatis. Sex Transm Dis. 1991;18:21–5. doi: 10.1097/00007435-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis. 2010;201(Suppl 2):S114–25. doi: 10.1086/652397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiviat NB, Peterson M, Kinney-Thomas E, Tam M, Stamm WE, Holmes KK. Cytologic manifestations of cervical and vaginal infections. II. Confirmation of Chlamydia trachomatis infection by direct immunofluorescence using monoclonal antibodies. JAMA. 1985;253:997–1000. doi: 10.1001/jama.253.7.997. [DOI] [PubMed] [Google Scholar]
- 25.Cline JM, Wood CE, Vidal JD, et al. Selected background findings and interpretation of common lesions in the female reproductive system in macaques. Toxicol Pathol. 2008;36:142S–163S. doi: 10.1177/0192623308327117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberle R, Microbiology of captive baboons . In: The baboon in biomedical research. VandeBerg JL, Williams-Blangero S, Tardif SD, editors. Chicago: Springer; 2009. pp. 11–138. [Google Scholar]