TLR stimulation results in polyclonal B cell activation, ectopic CLIP expression, release of T cell-derived TNFα, and reversal with high affinity peptide treatment.
Keywords: Toll-like receptors, cell death, polyclonal B cell activation
Abstract
Infectious pathogens produce compounds called Toll ligands that activate TLRs on lymphocytes. Acute activation triggered by certain TLRs appears to “jump start” the innate immune response, characterized by the release of inflammatory cytokines and cellular expansion. In some individuals, there is a failure to control acute inflammation, resulting in postinfectious, chronic inflammation. Susceptibility to chronic inflammation is strongly associated with an individual's MHC genes. Recent clinical trials for several autoimmune diseases characterized by chronic inflammation suggest that B lymphocyte depletion therapies dampen chronic immune activation. However, currently, there is no known mechanism that accounts for the correlation among TLR activation, MHC genetics, and a pathological role for B-lymphocytes. Our hypothesis is that TLR-activated B cells (B cells that have been polyclonally activated in the absence of antigen-specific signals) are not controlled properly by T cell-dependent B cell death, thereby causing B cell-dependent chronic inflammation. Here, we show that treatment with Toll ligands results in polyclonal B cell activation accompanied by ectopic expression of CLIP. Furthermore, by adoptively transferring purified CLIP+ B cells in syngeneic animals, we find that CLIP+ B cells induce production of TNF-α by host T cells. Finally, we demonstrate that CLIP-targeted peptide competition results in the death of polyclonally activated CLIP+ B cells.
Introduction
TLRs are innate pattern-detection receptors expressed on phagocytes, macrophages, lymphocytes, and other cells. TLRs can detect PAMPs, produced by bacteria, viruses, and parasites [1]. Common TLR ligands, or PAMPs, bind to and activate TLR2, TLR3, TLR4, TLR7/8, or TLR9 [2] (Table 1). TLRs induce cellular activation and a cascade of events that result in inflammation.
TABLE 1.
Toll Ligands and Their Receptors
| Toll ligand | TLR |
|---|---|
| Pam-3-Cys | TLR2 |
| Poly I:C | TLR3 |
| LPS | TLR4 |
| R848, CLO97 | TLR7/8 |
| CpG-ODN | TLR9 |
Susceptibility or resistance to pathogenic infection and most post-infectious syndromes [3, 4] strongly correlates with an individual's MHC genes, which are highly variable between individuals and reflect genetic diversity within a population. In contrast, CD74 (Ii) [5] and its cleavage products (CLIP [6]) are derived from the nonpolymorphic invariant protein, CD74, although multiple cleavage products can result from proteolysis in the endosomal/lysosomal compartments [5].
Some pathogens associated with TLR activation can cause long-term diseases characterized by chronic immune activation, as defined by an increase in pro-inflammatory cytokines such as TNF-α, greater numbers of white cells, and redistribution of lymphocytes between spleen and lymph node [7]. For example, recent clinical trials for several autoimmune diseases, such as Multiple Sclerosis [8], type I diabetes [9], Crohn's disease [10], and Lyme disease [11], suggest that B lymphocyte depletion therapies dampen chronic inflammation in some individuals. Interestingly, all of these diseases linked to TLR activation have also been genetically associated with immune-response genes, and all have inflammation as a common characteristic.
The consequence of TLR engagement on the B cell is polyclonal, TLR-dependent B cell activation in the absence of specific antigen [2]. Specific B cell antigen receptor engagement results in antigen internalization, increased lysosomal acidity associated with antigen processing, acidity-dependent inhibition of HLA-DO (H-2O in mouse) [12], activation of HLA-DM (H-2M in mouse) [12, 13], and HLA-DM-dependent replacement of CLIP in the groove of MHC class II molecules with antigenic peptides [14]. Specific antigen recognition by the B cell, followed by processing and presentation of the peptides to CD4+ T cells, helps focus B and T cell specificities, respectively, in adaptive immune responses [15]. Thus, it appears that B cells respond as key cellular players in adaptive, specific immunity or as mediators of the innate immune response to pathogens.
Mediated via byproducts of many infectious pathogens, polyclonal B cell activation occurs in the absence of antigen receptor engagement. However, polyclonal B cell activation, analogous to antigen-specific B cell activation, results in increased levels of B cell surface MHC class II [16–18]. Until antigen-specific activation occurs, the presence of HLA-DO in the endosomal/lysosomal compartments of B cells delays HLA-DM from replacing endogenous CLIP with peptidic antigen until the specific antigen receptor is engaged [19]. Although TLR-mediated activation of macrophages and DCs has been shown to drive maturation and loss of cell surface CLIP and improved antigen processing and presentation [20], the impact of TLR-mediated B cell activation on conventional B cell antigen processing and presentation remains unknown.
Because TLR activation of B cells does not necessarily trigger the same intracellular signals that lead to processing and presentation as antigen receptor-driven processing and presentation, we predicted that the physiological B cell response to TLR activation would result in presentation of cell surface CLIP in the groove of MHC class II molecules on B cells. We hypothesized further that if CLIP, rather than a specific antigen, is presented on the surface of the activated B cell, the consequence will be tightly regulated survival of the CLIP+ B cell. As CLIP is a self-antigen, survival of the CLIP+ B cell may be tightly controlled by peptide exchange, resulting in T cell receptor-mediated death of antigen-non-specific B cells, such that survival is restricted to antigen-specific, activated B cells. This hypothesis purports that CLIP+ B cells and MHC II-dependant ease of exchange of ectopic CLIP with another peptide may control the transition between acute and chronic inflammation.
Our hypothesis is that TLR activation of non-antigen primed B cells promotes activation/inflammation that can be reversed by selective B cell death of the TLR-activated B cells. We propose that tightly controlled B cell death or removal of TLR-activated B cells is not only necessary for those B cells that do not recognize antigen specifically but also preserves B cell antigen specificity. Survival is controlled by antigen specificity and antigen receptor-driven survival signals. Therefore, we feel that the conditions that determine whether the TLR-activated B cells live or die could play an important role in controlling chronic inflammation. The following experiments were designed and executed to test this hypothesis.
MATERIALS AND METHODS
Mice
C57Black6 and B6.129 mice were purchased from the Jackson Laboratories (Bar Harbor, ME, USA) and housed at the vivarium at the CU Institute of Bioenergetics and Immunology (Colorado Springs, CO, USA). Ii (CD74)-deficient mice and H-2M-deficient mice on the B6.129 syngeneic strain background were generously provided by Dr. Scott Zamvil (University of California San Francisco, CA, USA). MyD88−/− mice were provided by Shizuo, Akira (Osaka University, Osaka, Japan).
Antibodies
The following mAb were used in these studies: 15G4, a mAb directed against mouse MHC CLIP (I-Ab complex), only when CLIP is in the groove of mouse MHC class II I-Ab molecules (Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-mouse CD4 (GK4.5); anti-mouse CD8; anti-mouse TNF-α; and PE-conjugated monoclonal anti-mouse B220, obtained from BD PharMingen (San Diego, CA, USA). Mouse anti-human CLIP (clone CerCLIP), anti-human CD19-APC, anti-human CD20-APC, and anti-HLA-DR-PerCP Cy5.5 were all obtained from BD Biosciences (San Jose, CA, USA).
TLR-binding ligands
Toll ligands included Poly I:C (Sigma Chemical Co., St. Louis, MO, USA), Pam3Cys (Alexis, San Diego, CA, USA), imidazoquinoline resiquimod (R848, an analog of single-stranded viral RNA, also known as CLO97; Invivogen, San Diego, CA, USA) [7], LPS (Sigma Chemical Co.), and CpG-ODN (Invivogen, Alexis).
Peptides
Peptides used in these studies include the following (listed in Table 2): the synthetic MHC CLIP (Table 2), OVA 323–339 peptide (Sigma Chemical Co.), vaccinia virus sequences for known HB and LB to MHC class II I-Ab (Elim Biopharmaceuticals Inc., Hayward, CA, USA; Table 2), and MHC class II-targeted proprietary peptide synthesized by and purchased from Elim Biopharmaceuticals Inc. Briefly, using information provided in publicly available databases MHCPred and NetMHC, we computationally derived a 9mer peptide (TPP), which binds to the binding groove of MHC class II at a higher affinity than CLIP for MHC class II I-Ab. MHCPred uses IC50 comparisons and expresses this in nM concentrations. Although the programs do not calculate binding affinities directly, they can be used to compare peptide competition using the Cheng-Prussoff equation. Therefore, the lower the IC50, the more able it is to compete for binding of the MHC class II binding groove.
TABLE 2.
Peptides Used as CLIP Competitors
| Peptide | Sequence | IC50 (nM) |
|---|---|---|
| CLIP | MRMATPLLMQLY | 171.00 |
| High-binding TPP | Proprietary | 119.67 |
| Known HB | PGVMYAFTTPLISFF | 127.00 |
| Known LB | ENMLRSMPVKGKRKD | 347.8 |
| OVA peptide 323–339 | ISQAVHAAHAEINEAGR | 455.00 |
Isolation, purification, and activation of B and T lymphocytes
Splenocytes were harvested from select mouse strains and sorted or not for each cell type. CD4+T, CD8+T, and resting B cell isolations kits were purchased from Miltenyi Biotec (Germany) and sorted on a quadromacs magnet using the LS column, per the manufacturer's instruction. In brief, murine splenocytes were put through a 40-μ strainer in 2 mM EDTA in PBS. The cell isolation kits use biotinylated antibody to all cell-type markers except the cell of interest and then use an antibiotin magnetic bead-conjugated antibody to negatively select for the desired cell type. In the resting B cell kit, the activation marker CD43 is used to sort against activated B cells, thereby leaving a highly purified, resting B cell population, and cell populations were stained for CD4, CD8, or B220 before and after isolation to ensure 92%+ purified populations on all T cells and 96%+ purified populations on resting B cells.
Adoptive transfer of polyclonally activated B cells
B cells were isolated and activated for 48 h, harvested, and sampled for staining to confirm the induction of CLIP expression. Ten million CpG-activated, CLIP+ B cells were injected i.p. Spleens were harvested at 48 h and stained as described above for intracellular production of TNF-α (BD PharMingen) versus cell surface staining for CD3, B220, and CLIP.
In vivo TLR stimulations
C57Bl6 and B6.129 mice were injected with CpG-ODN (25 μg/mouse), with or without selected peptides (Table 2) at ∼5 μg/mouse, weighing ∼25 g, equivalent to 5 μM peptide. After 24, 72, or 96 h, animals were killed humanely, spleens and lymph nodes were removed and passed through nylon mesh to recover single cell suspensions, and the cells were counted. Purified, resting B cells or splenocytes activated with CpG-ODN were stained as indicated and analyzed flow-cytometrically using a Beckman Coulter Excel or Coulter FC500 flow cytometer (Beckman Coulter, Fullerton, CA, USA).
Antigen receptor-mediated B cell activation required culturing 5 × 107 freshly prepared, resting B cells with 500 μg rabbit anti-mouse IgG + IgM (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), and stimulation was confirmed by detection of increased levels of MHC class II on the B cells (Collaborative Biomedical Products, Becton Dickinson, Bedford, MA, USA). Cells were cultured in bulk overnight at 37°C at 1 × 106/ml in complete medium (RPMI 1640 supplemented with 10% FBS, penicillin, streptomycin, gentamycin, pyruvate, glutamine, and 50 μM 2-ME).
Mouse cell cultures
Freshly isolated splenocytes, single cell suspensions of lymph node cells, resting (or activated) B cells, or primed B cells were isolated from B6.129, Ii-deficient, or H-2M-deficient mice and cultured in 24-well plates in complete RPMI medium, with or without the appropriate Toll ligand, at 106 cells/ml. Cells were cultured for 24, 48, 72, or 96 h or up to 144 h, as indicated. Cells were harvested and stained by flow cytometric analysis using a Beckman Coulter Excel or a Beckman Coulter FC500 flow cytometer. Cells were stained by 2-color fluorescence using 15G4-FITC anti-mouse CLIP/I-Ab versus anti-mouse B220-PE.
B and T cell cocultures were performed by adding CD4+ and CD8+ T cells to an activated B cell culture at an E:T ratio of 1:5 prior to addition of selected peptides. CLIP positivity of B220+ cells was measured prior to coculture setup.
Cell culture with human PBMCs
Blood was taken from healthy donors and diluted with PBS. Diluted blood was layered on top of Ficoll-Paque (Amersham, Piscataway, NJ, USA) and centrifuged. Buffy coat was removed and washed in PBS.
PBMCs were prepared from a total of 5 normal donors to examine the effects of TLR binding on the percentage of B cells bearing ectopic CLIP and the changes in geometric MFI of the CLIP staining, resulting from TLR stimulation with CpG-ODN, LPS, Pam-3-Cys, and Poly I:C. For these experiments, the cells were cultured at ∼1 × 106/ml, and each TLR-binding ligand was added at 5 μg/ml for the indicated time periods. At the end of the appropriate culture period, cells were harvested and stained for cell surface CLIP using anti-human CLIP-FITC versus anti-human MHC class II HLA-DR-PE Cy5 or anti-human CLIP-FITC versus anti-human CD19-PE. Cells were analyzed using a BD FACSCalibur for the 5 samples or a Beckman Coulter FC500.
RESULTS
TLR activation causes ectopic CLIP expression on B lymphocytes
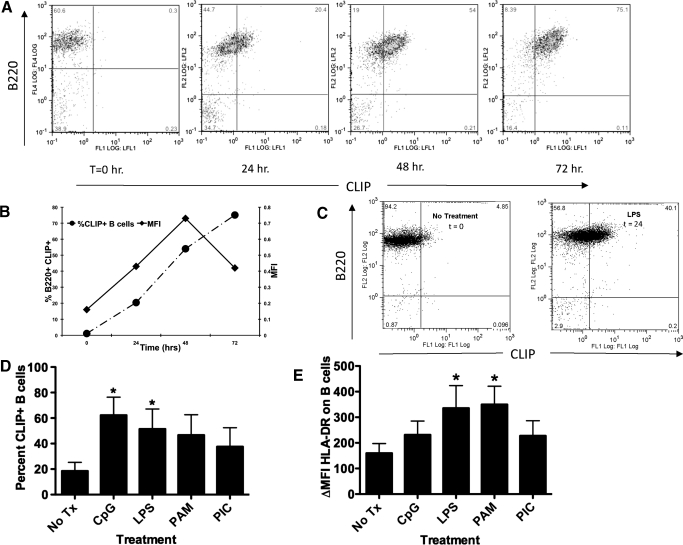
In vitro, LPS stimulation of mouse splenocytes resulted in a time-dependent increase in exogenous CLIP associated with MHC class II on B cells, as determined by staining with an anti-mouse CLIP/class II-specific antibody [21] versus anti-mouse B220 (Fig. 1A). We compared the increase in percent of CLIP+ B cells over time with the changes in geometric MFI and observed that the relative number of CLIP molecules per cell continues to rise over time, whereas the percentage of CLIP+ B cells over total number of B cells increases, peaking at 48 h, followed by a decline thereafter (Fig. 1B). This leads us to believe that MFI can be considered a measure of the relative increase in number of CLIP molecules per cell. Detection of CLIP in mouse studies, however, is limited by availability of antibody reagents to mouse CLIP; the one currently available is a mAb that recognizes CLIP only when associated with mouse MHC class II (I-Ab) and not CLIP associated with MHC class II from other alleles.
Figure 1. TLR activation of mouse spleen cells or human PBMCs results in ectopic CLIP in MHC class II on B cells.
(A) Immediately ex vivo, mouse splenocytes from B6.129 mice (H-2b) were treated with LPS for 24, 48, and 72 h as indicated. The cells were harvested at the indicated time-points and stained with 15G4-FITC and anti-mouse CLIP/I-Ab, as indicated on the x-axis, and PE-conjugated anti-mouse B220, antibody to the B cell molecule B220, y-axis, dually used to detect cell surface CLIP in the groove of I-Ab on B cells. The figure is representative of at least 4 experiments. FL1/2/4, Fluorescence 1/2/4; LFL½, laser fluorescence ½. (B) The percent CLIP+ B cells, left y-axis, dashed line with • (as determined by the staining method in A) was compared with the relative change in geometric MFI, right y-axis, solid line with ■, representing the increase in the amount of CLIP/I-Ab molecules per cell. (C) Purified resting B cells, isolated by magnetic bead separation (Miltenyi Biotec) from immediately ex vivo mouse splenocytes from B6.129 mice, were treated with LPS for 48 h. The cells were harvested and stained with 15G4-FITC, anti-mouse CLIP/I-Ab, as indicated on the x-axis, and PE-conjugated anti-mouse B220, antibody to the B cell molecule B220, y-axis, dually used to detect cell surface CLIP in the groove of I-Ab on B cells. (D) Human PBMC cells were stained using anti-human CLIP-FITC versus CD19-PE (values of isotype controls were subtracted from the specific stains, ΔMFI); nil vs. CpG, P = 0.06821; nil vs. LPS P = 0.0390; nil vs. PAM P = 0.0124. The data represent the percent of total PBMC that are CLIP+ B cells subsequent to each treatment. Each experiment represents at least 3 replicates. (E) Human PBMC from five donors were cultured for 24 hours with toll ligands (CpG-ODN, LPS, Pam3Cys, and Poly 1:C as indicated) and were stained with a pan anti-HLA-DR-FITC antibody (values of isotype controls were subtracted from the specific stains, ΔMF1).
TLRs are expressed on macrophages, DCs, and lymphocytes among other cells [1]. The increase in the number of B cells bearing ectopic CLIP and the increase in the number of molecules of CLIP per cell on the B cells may be attributed to the direct consequence of LPS binding TLR4 on the mouse B cell or the indirect effect of LPS on macrophages, other white cells, or their products. To distinguish between these possibilities, we isolated resting B cells using negative selection with antibody-coated magnetic beads (MACS, Miltenyi Biotec). For these experiments, purified and isolated, resting B cells were stimulated with LPS for 48 h and stained with antibodies to B220 versus CLIP/I-Ab. LPS stimulation induced an approximately 8-fold increase in the number of CLIP+ B cells from purified, CLIP+-resting B cells. This would suggest that induction of ectopic CLIP is the direct consequence of TLR engagement on the B cell (Fig. 1C).
As the commercially obtainable antibody to mouse CLIP peptide recognizes CLIP only when associated with MHC class II I-Ab, haplotype H-2b, we are limited to seeing only a 1:1 correspondence to I-Ab using this antibody. As there is an antibody to the human CLIP peptide that is not restricted to the detection of CLIP in the groove of HLA-Dr (MHC class II) molecules, but which rather recognizes CLIP independently, and as we wanted to investigate whether TLR activation would similarly induce CLIP on human B cells, we set up comparative CLIP experiments with human peripheral blood. We obtained PBMCs from 5 healthy donors, culturing the cells with no additional treatment, CpG-ODN, LPS, Pam3Cys, or Poly I:C, as indicated. We observed statistically significant increases in ectopic CLIP on the B cells treated with CpG-ODN and LPS (Fig. 1D). It is important to note that our ability to detect TLR-induced CLIP on purified human B cells requires the isolation of PBMC from freshly drawn human blood. The use of banked blood from filtered and stored blood appears to disallow detection of ectopic B cell CLIP. To rule out the possibility that ectopic CLIP resulted solely from coincident, increased levels of nascent MHC class II on the activated B cells, we counter-stained activated B cells with an MHC class II anti-human HLA-DR, -DP, and -DQ antibody (Fig. 1E). The increase in cell surface CLIP levels (Fig. 1D) does not correspond with the TLR-dependent changes in MHC class II (Fig. 1E), suggesting that TLR-mediated ectopic CLIP expression is not merely the consequence of increasing levels of cell surface MHC class II. In contrast, it is interesting to note that TLR (Poly I:C, Pam3Cys, R848, LPS, or CpG-ODN) activation of DCs and macrophages has been shown to result in MyD88-dependent maturation, increases in MHC class II, accelerated antigen processing and presentation, and loss of cell surface CLIP. In fact, cell surface CLIP is considered an indicator of immaturity in macrophages or DCs until they are activated by TLR engagement [22], at which point, cell surface CLIP expression decreases.
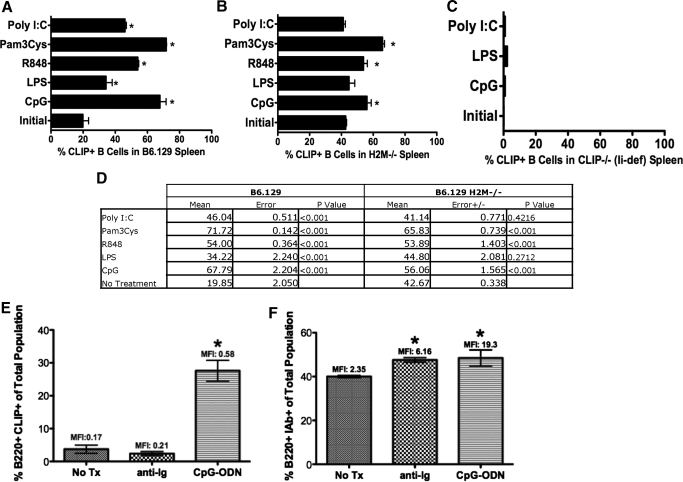
Treatment in vitro with all TLR ligands resulted in a statistically significant increase in CLIP+ B cells (Fig. 2A). As H-2M catalyzes the replacement of CLIP with peptide in lysosomes, we reasoned that ectopic CLIP on TLR-activated spleen cells from H-2M-deficient mice (H-2M−/− B6.129 background) might have higher numbers of CLIP+ B cells relative to strain-matched controls (Fig. 2B). As expected in mice less able to remove endogenously loaded CLIP, we observed that the initial levels of ectopic CLIP were higher on B cells from H-2M KO (Fig. 2B) animals than on the WT counterpart (initial: Fig. 2A). However, activation with TLR ligands CpG-ODN, R848, and Pam3Cys increased ectopic CLIP on H-2M-deficient B cells beyond initial levels in untreated, H-2M-deficient splenocytes. To rule out the possibility that the cell surface CLIP staining results from cross-reactivity or nonspecific binding, we performed fluorescent staining for CLIP on TLR-activated B cells from Ii-deficient animals (Fig. 2C) [23]. As expected, we detected little to no cell surface CLIP on B cells from the Ii-deficient animals (Fig. 2C).
Figure 2. Expression of cell surface CLIP on B cells is TLR-dependent, and antigen receptor engagement increases cell surface MHC class II but not ectopic CLIP on B cells.
(A) B6.129 mice (H-2b; P<0.001); (B) H-2M-deficient mice (P<0.001 for statistically different values); and (C) Ii, CD74, Ii-deficient mice, all on a B6.129 (H-2b) background, were injected with TLR-binding ligands [2, 7], Poly I:C, Pam3Cys, R848, LPS, and CpG-ODN, and no treatment, as indicated for 48 h. Spleens were harvested, and cells were stained with 15G4-FITC, anti-mouse CLIP/I-Ab, and PE-conjugated anti-mouse B220, antibody to the B cell molecule B220, and analyzed flow-cytometrically. Shown are percentages of CLIP+ B cells from splenocytes stimulated in vitro as indicated. (D) Table showing the mean percent CLIP+ B cells, sem, and P values for the graphs in A and B. (E) B6.129 splenocytes, untreated or treated in vitro with anti-Ig (as a surrogate for antigen) or CpG-ODN for 48 h. Cells were harvested and stained with anti-mouse B220-PE versus 15G4-FITC anti-mouse CLIP/I-Ab (P<0.001). (F) B6.129 splenocytes were untreated or treated in vitro with anti-Ig (as a surrogate for antigen) or with CpG-ODN for 48 h. Cells were harvested and were stained with anti-mouse B220-PE versus anti-mouse MHC class II, I-Ab (*P<0.001). Each experiment represents at least 3 replicates.
As B cell-specific antigen receptor engagement results in signals that increase the acidity in the lysosomes and inhibit the activity of H-2O, subsequently allowing H-2M to catalyze the replacement of CLIP with antigenic peptide-loading into MHC class II [24], we directly compared the effects of TLR versus antigen receptor engagement on cell surface expression of CLIP. We used anti-Ig stimulation as a known surrogate for antigen receptor signaling [25] and compared levels of ectopic CLIP and percentage of CLIP+ B cells with TLR-dependent B cell activation versus stimulation through the B cell antigen receptor. Splenocytes were treated in culture with anti-Ig or CpG-ODN, as indicated for 24 h, harvested, and stained for ectopic CLIP:MHC class II (Fig. 2D). As predicted, we observed significantly less ectopic CLIP per cell by measuring geometric MFI after antigen receptor-mediated stimulation. Similarly, the percent of CLIP+ B cells postantigen receptor engagement was reduced significantly relative to the percentage of CLIP+ B cells post-TLR activation (Fig. 2D). Anti-Ig and CpG-ODN stimulation resulted in significant and similar increases in MHC class II, as measured by changes in relative fluorescence intensity (Fig. 2E). These results indicate that antigen receptor engagement and TLR engagement of B cells result in a similar level of activations, as measured by increases in cell surface MHC class molecules. However, TLR engagement, but not antigen receptor engagement, significantly increases the percentage of cell surface, CLIP-positive B cells and the relative amount of CLIP per cell.
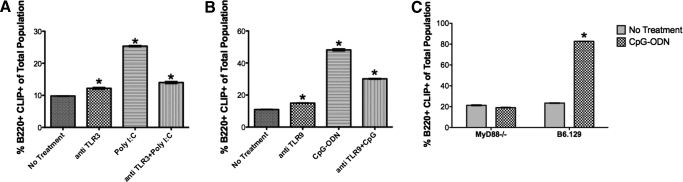
To address whether TLR-induced ectopic CLIP expression on B cells is the direct result of TLR engagement and signaling, we used two experimental approaches. In the first, we used antibody to TLR3 to block TLR3 (Poly I:C)-mediated increases in CLIP expression. In the second, as TLR2, -4, -7, -8, and -9 depend on the MyD88 pathway to signal cellular activation [1], we stimulated spleen cells from B6.129 or MyD88 KO animals on a B6.129 background with CpG (Fig. 3A). Cells were treated with antibody to Poly I:C (eBioscience, San Diego, CA, USA) for 30 min on ice, followed by treatment with Poly I:C for 24 h. Treatment with antibody to TLR3 prevented Poly I:C-induced, ectopic CLIP expression on B cells (Fig. 3A). CpG-ODN treatment of splenocytes from MyD88-deficient animals did not result in ectopic expression of CLIP, whereas CpG-ODN treatment of splenocytes from strain-matched B6.129 animals induced a statistically significant increase in CLIP+ B cells (Fig. 3B). These results suggest that ectopic CLIP induction requires TLR engagement and signaling.
Figure 3. CLIP induction requires TLR engagement and signaling.
(A) C57/B6 splenocytes were treated in vitro with anti-TLR3 antibody, Poly I:C, or anti-TLR3 + Poly I:C for 24 h and stained for B220-PE versus I-Ab/CLIP-FITC. The percentage of B220+CLIP+ cells is reported (P<0.001). (B) C57/B6 splenocytes were treated in vitro with anti-TLR9 antibody, CpG-ODN, or anti-TLR9 + CpG-ODN for 24 h and stained for B220-PE versus I-Ab/CLIP-FITC. The percentage of B220+CLIP+ cells is reported (*P<0.001). (C) MyD88−/− or B6.129 splenocytes were treated in vitro with CpG-ODN for 48 h and then stained for B220-PE versus I-Ab/CLIP-FITC. The percentage of B220+CLIP+ cells is reported (*P<0.001).
Peptide-dependent inhibition of immune activation
We reasoned that if B cells bearing ectopic CLIP were important for promoting immune activation (as defined by increased cellularity, movement from the periphery to the lymph node, and/or increased cytokine production of type I IFNs), then treatment with a competitive peptide with a higher predicted binding constant than CLIP for the peptide-binding groove in MHC class II molecules would result in decreased numbers of cells from spleen and lymph node.
As peptide and CLIP peptide affinity for MHC class II molecules is MHC allele-dependent [26], we used the MHCPred (http://www.darrenflower.info/MHCPred/) and NetMHC (http://www.cbs.dtu.dk/services/NetMHC/) databases to determine the differences in binding affinities between CLIP for molecules encoded by mouse or the known human MHC alleles. Using newly developed software, we predicted and later synthesized peptides with sequences of 11 aa, predicted by our software to have relatively higher binding constants than CLIP for all mouse and human MHC gene products (referred to as TPP, Table 2).
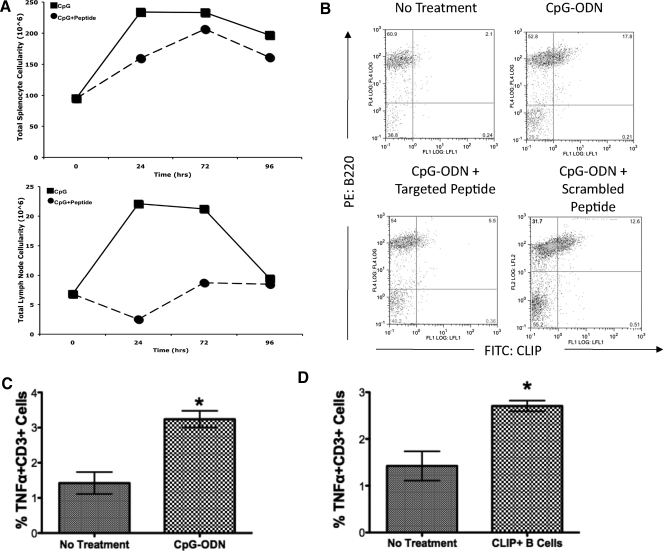
As controls for the novel peptide, we also synthesized known and well-characterized peptides that have higher or lower affinity-binding constants for the MHC class II I-Ab molecules in the B6.129 mice (Table 2). We injected B6.129 (H-2b) mice with CpG-ODN alone or CpG-ODN in combination with each of the peptides. As expected, the mice injected with CpG-ODN alone exhibited dramatic hyperplasia, as described previously [27], in spleen (Fig. 4A, upper panel) and lymph node (Fig. 4A, lower panel). Treatment with CpG-ODN caused an increase in the total numbers of splenic B-lymphocytes and splenic B cells that expressed cell surface CLIP (Fig. 4B, upper right panel). Increases were reversed with the high-binding peptide, not a scrambled control peptide (Fig. 4B, lower panels), indicating that peptide affinity affects characteristics of TLR activation. Treatment with TPP and CpG-ODN reversed the effects of CPG-ODN alone (Fig. 4B, lower left panel). However, treatment with sTPP or low-affinity peptide (Table 2) showed no reduction in the percent of CPG-induced CLIP+ B cells, (4B, lower right panel).
Figure 4. Administration of targeted peptide decreases inflammatory effects of TLR9 activation.
(A) B6.129 mice were injected with CpG-ODN alone (■, solid line) or with CpG-ODN and TPP (•, dashed line; see Table 2). Total spleen cell recoveries (upper panel) and lymph node cell recoveries (lower panel) at 24, 72, and 96 h are reported. (B) Immediately ex vivo mouse splenocytes from B6.129 mice (H-2b) were treated with CpG-ODN for 48 h. The cells were stained with 15G4-FITC, anti-mouse CLIP/I-Ab, as indicated on the x-axis, and PE-conjugated anti-mouse B220, antibody to the B cell molecule B220, y-axis. Spleen cells from untreated B6.129 (upper left panel) mice, spleen cells from mice injected with CpG-ODN for 48 h (upper right panel), spleen cells from mice injected with CpG-ODN + TPP (lower left panel), or spleen cells from mice injected with CpG-ODN + sTPP (lower right panel; see Table 2) were harvested and stained using anti-mouse B220-PE Cy5 versus 15G4-FITC anti-mouse CLIP/I-Ab. Cells were analyzed flow-cytometrically using a 2-dimensional dot-plot analysis. (C) C57B/6 mice were injected i.p. with CpG-ODN, and spleens were removed at 48 h postinjection. The percent of TNF-α-PE-Cy7+ CD3-PE+ cells is shown. (D) Purified B cells from C57B/6 were activated with CpG-ODN for 48 h, stained to confirm cell surface CLIP expression, and then adoptively transferred syngeneically by i.p. injection into C57B/6 host. TNF-α levels were measured by intracellular cytokine staining flow-cytometrically. These results represent the results of at least 3 separate experiments. *Statistical Significance.
Adoptive transfer of CLIP+ B cells into syngeneic animals induces TNF-α production in the host animals
We have shown that TLR activation results in expansion of CLIP+ B cells. As TLRs are known to induce secretion of the pro-inflammatory cytokine TNF-α, we sought to determine whether CLIP+ B cells contribute to, or are the consequence of, TLR-mediated TNF-α production. This experiment had 2 sequential parts. The first was to TLR-activate (using CpG-ODN, a TLR9 agonist) column-purified B cells to induce ectopic CLIP expression, confirming the induction by staining and flow cytometry analysis. For the first step in the protocol, we isolated purified, resting B cells using Miltenyi Biotec magnetic, negative selection for B cells, yielding >95% pure, resting B lymphocytes. The purified B cells were activated with CpG-ODN for 48 h and harvested, and a representative sample was stained and analyzed flow-cytometrically to confirm that the cells were CLIP+. The second part of the experimental design included injecting syngeneic mice with CpG-ODN alone (Fig. 4C) or adoptively transferring by injection the animals with the activated CLIP+ B cells. Forty-eight hours postinjection, splenocytes were harvested from the host animals and stained using a “cyto-perm/cyto-fix” kit with fluorochrome-conjugated antibody to TNF-α, staining for intracellular production of TNF-α. By comparing levels of TNF-α among unstimulated, CpG-ODN-activated, and adoptively transferred CLIP+ B cells, we detected significant increases in TNF-α levels with CpG-ODN injection, as expected. Injection of CLIP+ B cells into syngeneic animals resulted in significant increases in TNF-α production. The cells producing TNF-α were counter-stained with antibody to the TCR-CD3 complex and were determined to be a subpopulation of CD3+ cells. The data are reported as the percent TNF-α+, CD3+ cells. The data show that adoptive transfer/injection of syngeneic animals with CLIP+ B cells result in TNF-α production that is comparable with the production of TNF-α by injecting an animal directly with CpG-ODN.
Redistribution of CLIP+ B cells in spleen and lymph node
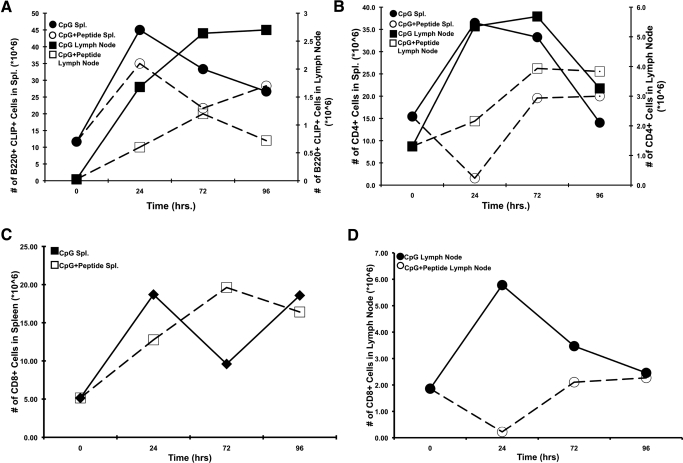
Following CpG-ODN injection, numbers of CLIP+ B cells in the spleen increased over time, peaking at 48 h (Fig. 5A). We observed a similar, but delayed, rise in the percentage and absolute number of CLIP+ B cells in the lymph nodes (Fig. 5A). This is consistent with reports of CpG-ODN-induced hyperplasia [27]. Conversely, the addition of TPP following CpG-ODN treatment caused a reduction in total cell numbers and in absolute numbers of B cells and significantly altered CLIP+ B cell distribution between spleen and lymph node (Fig. 5A). We note that TPP peptide treatment reduced the expansion of splenic and lymph node CD4+ T cells (Fig. 5B), reduced CD8+ T cell expansion in the lymph node (Fig. 5D), but did not block expansion of CD8+ T cells in the spleen (Fig. 5C).
Figure 5. Effects of targeted peptides on the distribution of TLR-activated lymphoid subsets of B cells, CD4+ cells, and CD8+ cells.
(A) B6.129 mice were injected with CpG-ODN alone (■/•) or CpG-ODN with TPP (□/○; Table 2). Spleen (Spl.; circles, left y-axis) and lymph nodes (squares, right y-axis) were harvested at 24, 72, and 96 h and stained using anti-mouse B220-PE versus 15G4-FITC anti-mouse CLIP/I-Ab. Data were plotted as percent CLIP+ B cells from spleen, left y-axis, or node, right y-axis. (B) B6.129 mice were injected with CpG-ODN alone (■/•) or CpG-ODN with TPP (□/○). Spleen (squares, left y-axis) and lymph nodes (circles, right y-axis) were harvested at 24, 72, and 96 h and stained using anti-mouse CD4, GK 1.5 FITC. Data were plotted as percent CD4+ T cells from spleen or node as indicated. (C) B6.129 mice were injected with CpG-ODN alone (■) or CpG-ODN with TPP (□). Spleen (squares) was harvested at 24, 72, and 96 h and stained using anti-mouse CD8-PE. Data were plotted as percent CD8+ T cells from spleen. (D) B6.129 mice were injected with CpG-ODN alone (•) or CpG-ODN with TPP (○). Lymph nodes were harvested at 24, 72, and 96 h and stained using anti-mouse CD8-PE. Each experiment represents at least 3 replicates.
Peptide-dependent B cell death
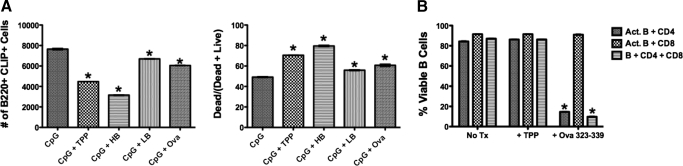
CD4+ T cells, T regulatory cells specifically, have been reported to kill polyclonally activated B cells [28]. As MHC engagement of nonantigen-primed B cells results in B cell death [29], we reasoned that peptide exchange for CLIP on activated B cells, in the absence of antigen receptor (or other) survival signals, may promote MHC class II-dependent B cell death by interaction with CD4+ T cells. To assess the possibility that exogenous loading of known peptides with high, but not low peptide-binding affinity (IC50; Table 2), would lead to an increase in B cell death of those treated with high-affinity peptides, but not in those treated with low or scrambled peptides, we monitored the number and percentage of CLIP+ B220-positive cells and percent live or dead B cells after treatment with CpG-ODN, with or without peptides as indicated (Fig. 6A). These results demonstrate that high-affinity, but not low or scrambled high-affinity, peptides lead to death of polyclonally activated B cells.
Figure 6. Peptide treatment results in death of TLR-activated B cells and requires TCR specificity.
Purified B, CD4+ T, and CD8+ T cells were isolated from C57BL/6 murine spleens using magnetic bead separation (Miltenyi Biotec). Isolated cells, combined as indicated, were cultured (106 cells/ml) in the presence of CpG-ODN, with or without peptides (Table 2), including TPP, HB, LB, or OVA 323–339 at an E:T ratio of 1:5. The cells were harvested and stained with 15G4-FITC, anti-mouse CLIP/I-Ab, and PE-conjugated anti-mouse B220, antibody to the B cell molecule B220. (A, left) The number of live B220+, CLIP+ population (*P<0.001). (A, right) The percent of dead cells resulting from treatments as indicated (*P<0.001). (B) B cells were isolated from C57BL/6 spleen and cultured with or without purified CD4+ T cells isolated from OT-2 transgenic mice expressing only TCRs specific for the OVA peptide 323–339. Cells were cultured with and/or without CD8+ T cells derived from C57Bl6. All cells were cultured at 106 cells/ml in the presence of CpG, with or without TPP or OVA 323–339 at an E:T ratio of 1:5 (*P <0.001). Act. B, Activated B cell.
To determine whether death of the B cells requires CD4 TCR recognition of the peptide in the groove of MHC class II molecules, we obtained transgenic mice (OT-2 transgenics). These mice have been generated to express a single TCR specificity for a peptide derived from the protein OVA (Table 2) when the peptide is displayed in the peptide-binding groove of mouse MHC class II I-Ab. We isolated B cells from nontransgenic C57B16 (H-2b) mice or from the OT-2 transgenics (on the C57B16, H-2b background). B cells activated with CpG-ODN, from WT and OT-2 transgenics, expressed high levels of ectopic CLIP. Treatment of CpG-activated CLIP+ B cells with the OVA peptide resulted in death of the activated B cells only when the activated cells were treated with the OVA peptide and only in the presence of CD4+ T cells from the OT-2 transgenic mice. These data confirm that peptide-dependent death of TLR-activated B cells results from CD4+ T cell recognition of a specific peptide in the peptide-binding groove of I-Ab, formerly occupied by CLIP (Fig. 6B).
Our results reveal that treatment with Toll ligands results in polyclonal B cell activation accompanied by ectopic expression of CLIP. Furthermore, we demonstrate that targeted peptide treatment results in inhibition of cytokine production, decreased cellularity, and peptide-dependent death of TLR-activated B cells.
DISCUSSION
Our work offers a novel and previously unpredicted connection as to what may cause post-infection inflammation to become chronic in some people. Our work suggests that MHC genes determine if TLR-activated B cells can be controlled properly by T cell-receptor (TCR)-dependent B cell death. This theory supports the hypothesis that treatment of lymphocytes with Toll ligands results in polyclonal B and T cell activation and ectopic, cell-surface expression of CLIP. We propose that TLR-dependent, ectopic CLIP on the surfaces of B and T cells is a primordial response to acute infections and signals potential harm to the host, thereby initiating an inflammatory response. Subsequently, antigenic specificity triggers the transition from an acute, innate response to an adaptive, specific response by supporting the survival of antigen-specific cells and MHC-dependent death of non-specific immune cells. Although intra-lysosomal CLIP exchange is well studied, our findings that TLR engagement results consistently in ectopic, class II/CLIP complexes on B cells suggest a new and distinct immunological process that when inadequately controlled, may contribute to chronic inflammation.
These results suggest a novel therapeutic approach for redirecting immune imbalances using targeted peptides. We have computationally predicted peptides that bind to an individual's MHC gene products with higher affinity than the CLIP peptide, and we have had these target peptides individually synthesized. In mouse models, treatment of polyclonally activated CLIP+ B cells with synthesized targeted peptides results in significant reduction in the percentages of TLR-activated cells, inhibition of TLR-mediated hyperplasia in spleen and lymph nodes in mice, death of B cells, and a reduction of TLR-dependent cytokine production (Fig. 5).
We propose that specific antigen-receptor engagement generates a survival signal. Reciprocally, when T cells recognize MHC class II, and B cells are polyclonally activated by TLRs, the result is death of the activated B cell [30, 31]. This mechanism could serve to prevent the production of potentially dangerous autoreactive antibodies, as generally acknowledged studies would appear to corroborate [32].
Furthermore, we propose that the transition between acute inflammation and a specific adaptive immune response is mediated by polyclonal B cell and T cell activation. Relatively nonspecific, antipathogenic responses and inflammation can quickly promote an antimicrobial response as a part of innate immunity. For example, macrophages, γ δ T cells, and NK cells have all been shown to produce defensins as antimicrobial products [33] subsequent to infection. The human antimicrobial and chemotactic peptides, such as LL-37 and α-defensins, are expressed by certain lymphocyte and monocyte populations [33]. Once the acute response subsides, we suggest that an adaptive, acquired, and specific immune response is facilitated by an antigenic peptide-dependent death of the polyclonally expanded cells, while leaving a focused, specific antipeptide response, thereby limiting acute inflammation and selectively supporting survival of the antigen-specific B cells.
Conversely, failure to control the initial innate response, including exchange or loss of CLIP+ B and T cells, may be a contributing factor to chronic immune activation and inflammation. Although the definitive role of ectopic CLIP on activated B cells has yet to be elucidated, our observations not only reflect but serve to broaden current findings that B cell depletion is an effective therapy for diseases such as Multiple Sclerosis, type I diabetes, Crohn's disease, and Lyme disease, all characterized by chronic, immune activation. We feel our hypothesis finally sheds some light on the mechanism that triggers chronic inflammation and lends further corroboration that B cell depletion is effective because of the depletion of a subset of B cells that is causally related to inflammation [34].
ACKNOWLEDGMENTS
Support for this work was provided by the University of Colorado Technology Transfer Office (University of Colorado, Colorado Springs, CO, USA); The Mary K. Chapman Foundation (Tulsa, OK, USA); The Coleman Institute for Cognitive Disabilities (Boulder, CO, USA); Viral Genetics (San Marino, CA, USA; peptide products of Viral Genetics are included in the series of biologics, including TPP); the Time for Lyme Foundation; and the Turn the Corner Foundation. We gratefully acknowledge Evan Newell, Ph.D., for computational predictions of peptides and peptide binding; Jeff Rogers for technical assistance; Kathleen McKee for extensive editorial and writing assistance; Robert Melamede, Ph.D., for his helpful review of our manuscript; and Monica Ord and Haig Keledjian of Viral Genetics for helpful discussions and support.
Footnotes
- APC
- allophycocyanin
- CLIP
- class II-associated invariant peptide
- HB
- high binder
- Ii
- invariant chain
- KO
- knockout
- LB
- low binder
- MFI
- mean fluorescence intensity
- ODN
- oligodeoxynucleotide
- Pam3Cys
- tripalmitoyl-S-glyceryl-cysteine
- Poly I:C
- polyinosinic:polycy-tidylic acid
- sTPP
- scrambled targeted proprietary peptide
- TPP
- targeted proprietary peptide
AUTHORSHIP
M.K.N.—conceived the hypothesis, designed experiments, evaluated data, and contributed to the writing and preparation of the manuscript. R.P.T.—contributed to the design and performance of the experiments and manuscript preparation and writing. J.H.C.—contributed to the design and performance of the experiments and manuscript preparation and writing. M.B.S.—contributed to performance of the experiments and manuscript preparation and writing. A.H.—contributed to the design and performance of the experiments and manuscript preparation and writing. E.M.V-M.—contributed to performance of the experiments. M.B.—contributed to manuscript preparation and writing. E.M.—contributed to the design and performance of the experiments. C.P.H.—contributed to the design and performance of the experiments. A.B.—contributed to performance of the experiments. A.B-O.—contributed to the conception of ideas and manuscript review. M.S.F.—contributed to the conception of ideas, cell lines, and manuscript review. J.N.—contributed to the conception of ideas and manuscript review. N.A.—contributed to the design and performance of the experiments and manuscript preparation and writing. S.S.Z.—contributed to the conception of ideas and manuscript review and provided transgenic mice. J.P.A.—conception of the idea, contributed to the design of the experiments, manuscript revisions and review.
REFERENCES
- 1. Takeuchi O., Akira S. (2010) Pattern recognition receptors and inflammation. Cell 140, 805–820 [DOI] [PubMed] [Google Scholar]
- 2.McGettrick A. F., O'Neill L. A. (2007) Toll-like receptors: key activators of leucocytes and regulator of haematopoiesis. Br. J. Haematol. 139, 185–193 [DOI] [PubMed] [Google Scholar]
- 3. Steere A. C., Klitz W., Drouin E. E., Falk B. A., Kwok W. W., Nepom G. T., Baxter-Lowe L. A. (2006) Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J. Exp. Med. 203, 961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghans J. A., Molgaard A., de Boer R. J., Kesmir C. (2007) HLA alleles associated with slow progression to AIDS truly prefer to present HIV-1 p24. PLoS One 2, e920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glimcher L. H., Polla B. S., Poljak A., Morton C. C., McKean D. J. (1987) Murine class II (Ia) molecules associate with human invariant chain. J. Immunol. 138, 1519–1523 [PubMed] [Google Scholar]
- 6. Roche P. A. (1996) Out damned CLIP! Out, I say! Science 274, 526–527 [DOI] [PubMed] [Google Scholar]
- 7.Baenziger S., Heikenwalder M., Johansen P., Schlaepfer E., Hofer U., Miller R. C., Diemand S., Honda K., Kundig T. M., Aguzzi A., Speck R. F. (2009) Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood 113, 377–388 [DOI] [PubMed] [Google Scholar]
- 8. Cross A. H., Stark J. L., Lauber J., Ramsbottom M. J., Lyons J. A. (2006) Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J. Neuroimmunol. 180, 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bour-Jordan H., Bluestone J. A. (2007) B cell depletion: a novel therapy for autoimmune diabetes? J. Clin. Invest. 117, 3642–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuek A., Hazleman B. L., Ostor A. J. (2007) Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad. Med. J. 83, 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soulas P., Woods A., Jaulhac B., Knapp A. M., Pasquali J. L., Martin T., Korganow A. S. (2005) Autoantigen, innate immunity, and T cells cooperate to break B cell tolerance during bacterial infection. J. Clin. Invest. 115, 2257–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Ham S. M., Tjin E. P., Lillemeier B. F., Gruneberg U., van Meijgaarden K. E., Pastoors L., Verwoerd D., Tulp A., Canas B., Rahman D., Ottenhoff T. H., Pappin D. J., Trowsdale J., Neefjes J. (1997) HLA-DO is a negative modulator of HLA-DM-mediated MHC class II peptide loading. Curr. Biol. 7, 950–957 [DOI] [PubMed] [Google Scholar]
- 13.Van Ham S. M., Gruneberg U., Malcherek G., Broker I., Melms A., Trowsdale J. (1996) Human histocompatibility leukocyte antigen (HLA)-DM edits peptides presented by HLA-DR according to their ligand binding motifs. J. Exp. Med. 184, 2019–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weber D. A., Evavold B. D., Jensen P. E. (1996) Enhanced dissociation of HLA-DR-bound peptides in the presence of HLA-DM. Science 274, 618–620 [DOI] [PubMed] [Google Scholar]
- 15.Grey H. M., Colon S. M., Chesnut R. W. (1982) Requirements for the processing of antigen by antigen-presenting B cells. II. Biochemical comparison of the fate of antigen in B cell tumors and macrophages. J. Immunol. 129, 2389–2395 [PubMed] [Google Scholar]
- 16. Roehm N. W., Leibson H. J., Zlotnik A., Kappler J., Marrack P., Cambier J. C. (1984) Interleukin-induced increase in Ia expression by normal mouse B cells. J. Exp. Med. 160, 679–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falkoff R. J., Zhu L. P., Fauci A. S. (1982) Separate signals for human B cell proliferation and differentiation in response to Staphylococcus aureus: evidence for a two-signal model of B cell activation. J. Immunol. 129, 97–102 [PubMed] [Google Scholar]
- 18. Barrachina M., Gonalons E., Celada A. (1999) LPS upregulates MHC class II I-A expression in B lymphocytes at transcriptional and at translational levels. Tissue Antigens 54, 461–470 [DOI] [PubMed] [Google Scholar]
- 19.Xu X., Press B., Wagle N. M., Cho H., Wandinger-Ness A., Pierce S. K. (1996) B cell antigen receptor signaling links biochemical changes in the class II peptide-loading compartment to enhanced processing. Int. Immunol. 8, 1867–1876 [DOI] [PubMed] [Google Scholar]
- 20. Torres-Aguilar H., Aguilar-Ruiz S. R., Gonzalez-Perez G., Munguia R., Bajana S., Meraz-Rios M. A., Sanchez-Torres C. (2010) Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J. Immunol. 184, 1765–1775 [DOI] [PubMed] [Google Scholar]
- 21.Farr A., DeRoos P. C., Eastman S., Rudensky A. Y. (1996) Differential expression of CLIP:MHC class II and conventional endogenous peptide:MHC class II complexes by thymic epithelial cells and peripheral antigen-presenting cells. Eur. J. Immunol. 26, 3185–3193 [DOI] [PubMed] [Google Scholar]
- 22. West M. A., Wallin R. P., Matthews S. P., Svensson H. G., Zaru R., Ljunggren H. G., Prescott A. R., Watts C. (2004) Enhanced dendritic cell antigen capture via Toll-like receptor-induced actin remodeling. Science 305, 1153–1157 [DOI] [PubMed] [Google Scholar]
- 23.Slavin A. J., Soos J. M., Stuve O., Patarroyo J. C., Weiner H. L., Fontana A., Bikoff E. K., Zamvil S. S. (2001) Requirement for endocytic antigen processing and influence of invariant chain and H-2M deficiencies in CNS autoimmunity. J. Clin. Invest. 108, 1133–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alfonso C., Liljedahl M., Winqvist O., Surh C. D., Peterson P. A., Fung-Leung W. P., Karlsson L. (1999) The role of H2-O and HLA-DO in major histocompatibility complex class II-restricted antigen processing and presentation. Immunol. Rev. 172, 255–266 [DOI] [PubMed] [Google Scholar]
- 25.Finkelman F. D., Mond J. J., Woods V. L., Wilburn S. B., Berning A., Sehgal E., Scher I. (1980) Effects of anti-immunoglobulin antibodies on murine B lymphocytes and humoral immune responses. Immunol. Rev. 52, 55–74 [DOI] [PubMed] [Google Scholar]
- 26. Gelin C., Sloma I., Charron D., Mooney N. (2009) Regulation of MHC II and CD1 antigen presentation: from ubiquity to security. J. Leukoc. Biol. 85, 215–224 [DOI] [PubMed] [Google Scholar]
- 27.Baker C. A., Clark R., Ventura F., Jones N. G., Guzman D., Bangsberg D. R., Cao H. (2007) Peripheral CD4 loss of regulatory T cells is associated with persistent viraemia in chronic HIV infection. Clin. Exp. Immunol. 147, 533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao D. M., Thornton A. M., DiPaolo R. J., Shevach E. M. (2006) Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood 107, 3925–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newell M. K., VanderWall J., Beard K. S., Freed J. H. (1993) Ligation of major histocompatibility complex class II molecules mediates apoptotic cell death in resting B lymphocytes. Proc. Natl. Acad. Sci. USA 90, 10459–10463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blanchereau V., Charron D., Mooney N. (2002) HLA class II signals sensitize B lymphocytes to apoptosis via Fas/CD95 by increasing FADD recruitment to activated Fas and activation of caspases. Hum. Immunol. 63, 375–383 [DOI] [PubMed] [Google Scholar]
- 31.Truman J. P., Ericson M. L., Choqueux-Seebold C. J., Charron D. J., Mooney N. A. (1994) Lymphocyte programmed cell death is mediated via HLA class II DR. Int. Immunol. 6, 887–896 [DOI] [PubMed] [Google Scholar]
- 32. Catron D. M., Itano A. A., Pape K. A., Mueller D. L., Jenkins M. K. (2004) Visualizing the first 50 hr of the primary immune response to a soluble antigen. Immunity 21, 341–347 [DOI] [PubMed] [Google Scholar]
- 33.Agerberth B., Charo J., Werr J., Olsson B., Idali F., Lindbom L., Kiessling R., Jornvall H., Wigzell H., Gudmundsson G. H. (2000) The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96, 3086–3093 [PubMed] [Google Scholar]
- 34. Nikolajczyk B. S. (2010) B cells as under-appreciated mediators of non-auto-immune inflammatory disease. Cytokine 50, 234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]