Abstract
Background. Failure to normalize CD4+ T-cell numbers despite effective antiretroviral therapy is an important problem in human immunodeficiency virus (HIV) infection.
Methods. To evaluate potential determinants of immune failure in this setting, we performed a comprehensive immunophenotypic characterization of patients with immune failure despite HIV suppression, persons who experienced CD4+ T-cell restoration with therapy, and healthy controls.
Results. Profound depletion of all CD4+ T-cell maturation subsets and depletion of naive CD8+ T cells was found in immune failure, implying failure of T-cell production/expansion. In immune failure, both CD4+ and CD8+ cells were activated but only memory CD4+ cells were cycling at increased frequency. This may be the consequence of inflammation induced by in vivo exposure to microbial products, as soluble levels of the endotoxin receptor CD14+ and interleukin 6 were elevated in immune failure. In multivariate analyses, naive T-cell depletion, phenotypic activation (CD38+ and HLA-DR expression), cycling of memory CD4+ T cells, and levels of soluble CD14 (sCD14) distinguished immune failure from immune success, even when adjusted for CD4+ T-cell nadir, age at treatment initiation, and other clinical indices.
Conclusions. Immune activation that appears related to exposure to microbial elements distinguishes immune failure from immune success in treated HIV infection.
The introduction of combination antiretroviral therapies has changed the landscape of human immunodeficiency virus (HIV) treatment. With sustained suppression of HIV replication, immune function improves and morbidity and mortality decrease dramatically [1]. Though survival has improved, it is not comparable to that of healthy uninfected subjects [2], and treated persons remain at increased risk for serious cardiovascular, malignant, and hepatic diseases [1, 3], especially among those with low CD4+ T-cell counts [3, 4]. Despite effective control of HIV replication, a substantial minority of persons receiving HIV care fail to increase CD4+ T-cell counts to “normal” levels [5, 6]. Immune failure in the setting of treatment-induced suppression of HIV replication has been studied extensively, but these studies have largely been either epidemiologic with no [5, 7, 8] or limited immunologic analyses [9], or small studies [10–17] often lacking normative data from healthy controls [11–15, 18] and/or performed among persons with either limited or unknown durations of virologic suppression [9, 11–14, 16, 17, 19–21]. To better define “immune failure” despite virologic control, we initiated a comprehensive evaluation of immune failure despite drug-induced suppression of HIV replication. As reported by us and by others in smaller studies, immune failure was associated with increased T-cell activation [6, 9, 14, 17], here defined as coexpression of CD38+ and HLA-DR on CD4+ and CD8+ T cells, and by profound decreases in circulating naive CD4+ and CD8+ T cells [6, 16, 17]. We also show that immune failure is linked to indices of microbial translocation and inflammation, as levels of the soluble receptor for LPS (sCD14+) and levels of interleukin 6 are significantly elevated. In addition, we find that in immune failure, activation of CD4+ T cells is accompanied by increased cell cycling, especially in the memory compartment, while among CD8+ T cells that are even more frequently activated, cycling is not increased above levels seen in healthy controls or in treated patients who have “normalized” circulating CD4+ T cells. These in vivo findings are strikingly similar to the immune phenotype induced by exposure to microbial Toll-like receptor ligands in vitro [22], providing an important link between exposure to microbial elements and immune homeostasis in HIV infection.
METHODS
This work was approved by institutional review boards at University Hospitals/Case Medical Center and the Cleveland Clinic Foundation. Using cut points proposed to determine guidelines for treatment initiation, immune failure subjects had CD4+ T-cell counts <350/μL and immune success subjects had CD4+ T-cell counts >500/μL after at least 24 months of virologic control, defined by plasma HIV RNA levels below detection limits (typically 50 copies per mL). Transient viremic blips did not exclude participation if flanked by viral levels below detection limits. Controls were not matched to HIV+ subjects, but variables significantly different among the groups on univariate analysis were controlled for in subsequent multivariable analyses.
With written informed consent, blood was drawn into tubes containing ethylenediaminetetraacetic acid (EDTA). Lymphocyte phenotypes were identified using fluorochrome-labeled monoclonal antibodies (BD Biosciences and BD Pharmingen) and were enumerated by flow cytometry. Plasma was removed after centrifugation, frozen at −80oC, thawed and analyzed in batch for levels of D-dimers (Diagnostica Stago), soluble CD14 (sCD14), interleukin 6 (IL-6; R&D Systems), and bacterial lipopolysaccharide (LPS; QCL-1000, Lonza) according to manufacturers’ protocols.
We compared continuous variables using Mann–Whitney U test, Kruskal–Wallis H test, or the Jonckheere-Terpstra test as needed according to number and independence of groups compared. We fitted multiple linear regression models to control for potential confounders in comparisons of each readout; we used mixed effects models to estimate the slope of CD4+ T-cell change, controlling for potential confounders. To assess the contribution of each predictor to immunologic outcome, we fitted logistic regression models and tested for significant interaction and confounding among the predictors. All tests were 2-sided with a significance cutoff of 0.05, without formal correction for multiple comparisons.
RESULTS
Sixty immune failure patients, 20 immune success patients, and 21 healthy controls underwent detailed immunologic studies. Another 168 immune success subjects who met study criteria were identified from the Special Immunology Unit Patient Care and Research Database, and are included in all analyses except those involving immune and inflammatory markers. Characteristics of the subjects are shown in Table 1.
Table 1.
Patient Characteristics
| HIV-negative controls | Immune failures | Immune successes |
||
| Immune analysis subset | Overalla | |||
| No. of patients | 21 | 60 | 20 | 188 |
| Male sex, n (%) | 10 (48) | 49 (81) | 12 (60) | 132 (70) |
| Race/ethnicity, n (%) | ||||
| White | 17 (81) | 36 (60) | 9 (45) | 84 (45) |
| African-American | 2 (10) | 14 (23) | 10 (50) | 95 (50) |
| Hispanic | 1 (5) | 3 (5) | 1 (5) | 3 (2) |
| Other/unknown | 1 (5) | 7 (12) | 0 (0) | 6 (4) |
| Age, years (range) | ||||
| At study enrollment | 37 (32–47) | 48 (43–56) | 44 (40–49) | … |
| At HAART start | … | 41 (34–47) | 35 (32–41) | 37 (33–43) |
| Years with plasma HIV RNA <400 | … | 4.4 (2.6–6.7) | 7.5 (5.7–11.4) | 4.6 (3.3–7.1) |
| HCV seropositive, n (%) | … | 8 (13) | 1 (5) | 23 (12.2) |
| Nadir CD4+ T cells/μL | NA | 35 (10–129) | 175 (19–301) | 199 (82–250) |
| Peak viremia, copies/mL | NA | 227 000 | 185 147 | 99 394 |
| CD4+ T cells/μL | 907 (726–1043) | 258 (186–-303) | 775 (662–961) | … |
| CD8+ T cells/μL | 451 (363–732) | 620 (450–840) | 820 (620–1020) | … |
Abbreviations: HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NA, not applicable.
Includes the immune analysis subset and additional subjects identified through database search.
Immune failure patients were more likely to be male (81%) and white (60%) than immune success patients (70% and 45%, P = .04 and P = .01, respectively). They were older at the initiation of combination antiretroviral therapies (41 years vs 37 years, P = .011). Median CD4+ T-cell count in immune failures was 258 cells/μL, 775/μL in immune successes, and 907/μL among healthy controls. CD8+ T-cell counts were higher in immune successes (820/μL) than in immune failures (620/μL, P = .038) and healthy controls (451/μL, P = .004). Not surprisingly, the CD4+ T-cell nadir was lower in immune failures than in immune successes (35 vs 199/μL, P < .001). Peak viremia tended to be higher in immune failures (227 000 vs 99 394 copies/mL), but not significantly. Median time with undetectable viremia was lower in immune failures than in the immune analysis subset of the immune success group (4.4 vs 7.5 years, P = .001), but not different from the overall immune success group (4.6 years, P = .16).
CD4+ T-Cell Maturation Subsets
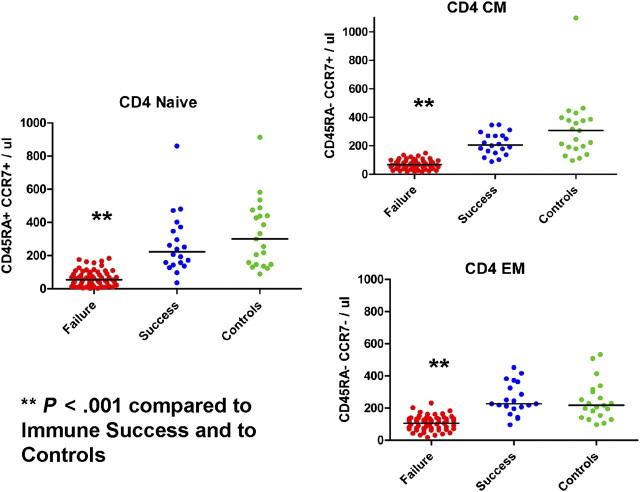
The median numbers of each CD4+ T-cell maturation subset (naive = 54/μL, central memory = 67/μL, effector memory = 109/μL) were significantly lower in immune failures than in healthy controls (naive = 300/μL, central memory = 307/μL, effector memory = 218/μL; P < .001 for each) and immune successes (naive = 223/μL; central memory = 206/μL, effector memory = 227/μL; P <.001 for each; Figure 1). These numbers were comparable in controls and immune successes, underscoring effective numerical CD4+ T cell restoration in this group.
Figure 1.
CD4+ lymphocyte maturation subsets. Absolute numbers of circulating naive (CD45RA+/CCR7+), central memory (CM; CD45RA–/CCR7+), and effector memory (EM; CD45RA–/CCR7–) CD4+ lymphocytes in immune successes, immune failures, and healthy controls. Lymphocytes were identified by forward and right-angle light scatter and were gated according to high-level expression of CD4.** lower than numbers in immune successes and healthy controls (P < .001).
CD8+ T-Cell Maturation Subsets
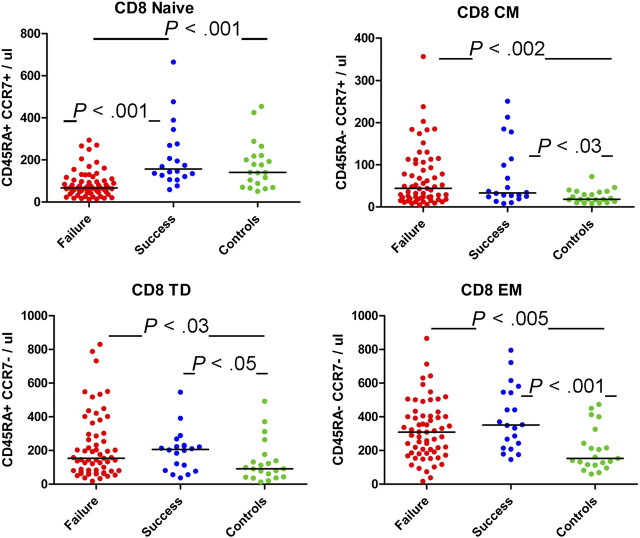
In contrast, among CD8+ T cells, only naive cell numbers were diminished in immune failure (68/μL) compared with immune successes (157/μL, P < .001) and healthy controls (141/μL, P = .001; Figure 2). All other CD8+ maturation subsets were increased in both patient groups compared with healthy controls, reflecting the global expansion of CD8+ memory cells characteristic of chronic HIV infection.
Figure 2.
CD8+ lymphocyte maturation subsets. Absolute numbers of circulating central memory (CM), effector memory (EM), and terminally differentiated memory (TM; CD45RA+/CCR7–) cells were lower in healthy controls than in immune success and immune failure subjects. Naive cell numbers were lower in immune failure subjects than among immune success subjects and healthy controls.
T-Cell Activation
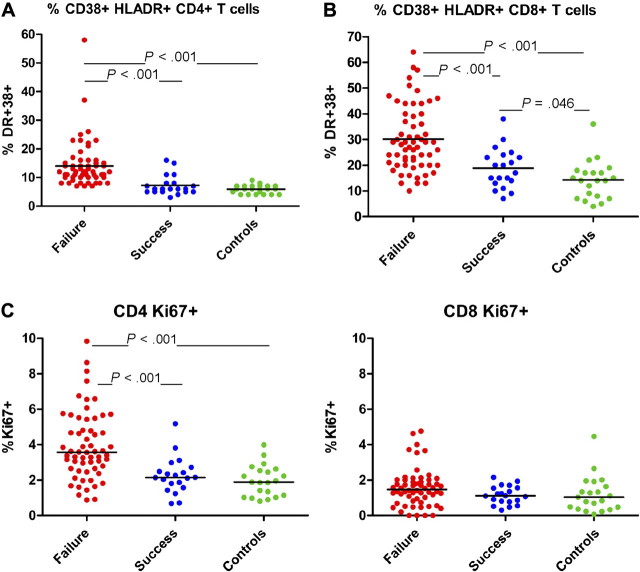
T-cell activation is a hallmark of HIV infection, and expression of the activation marker CD38+ has been linked to disease progression [23]. As naive T cells may express CD38 without activation [24], we analyzed activation as reflected by coexpression of CD38 and HLA-DR. The proportion of activated CD4+ T cells in immune failures (12%) was significantly greater (P < .001) than in immune successes and in healthy controls (6% for each, Figure 3A). Proportions of activated CD8+ T cells in immune failures (29%) were greater (P < .001) than in immune successes (19%) and healthy controls (14%; Figure 3B). The proportion of activated CD8+ T cells in immune successes was marginally higher than among healthy controls (P = .046).
Figure 3.
A, B, Proportions of activated (CD38+, HLA-DR+) CD4+ and CD8+ lymphocytes. Among both CD4+ and CD8+ cell populations, proportions of activated cells were higher in immune failure patients than among immune success patients and healthy controls. C, Proportions of CD4+ and CD8+ lymphocytes in cell cycle. The proportions of CD4+ lymphocytes in cell cycle (expressing the nuclear antigen Ki-67) were higher in immune failure patients than among immune success patients and healthy controls, while these proportions were not different among CD8+ lymphocytes.
Cell Cycling
As we [25, 26] and others [27, 28] have identified cycling and turnover of central memory CD4+ T cells as a possible driver of immune deficiency in chronic untreated HIV or simian immunodeficiency virus (SIV) infection, we examined the proportions of cells expressing the nuclear antigen Ki-67. While immune success patients and healthy controls had similar proportions of Ki-67+ CD4+ T cells (2.2% and 1.9%, respectively), 3.6% of CD4+ T cells in immune failures were Ki-67+, and this was greater than in both the controls and immune successes (P < .001, Figure 3C). Among CD8+ T cells, however, and despite the fact that proportionally more of these cells were activated than were CD4+ T cells, the frequencies of cycling cells were not different in immune failures, immune successes, and healthy controls (1.5%, 1.1%, and 1.0%, respectively).
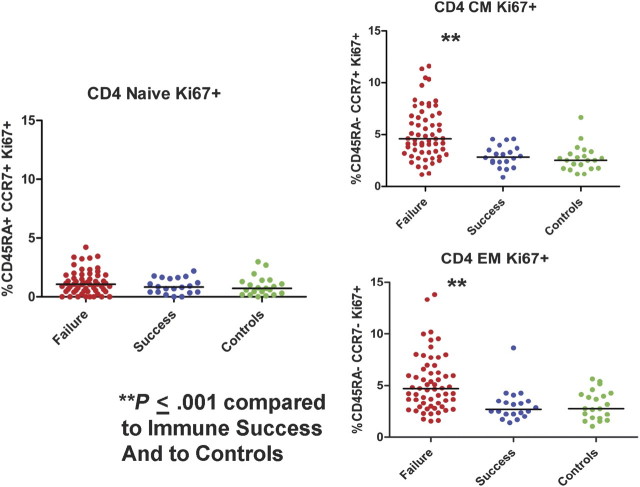
We therefore next examined Ki-67 expression among the different CD4+ T cell maturation subsets (Figure 4). While proportions of cycling naive CD4+ T cells were comparable in all groups, proportions of cycling central memory and effector memory CD4+ T cells were significantly greater in immune failures (4.6 and 4.7%, respectively) than in immune successes (2.9 and 2.7%, P < .001) and in healthy controls (2.5% and 2.8%, P = .001). Proportions were comparable in immune successes and in controls.
Figure 4.
Proportions of CD4+ lymphocyte maturation subsets in cell cycle. The proportions of both central memory (CM) and effector memory (EM) CD4+ lymphocytes in cycle was greater in immune failure patients than among immune success patients and healthy controls.
Soluble Markers of Microbial Translocation and Inflammation
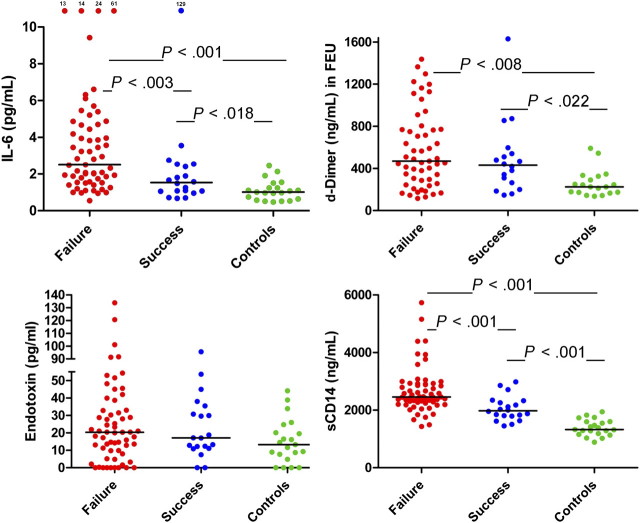
An increasing body of evidence is linking inflammation and coagulation to morbidity and mortality in chronic HIV infection [29], and work from our research collaboration has implicated translocation of microbial products from the gut as a major correlate of immune activation, coagulation, and CD4+ T-cell losses in chronic HIV infection [30–32]. We found that the plasma level of IL-6 in immune failures (2.5 pg/mL) was higher than in immune successes (1.53 pg/mL, P = .003, Figure 5), and both were higher than in controls (1.01 pg/mL, P = .001, P = .018). D-dimer levels were similar in immune failures and immune successes (427 and 401 ng/mL, respectively), and both were higher than in controls (224 ng/mL, P = .008, P = .022). Levels of LPS tended to be higher in immune failures (20.2 pg/mL) and immune successes (17.1 pg/mL) than in controls (13.3 pg/mL), but not significantly (P = .16 and 0.1). Levels of soluble CD14+, the LPS coreceptor, were higher in immune failures (2453 ng/mL) than in immune successes (2056 ng/mL, P < .001), and both were higher than in controls (1390 ng/mL, P < .001).
Figure 5.
Soluble markers of microbial translocation, inflammation, and coagulation. Plasma levels of IL-6 were higher in immune failure patients than in immune success patients, and the levels in both patient groups were higher than in controls. D-dimer levels were comparable in both patient groups but higher than among controls. Levels of bacterial lipopolysaccharide (LPS), though nominally higher in immune failure patients than in immune success patients and controls, were not significantly elevated, while levels of the soluble LPS receptor CD14+ were higher in immune failure patients than in immune success patients and were higher in each patient group than in controls.
Coinfections and Other Illness
Among subjects with available serologies, there were no differences in prevalence of infection with hepatitis B virus, hepatitis C virus, or cytomegalovirus in immune success and immune failure. As both malignant outcomes are more common in patients with lower CD4+ T-cell counts, and treatment for malignancy might affect the magnitude of immune restoration, we found that malignancy was more frequent in immune failures (18%) than in immune successes (5.3%, P < .01), but in multivariable analysis (below), history of malignancy did not significantly affect the results of the study.
Multivariable Analyses
To exclude potential confounders, we considered the possibility that immune failure subjects could represent a subset of patients who would continue to increase CD4+ T-cell counts and would have been classified as immune successes had they been enrolled into the study later during therapy. We also entertained the possibility that our results could be explained by shorter periods of virologic control or by lower nadir CD4+ T-cell counts, which are linked to both higher residual inflammation/activation [12] and poor CD4+ T-cell increases after highly active antiretroviral therapy (HAART) [7, 33, 34]. We found the CD4+ T-cell trajectory after HAART was dramatically different in the 2 groups (Supplementary Figure 1). Mixed-effect modeling showed a significantly greater slope of CD4+ T-cell gain among immune success subjects, even after controlling for nadir CD4+ T-cell count, time from HAART initiation, and years with an undetectable HIV RNA level (adjusted P value < .001). With evidence that group classifications represented a true difference in the rate of CD4+ T-cell reconstitution between immune failures and immune successes, we then asked whether these factors affected any of the other differences we found between the groups. As shown on Table 2, our conclusions about T-cell activation, cycling, and soluble CD14+ levels remained unchanged after adjusting for nadir CD4+ T-cell count, time since HAART initiation, and duration of complete virologic control.
Table 2.
Multivariate Comparisons of Immunologic and Inflammatory Readouts, Adjusting for Nadir CD4+ T-Cell Count and for Time Since Initiation of HAART
| Readout | Comparison of immune failure vs immune success groups |
|||
| Crude P value | Adjusted P values |
|||
| Controlling for nadir CD4+ T cells | Controlling for time between HAART and study enrollment | Controlling for time with an HIV RNA below the limit of detection since HAART initiation | ||
| CD4+ that are Ki67+ % | .000 | .011 | .002 | .026 |
| CD4+ that are CD28+ % | .785 | .515 | .294 | .546 |
| CD8+ that are CD28+ % | .050 | .237 | .149 | .223 |
| CD4+ that are HLA-DR+, CD38+ % | .000 | .013 | .001 | .002 |
| CD8+ that are HLA-DR+, CD38+ % | .000 | .006 | .001 | .002 |
| Naive CD4+ (CD45RA+/CCR7+) count | .000 | .000 | .000 | .000 |
| EM CD4+ (CD45RA–/CCR7–) count | .000 | .000 | .000 | .000 |
| CM CD4+ (CD45RA–/CCR7+) count | .000 | .000 | .000 | .000 |
| Cycling EM CD4+ (CD45RA–/CCR7–/Ki67+) % | .000 | .022 | .007 | .065 |
| Cycling CM CD4+ (CD45RA–/CCR7+/Ki67+) % | .000 | .003 | .001 | .011 |
| Naive CD8+ (CD45RA+/CCR7+) count | .000 | .000 | .000 | .000 |
| sCD14 | .000 | .000 | .000 | .000 |
| IL-6 | .003 | .242 | .375 | .657 |
| D-dimer | .781 | .249 | .450 | .604 |
| LPS | .982 | .940 | .732 | .360 |
Abbreviations: CM, central memory; EM, effector memory; HAART, highly active antiretroviral theraphy; HIV, human immunodeficiency virus; IL-6, interleukin 6; LPS, lipopolysaccharide.
Having excluded major design bias, we asked whether associations among markers of activation, microbial translocation, and immune failure were independent of other correlates of immune failure identified in the univariate analyses shown in the figures. Logistic regression analyses showed that the number and proportion of activated (CD38+, HLA-DR+) CD4+ T cells (adjusted P = .025 and .039), the number and proportion of activated (CD38+, HLA-DR+) CD8+ T cells (adjusted P = .027 and .005), the proportion of total, central memory, and effector memory CD4+ T cells in cell cycle, and plasma levels of sCD14+ (adjusted P = .011, .013, .014, and .008) all remained significantly associated with immune failure after adjusting for nadir CD4+ T-cell count, age at HAART initiation, race, and history of malignancy, suggesting that these differences could not be accounted for on the basis of those additional predictors.
DISCUSSION
In this study, we have established a phenotypic characterization of immune failure despite HAART-induced control of HIV replication. We recognize that some in this group will continue to increase CD4+ T-cell counts [5, 35], yet the median time since initiation of antiretroviral therapy in immune failure patients was greater than 7 years, and analysis of the average CD4+ T-cell increases in immune failure and immune success patients demonstrates a highly significant difference in CD4+ T-cell trajectory (supplementary Figure 1), clearly indicating these patient populations are distinguishable. Thus, immune failure patients represent a population experiencing sustained periods of subclinical immune deficiency. The long-term risks of subclinical immune deficiency in this setting are not entirely known, but available data suggest that heightened morbidity and mortality is seen in persons who fail to increase their CD4+ T-cell counts while receiving effective antiretroviral therapies [3, 4, 36].
This study represents the largest and most comprehensive characterization of immunophenotype, inflammation, and coagulation in persons experiencing immune failure despite long-term suppression of HIV replication. As we [16] and others [6] had earlier reported in smaller studies with shorter durations of post-treatment follow up, both naive and memory CD4+ T-cell populations remain diminished in immune failure patients, and here we show that this is the case in both the central memory and effector memory compartments.
We also show that immune success patients have persistent expansions of total CD8+ T-cell populations, and in both immune successes and immune failures, this comprises predominantly an expansion of more differentiated effector and terminally differentiated populations, while naive CD8+ T cells remain diminished in numbers. The determinants of persistent memory CD8+ T-cell expansion in chronic treated HIV infection are incompletely understood. Whether their persistence reflects sustained exposure to antigenic stimuli (and if so, which antigenic stimuli?) the residuum of bystander expansion in response to common gamma-chain receptor cytokines [37], or persistence as a result of replicative senescence and resistance to programmed cell death remains uncertain.
In contrast to the persistent expansion of memory CD8+ T cells in both treated populations, immune failure patients but not immune success patients have profound depletion of both CD8+ and CD4+ naive T cells. This may reflect impaired thymic output seen in aging and in HIV infection [16, 38, 39], the impaired naive T-cell expansion capacity described in HIV infection [40, 41], or a compound defect that is the result of both. Both thymic failure [11, 14, 16] and naive CD4+ T-cell depletion have been linked to immune failure after application of antiretroviral therapies [11, 16, 42]. As lymphoid tissue fibrosis is associated with both diminished naive T-cell numbers and failure of immune restoration with administration of antiretroviral therapies [42, 43], it is tempting to propose that interference with maturation and homeostatic signaling in disordered lymphoid tissues may contribute to the failure of naive T-cell expansion capacity observed in vitro [40, 44, 45] and to the diminished naive T-cell numbers seen in patients with immune failure studied here.
As was shown earlier [9, 13, 14, 17, 19, 20], immune failure in treated HIV infection is associated with immune activation, here reflected in coexpression of CD38 and HLA-DR on both CD4+ and CD8+ T cells. What, then, is driving T-cell activation in the setting of virologic suppression? Conceivably, immune failure patients could have higher levels of viral replication, though still below the limits of detection by the commercial assays used in this study. We did not collect sufficient volumes of plasma to perform these assays, thus this hypothesis remains untested in this data set.
On the other hand, our data indicate that systemic translocation of microbial products may distinguish immune failure from immune success in the setting of antiretroviral therapies. Phenotypic indices of T-cell activation (specifically CD38+) and plasma IL-6 levels are predictive of disease progression [29, 46], and expression of both can be induced by microbial products in vitro [22, 31]. Not surprisingly, plasma levels of IL-6 are higher in immune failure than in immune success patients, and in both populations, levels are higher than in healthy controls. LPS levels tended to be higher in both patient populations than among controls, although these differences did not achieve statistical significance. And soluble levels of the LPS coreceptor (sCD14) in immune failures were higher than levels in immune successes, and in both patient populations, these levels were higher than among healthy controls. Microbial products may contribute to immune activation in vivo, as both indices were significantly correlated with plasma levels of IL-6 and both CD4+ and CD8+ T-cell expression of CD38 and HLA-DR (not shown). Interestingly, in this study, we did not find a significant correlation between plasma levels of LPS and sCD14+ as we had previously [30, 31]. In this study and in a recent analysis of the Strategies for Management of Antiretroviral Therapy (SMART) study [47], sCD14 appears to be a better correlate of impaired immune restoration and of clinical outcomes than are levels of LPS. This may be related to the complexities of current bioassays for LPS activity or, alternatively, the release of the LPS coreceptor in vivo may be a better readout of in vivo LPS activity or indeed of monocyte activation than are bioactive levels of LPS that may be bound by plasma levels of LPS binding protein (LBP), endotoxin core antibody (EndoCAb), or sCD14 itself.
Importantly, the activation phenotype of CD4+ and CD8+ T cells is quite distinguishable. Whereas in untreated HIV infection, increased cell cycling is demonstrable in both CD4+ and CD8+ T cells and especially in central memory T cells [25, 26], here we show that despite high-level phenotypic activation of both CD4+ and CD8+ T cells in immune failure, suppression of HIV replication results in apparent normalization of CD8+ T-cell cycling while CD4+ T-cell cycling remains high, especially among central memory and effector memory CD4+ T cells. This is the phenotype that is demonstrable in vitro when peripheral blood mononuclear cells (PBMCs) of healthy controls are exposed to microbial Toll-like receptor (TLR) ligands [22]. TLR ligand exposure induces phenotypic markers of both CD4+ and CD8+ T-cell activation [22], yet only CD4+ T cells (and especially central memory and effector memory CD4+ T cells) are activated to enter the cell cycle while effects on CD8+ T-cell cycling are minimal. We interpret these findings to indicate that in untreated HIV infection, increased cycling of CD8+ T cells is largely a direct and indirect consequence of HIV replication, specifically driving the expansion of HIV-reactive CD8+ T cells and also concurrently driving the expansion of CD8+ T cells of diverse specificities [26] through sustained bystander activation in lymphoid tissues [37]. In this proposed model, once HIV replication is substantially suppressed by antiretroviral therapies, persistent T-cell activation is related more to sustained exposure to translocated microbial products that activate both CD4+ and CD8+ T cells, but drive cycling primarily of memory CD4+ T cells [22].
In both immune failures and immune successes, plasma D-dimer levels were elevated, underscoring the recognized increased risks for thrombotic events seen in persons with HIV infection, even in the HAART era [48]. Earlier, we found expression of the procoagulant tissue factor (TF) increased in persons with HIV infection. TF levels were correlated to plasma levels of sCD14, and monocyte expression of TF could be induced by exposure to bacterial LPS and flagellin in vitro [31]. The modest but significant relationship between levels of D-dimers and sCD14 (r = 0.26, P = .019) provides some additional indication that may link microbial translocation to thromboses, even with sustained suppression of HIV replication.
In this study, immune failure patients were more likely to be male and tended to be older at the initiation of antiretroviral therapies. While it may be premature to conclude from our work that gender determines the magnitude of cellular restoration, Gandhi et al. [20] also found better immune recovery in HAART-treated women. This should be explored further. It is not surprising that age is linked to immune failure because both thymic function and T-cell responsiveness to homeostatic cytokines [49] diminish with age.
Perhaps the most important, if not most obvious, predictor of immune failure is CD4+ T-cell nadir. While a low nadir does not universally predict immune failure (half of our immune success patients began antiretroviral therapies with CD4+ T-cell nadirs less than 200/μL), initiating HAART at higher CD4+ T-cell numbers is virtually guaranteed to sustain and will typically increase CD4+ T-cell counts. It should also be noted that initiating antiretroviral therapies at higher CD4+ T-cell counts also predicts a better in vivo immune response (as determined by immunization responses), even in subjects with “normal” circulating CD4+ T-cell counts [50]. Immune function assays and measures of inflammation and coagulation may help to provide more discriminating readouts of immune restoration that predict clinical outcome than do circulating CD4+ T-cell counts alone. Our data support the importance of early recognition of HIV infection so that infected persons can be offered HAART before irreversible immune deficiency develops.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/).
Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
The authors thank Drs Steven Bass, Martine Binstock, Scott Fulton, K. V. Gopalkrishna, and Gopal Yadavalli for referral of patients.
Financial support. This work was supported by grants from the National Institutes of Health (NIH); the Case Western Reserve University (CWRU) Center for AIDS Research (AI-36219); the AIDS Clinical Trials Group Core Immunology Service Laboratory (AI-68636); the Centers for Aids Research (CFAR) Network of Integrated Clinical Systems (CNICS; AI 67039); the Cleveland Immunopathogenesis Consortium (AI- 076174); and by the Richard J. Fasenmyer Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Keiser O, Taffe P, Zwahlen M, et al. All cause mortality in the Swiss HIV Cohort Study from 1990 to 2001 in comparison with the Swiss population. AIDS. 2004;18:1835–43. doi: 10.1097/00002030-200409030-00013. [DOI] [PubMed] [Google Scholar]
- 3.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–8. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewden C, Chene G, Morlat P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46:72–7. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- 5.Kelley CF, Kitchen CM, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–94. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins GK, Spritzler JG, Chan ES, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48:350–61. doi: 10.1086/595888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–6. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 8.Tuboi SH, Brinkhof MW, Egger M, et al. Discordant responses to potent antiretroviral treatment in previously naive HIV-1-infected adults initiating treatment in resource-constrained countries: the antiretroviral therapy in low-income countries (ART-LINC) collaboration. J Acquir Immune Defic Syndr. 2007;45:52–9. doi: 10.1097/QAI.0b013e318042e1c3. [DOI] [PubMed] [Google Scholar]
- 9.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 10.Anthony DD, Yonkers NL, Post AB, et al. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–16. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 11.Benveniste O, Flahault A, Rollot F, et al. Mechanisms involved in the low-level regeneration of CD4+ cells in HIV-1-infected patients receiving highly active antiretroviral therapy who have prolonged undetectable plasma viral loads. J Infect Dis. 2005;191:1670–9. doi: 10.1086/429670. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez S, Price P, McKinnon EJ, Nolan RC, French MA. Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clin Immunol. 2006;120:163–70. doi: 10.1016/j.clim.2006.04.570. [DOI] [PubMed] [Google Scholar]
- 13.Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–8. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 14.Marchetti G, Gori A, Casabianca A, et al. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS. 2006;20:1727–36. doi: 10.1097/01.aids.0000242819.72839.db. [DOI] [PubMed] [Google Scholar]
- 15.Molina-Pinelo S, Vallejo A, Diaz L, et al. Premature immunosenescence in HIV-infected patients on highly active antiretroviral therapy with low-level CD4 T cell repopulation. J Antimicrob Chemother. 2009;64:579–88. doi: 10.1093/jac/dkp248. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS. 2001;15:1749–56. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 17.Valdez H, Connick E, Smith KY, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–66. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 18.Negredo E, Massanella M, Puig J, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: clinical implications. Clin Infect Dis. 2010;50:1300–8. doi: 10.1086/651689. [DOI] [PubMed] [Google Scholar]
- 19.Anthony KB, Yoder C, Metcalf JA, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J Acquir Immune Defic Syndr. 2003;33:125–33. doi: 10.1097/00126334-200306010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Gandhi RT, Spritzler J, Chan E, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42:426–34. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 21.Rajasuriar R, Booth D, Solomon A, et al. Biological determinants of immune reconstitution in HIV-infected patients receiving antiretroviral therapy: the role of interleukin 7 and interleukin 7 receptor alpha and microbial translocation. J Infect Dis. 2010;202:1254–64. doi: 10.1086/656369. [DOI] [PubMed] [Google Scholar]
- 22.Funderburg N, Luciano AA, Jiang W, Rodriguez B, Sieg SF, Lederman MM. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS One. 2008;3:e1915. doi: 10.1371/journal.pone.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 24.Clement LT, Vink PE, Bradley GE. Novel immunoregulatory functions of phenotypically distinct subpopulations of CD4+ cells in the human neonate. J Immunol. 1990;145:102–8. [PubMed] [Google Scholar]
- 25.Sieg SF, Bazdar DA, Lederman MM. S-phase entry leads to cell death in circulating T cells from HIV-infected persons. J Leukoc Biol. 2008;83:1382–7. doi: 10.1189/jlb.0907643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieg SF, Rodriguez B, Asaad R, Jiang W, Bazdar DA, Lederman MM. Peripheral S-phase T cells in HIV disease have a central memory phenotype and rarely have evidence of recent T cell receptor engagement. J Infect Dis. 2005;192:62–70. doi: 10.1086/430620. [DOI] [PubMed] [Google Scholar]
- 27.Lempicki RA, Kovacs JA, Baseler MW, et al. Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients. Proc Natl Acad Sci USA. 2000;97:13778–83. doi: 10.1073/pnas.250472097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picker LJ, Hagen SI, Lum R, et al. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 31.Funderburg NT, Mayne E, Sieg SF, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115:161–7. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–85. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann GR, Perrin L, Pantaleo G, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163:2187–95. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 35.Hunt PW, Deeks SG, Rodriguez B, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17:1907–15. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 36.Gutierrez F, Padilla S, Masia M, et al. Patients’ characteristics and clinical implications of suboptimal CD4 T-cell gains after 1 year of successful antiretroviral therapy. Curr HIV Res. 2008;6:100–7. doi: 10.2174/157016208783885038. [DOI] [PubMed] [Google Scholar]
- 37.Biancotto A, Grivel JC, Iglehart SJ, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–9. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 39.Dion ML, Poulin JF, Bordi R, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21:757–68. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Luciano AA, Lederman MM, Valentin-Torres A, Bazdar DA, Sieg SF. Impaired induction of CD27 and CD28 predicts naive CD4 T cell proliferation defects in HIV disease. J Immunol. 2007;179:3543–9. doi: 10.4049/jimmunol.179.6.3543. [DOI] [PubMed] [Google Scholar]
- 41.Sieg SF, Bazdar DA, Harding CV, Lederman MM. Differential expression of interleukin-2 and gamma interferon in human immunodeficiency virus disease. J Virol. 2001;75:9983–5. doi: 10.1128/JVI.75.20.9983-9985.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schacker TW, Brenchley JM, Beilman GJ, et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2006;13:556–60. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schacker TW, Reilly C, Beilman GJ, et al. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. AIDS. 2005;19:2169–71. doi: 10.1097/01.aids.0000194801.51422.03. [DOI] [PubMed] [Google Scholar]
- 44.Sieg SF, Bazdar DA, Lederman MM. Impaired TCR-mediated induction of Ki67 by naive CD4+ T cells is only occasionally corrected by exogenous IL-2 in HIV-1 infection. J Immunol. 2003;171:5208–14. doi: 10.4049/jimmunol.171.10.5208. [DOI] [PubMed] [Google Scholar]
- 45.Sieg SF, Harding CV, Lederman MM. HIV-1 infection impairs cell cycle progression of CD4+ T cells without affecting early activation responses. J Clin Invest. 2001;108:757–64. doi: 10.1172/JCI12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1993;6:904–12. [PubMed] [Google Scholar]
- 47.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–24. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bazdar DA, Kalinowska M, Sieg SF. Interleukin-7 receptor signaling is deficient in CD4+ T cells from HIV-infected persons and is inversely associated with aging. J Infect Dis. 2009;199:1019–28. doi: 10.1086/597210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lange CG, Lederman MM, Medvik K, et al. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS. 2003;17:2015–23. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]