Lnk physically interacts with c-Fms and blunts its activity in-cluding proliferation of macrophage progenitor cells, M-CSF stimulated migration, and generaton of ROS.
Keywords: receptor tyrosine kinase, cytokine, signaling, ROS, migration
Abstract
The M-CSFR (c-Fms) participates in proliferation, differentiation, and survival of macrophages and is involved in the regulation of distinct macrophage functions. Interaction with the ligand M-CSF results in phosphorylation of tyrosine residues on c-Fms, thereby creating binding sites for molecules containing SH2 domains. Lnk is a SH2 domain adaptor protein that negatively regulates hematopoietic cytokine receptors. Here, we show that Lnk binds to c-Fms. Biological and functional effects of this interaction were examined in macrophages from Lnk-deficient (KO) and WT mice. Clonogenic assays demonstrated an elevated number of M-CFUs in the bone marrow of Lnk KO mice. Furthermore, the M-CSF-induced phosphorylation of Akt in Lnk KO macrophages was increased and prolonged, whereas phosphorylation of Erk was diminished. Zymosan-stimulated production of ROS was increased dramatically in a M-CSF-dependent manner in Lnk KO macrophages. Lastly, Lnk inhibited M-CSF-induced migration of macrophages. In summary, we show that Lnk binds to c-Fms and can blunt M-CSF stimulation. Modulation of levels of Lnk in macrophages may provide a unique therapeutic approach to increase innate host defenses.
Introduction
Lnk is expressed in hematopoietic cells and plays a critical role in early hematopoiesis [1–4]. The phenotype of Lnk-deficient mice revealed critical functions of Lnk in B-lymphopoiesis, erythropoiesis, and megakaryopoiesis [5–8]. Together with its family members, adaptor molecule containing PH and SH2 domains (APS) and SH2-B, Lnk shares several structural motifs, including a proline-rich N terminus, a PH domain, a SH2 domain, and a conserved tyrosine near the carboxyl-terminus [9]. Ligand-mediated phosphorylation of tyrosine residues within the thrombopoietin receptor (Mpl) [7] or stem cell factor receptor (c-Kit) [2, 10] leads to interaction of Lnk with the receptors, and thereby, Lnk inhibits their downstream signaling [4, 7, 8]. An intact SH2 domain is essential for the inhibitory effects of Lnk, and the SH2 domain mutant R392E Lnk fails to interact with the activated receptors [2, 7, 8]. Proliferation and differentiation of hematopoietic precursors are markedly enhanced by decreased expression of Lnk and are prominently inhibited by increased levels of Lnk [2, 3]. Therefore, lack of Lnk binding to cytokine receptors makes hematopoietic progenitor cells more sensitive to cytokine signals. Furthermore, Lnk mRNA is up-regulated by TNF-α in endothelial cells, which indicates a role of Lnk in inflammation [11].
Previously, we have shown that Lnk interacts with the juxtamembrane domain of the human stem cell receptor c-Kit [12], which with the receptor for M-CSF, c-Fms, belongs to the class III family of receptor tyrosine kinases that are structurally characterized by a cytoplasmic tyrosine kinase domain, activated by binding of the specific ligand. c-Kit and c-Fms share a conserved sequence in the juxtamembrane domain. This prompted us to investigate whether Lnk interacts with c-Fms and is involved in signaling and cellular functions mediated by M-CSF.
c-Fms is expressed mainly on the surface of mononuclear phagocytes, such as macrophages and monocytes that are part of the innate immune system [13, 14]. In response to triggers such as cytokines, pathogens, or damaged cells, macrophages are able to induce or enhance their functions, including phagocytosis, chemotaxis, antigen presentation, and killing of microbial or tumor cells [15]. Furthermore, macrophages influence the surrounding cells or themselves by secretion of biologically active products, such as ROS, proteases, and cytokines, which are important mediators for immune responses. Interaction of c-Fms with the ligand M-CSF results in dimerization of the receptor leading to activation of the intracellular tyrosine kinase domain and phosphorylation of tyrosine residues [16] that serve as binding sites for several molecules containing SH2 domains, such as Src-family kinases, growth factor receptor-bound protein 2, and PI3K. These then activate signal transduction pathways that control macrophage proliferation, differentiation [17, 18], and distinct macrophage functions [19, 20]. For example, activation of Erk signaling promotes macrophage proliferation and cytokine production [21, 22], and activation of PI3K enhances macrophage survival and migration via Akt [23–25]. Recently, Yu et al. [26] reported that the phosphorylation of different tyrosine residues of c-Fms results in different activities of the receptor, thereby influencing distinct macrophage functions.
Macrophage activation in response to bacterial products is mediated primarily by members of the TLR family consisting of 10 different members (TLR1–10) that recognize structurally conserved molecules derived from pathogens. For example, bacterial cell-surface LPS binds to TLR4, lipoproteins such as Pam3CSK4 are recognized by TLR2, and unmethylated, double-stranded bacterial CpG DNA stimulates TLR9. In contrast, yeast cell wall particles (zymosan) interact with TLR2 and TLR6 [27], as well as the C-type lectin Dectin-1 [28]. TLR signaling mainly results in activation of the MyD88-IL-1R-associated kinase-TRAF6 pathway to regulate proinflammatory responses [29]. Most of the TLRs can activate additional pathways, and in the absence of M-CSF, bacterial products such as LPS and CpG DNA can mediate survival of macrophages and activate the MAPK [30] and PI3K/Akt pathways [31]. After stimulation with LPS, M-CSF is induced and primes macrophages to respond to further LPS stimulation with enhanced cytokine production [29]. Furthermore, M-CSF signaling can reduce expression levels of TLR9 and can decrease production of cytokines upon stimulation with CpG DNA [32]. Thus, the pathways of c-Fms and TLRs in macrophages are closely related, share downstream targets, and thereby, interact with each other.
In this study, we report for the first time that Lnk physically interacts with c-Fms and profoundly affects c-Fms function, as shown in macrophages from Lnk-deficient mice.
MATERIALS AND METHODS
Mice and cells
WT and Lnk-deficient (KO) 129/Sv mice were kindly provided by Tony Pawson (University of Toronto, Ontario, Canada) and maintained at Cedars-Sinai Medical Center (Los Angeles, CA, USA). Bone marrow-derived macrophages were prepared from the femurs of WT and KO mice by culture of bone marrow cells in complete medium (RPMI 1640, 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine), supplemented with 50 ng/mL rM-CSF (PeproTech, Rocky Hill, NJ, USA) for 7 days. Peritoneum-derived macrophages were harvested 96 h after i.p. injection of 4% thioglycollate. HEK293T cells were maintained in complete DMEM medium. The murine macrophage cell line RAW 264.7 was cultured in complete RPMI-1640 medium.
Coimmunoprecipitation
HEK293T cells were cotransfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocol. Forty-eight hours after transfection, serum-starved cells were treated with 10 ng/mL rM-CSF for 10 min and lysed in lysis buffer containing 0.5% Nonidet P-40, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, and Complete protease inhibitor cocktail (Roche Applied Science, Indianapoils, IN, USA). Coimmunoprecipitation was performed in lysis buffer. Protein (500 μg) was precipitated using 1 μg V5-tag (Applied Biological Materials, Richmond, BC, Canada) or c-Fms antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 15 μL Protein A/G PLUS agarose (Santa Cruz Biotechnology) at 4°C for 16 h, and then washed with PBS, eluted with SDS sample buffer, and resolved by SDS-PAGE. Precipitated proteins were detected with c-Fms or V5-tag antibodies. For coimmunoprecipitation of endogenously expressed Lnk and c-Fms, RAW 264.7 cells were serum-starved, treated with 10 ng/ml rM-CSF for 5 min, and lysed in lysis buffer, and 1 mg protein was coimmunoprecipitated using c-Fms or IgG isotype antibodies (Santa Cruz Biotechnology). Precipitated proteins were separated by SDS-PAGE, and proteins were detected by Lnk or c-Fms antibodies.
Clonogenic assay
Resuspended mononuclear bone marrow cells (2×104 cells/mL) from WT and Lnk KO mice and growth factors were added to methylcellulose medium M3234 (Stem Cell Technologies Inc., Vancouver, BC, Canada) to yield a final concentration of 1% methylcellulose, 30% FCS, 1% BSA, 10−4 M ME, and 2 mM L-glutamine. The final concentration of 10 ng/mL rM-CSF was added. One thousand cells were plated in six-well plates in a volume of 1.1 mL/well and incubated at 37°C in a humidified atmosphere containing 5% CO2. Colonies were counted after 7 days.
Real-time PCR
Total RNA was extracted from murine cells using TRIZOL reagent (Invitrogen), according to the standard protocol. Total RNA (3 μg) was processed to cDNA by RT with Superscript II (Invitrogen), as described in the manufacturer's protocol. Primers with the following sequences were synthesized by Invitrogen: murine Lnk, cctggtgcttcggtctccag (forward) and aacaggcctcttggagcttgg (reverse); murine c-Fms, tcacccaaagggccatatac (forward) and tgccttgttttgtgccatta (reverse); and 18S, aaacggctaccacatccaag (forward) and cctccaatggatcctcgtta (reverse). The expression levels of murine Lnk, murine c-Fms, and 18S (for endogenous reference) were measured with Platinum Taq DNA polymerase (Invitrogen) and SYBR Green I (Invitrogen). The reactions were done in triplicates in an iCycler iQ system (Bio-Rad, Hercules, CA, USA). The thermal cycling conditions were as follows: 2 min at 95°C, followed by 50 cycles of 95°C for 20 s, 60°C for 20 s, 72°C for 20 s, and fluorescence determination at the melting temperature of the product for 20 s.
Surface markers
Peritoneum- and bone marrow-derived macrophages were gently scraped from the surface of a 24-well plate and stained with antibodies specific for F4/80, CD11b, or CD11c, along with the relevant isotype controls (BD Biosciences PharMingen, San Diego, CA, USA). Flow cytometry was conducted using a FACSCalibur immunocytometry system.
Western blot
Two hundred fifty thousand murine macrophages from WT or Lnk KO mice were plated per well in 24-well plates, serum-starved overnight, and induced with 50 ng/mL rM-CSF. At the indicated time-points, cells were lysed on ice in Laemmli sample buffer, and lysates from 100,000 cells were loaded/lane in a SDS-PAGE gel (Invitrogen). After blotting to a PVDF membrane, protein levels were detected using the following antibodies: phospho-Akt and Akt (Cell Signaling Technology, Danvers, MA, USA), phospho-Erk and Erk (Millipore, Billerica, MA, USA), phosphotyrosine, c-Fms, and Lnk (Santa Cruz Biotechnology), and β-actin (Sigma-Aldrich, St. Louis, MO, USA).
Phagocytosis assay
FITC-labeled zymosan particles (100 μg/mL) were fed to bone marrow-derived macrophages from WT and Lnk KO mice for 30 min, and bound but uninternalized zymosan was removed by treating cells with proteinase K prior to assessment of zymosan internalization by flow cytometry.
Measurement of ROS
Fifty thousand macrophages were plated in each well in a 96-well luminometer plate (Corning, Lowell, MA, USA), treated with 50 ng/mL M-CSF or 25 U/mL IFN-γ (PeproTech) or without any cytokines for 18 h before stimulation with 10 μg/mL zymosan, and the production of ROS was assayed by luminol-enhanced chemiluminescence with readings taken at 3-min intervals.
ELISA
Fifty thousand murine macrophages were plated in each well in a 96-well plate in complete medium containing 50 ng/mL rM-CSF. Cells were stimulated for 18 h with 10 μg/mL zymosan (Sigma-Aldrich), 10 ng/mL LPS (List Biological Laboratories, Campbell, CA, USA), 600 nM CpG, or 10 ng/mL Pam3CSK4 (EMC Microcollections, Tuebingen, Germany), respectively. Supernatants were collected, and levels of murine TNF-α or murine IL-6 were measured using OptEIA mouse TNF or IL-6 ELISA sets (Becton Dickinson, Franklin Lakes, NJ, USA), according to the manufacturer's recommendations. Equality of cell numbers of WT and Lnk KO macrophages was determined using a MTT assay. Briefly, cells were cultured with medium containing 1 mg/mL MTT (Sigma-Aldrich) for 3 h, and after resuspension in isopropanol, the OD at 570 nm was measured.
Migration assay
Peritoneum-derived macrophages were harvested and plated in a Petri dish overnight. After detachment with 1 mM EDTA in PBS, adherent cells were resuspended in RPMI, and 250,000 cells were plated onto Transwell polycarbonate membranes containing 5.0 μm pores (Corning), placed in a 24-well plate, each well containing RPMI, RPMI with 100 ng/mL rM-CSF, or RPMI with 10% FCS. Cells were cultured at 37°C for 20 h. This was followed by removing the remaining cells from the upper side of the Transwell by wiping them off with a cotton swab. The membranes were stained with a Diff-Quik stain set (Dade Behring, Deerfield, IL, USA). With an inverted light microscope, cells were counted in 5 random fields of each membrane.
Statistical analyses
Paired and unpaired Student's t tests were used for ELISA experiments and for the migration and the clonogenic assays. P values were calculated based on comparisons of Lnk KO and WT cells, which were tested at the same time.
RESULTS
Lnk binds to c-Fms upon exposure of cells to M-CSF
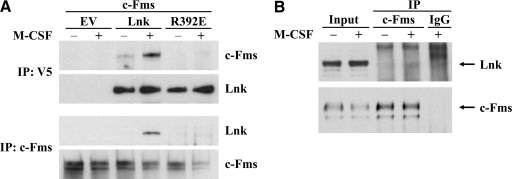
We found previously that Lnk binds to the juxtamembrane domain of c-Kit [12]. As a result of sequence similarities in the juxtamembrane domains of c-Kit and c-Fms, we hypothesized that Lnk might also be a binding partner of c-Fms. Therefore, Lnk and c-Fms were coexpressed in HEK293T cells, and coimmunoprecipitation was performed with lysates of untreated and M-CSF-treated cells. We show that Lnk interacts physically with c-Fms after stimulation of the receptor with its ligand (Fig. 1A). In untreated, serum-starved cells, only minor binding could be observed. Similarly, weak but reproducible interaction of endogenously expressed Lnk and c-Fms could be observed in murine RAW 264.7 macrophages after treatment with M-CSF (Fig. 1B).
Figure 1. Lnk binds to activated c-Fms with its SH2 domain.
(A) HEK293T cells were cotransfected with cDNAs for c-Fms, V5-tagged Lnk, R392E mutant Lnk, or empty control vector (EV). After starving overnight (–), cells were treated with M-CSF for 10 min (+), lysed, and subjected to coimmunoprecipitation (IP) with V5-tag or c-Fms antibodies. Proteins were resolved by SDS-PAGE, and binding was detected using Western blot probed with c-Fms or V5-tag (Lnk) antibodies. (B) Endogenously expressed Lnk was coimmunoprecipitated from lysates (Input) of the murine macrophage cell line RAW 264.7 after M-CSF treatment (+) using c-Fms antibodies and detected by Western blot. –, Serum-starved cells; IgG, isotype control.
The SH2 domain of Lnk is responsible for the interaction with c-Fms
Similarly to its family members, Lnk contains a SH2 domain, which is responsible for its binding to tyrosine residues of several cytokine receptors (e.g., EpoR, Mpl, c-Kit). A point mutation in the SH2 domain of Lnk (R392E) abolishes the binding to these receptors and the downstream effects of these receptors. To elucidate if the SH2 domain of Lnk is the responsible element for its interaction with c-Fms, mutated R392E Lnk was coexpressed in HEK293T cells together with c-Fms, and coimmunoprecipitation was performed as described before. As shown in Fig. 1A, the SH2 domain of Lnk is essential for its binding to c-Fms. Replacement of a critical amino acid within this domain leads to complete loss of interaction between these two proteins.
Number of M-CFUs is elevated in Lnk KO bone marrow
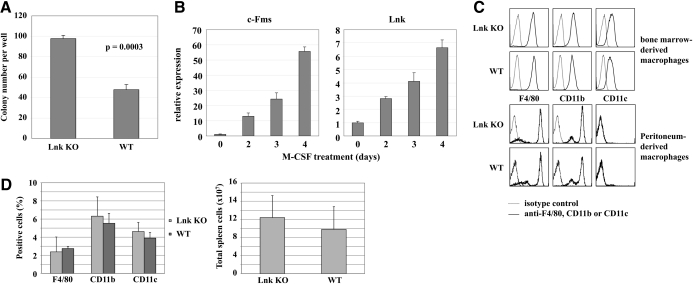
Using mononuclear cells from WT and Lnk-deficient bone marrow, clonogenic assays were performed. Cells were plated in triplicates in methylcellulose medium containing 100 ng/mL M-CSF and incubated for 7 days prior to counting (Fig. 2A). The number of M-CSF-induced colonies was increased significantly in Lnk KO as compared with WT mice (P=0.0003).
Figure 2. Effect of Lnk on proliferation and differentiation of macrophages.
(A) M-CFUs in Lnk KO and WT bone marrow. Mononuclear bone marrow cells were harvested from Lnk KO and WT mice, and 1000 cells/well were plated in methylcellulose medium supplemented with M-CSF in triplicates. After 7 days, colonies were counted. The graph demonstrates 1 of 3 independent experiments with similar results. Results represent the means and sd of 3 plates/experimental round. (B) Lnk mRNA expression during macrophage differentiation. Mononuclear cells were isolated from bone marrow of WT 129/Sv mice, plated in complete RPMI medium supplemented with M-CSF for 2–4 days, and total RNA was extracted from the cells at the indicated time-points. Expression levels of c-Fms and Lnk are shown as the means and sd of triplicate culture dishes using real-time PCR and normalized to 18S expression. The graph shows results from 1 of 2 similar experiments. (C) Surface marker expression of Lnk KO and WT macrophages. Bone marrow-derived macrophages from WT and Lnk KO mice were differentiated in vitro for 7 days (upper chamber). Peritoneum-derived macrophages (lower chamber) were harvested 96 h after i.p. injection of thioglycollate. Cells were stained with fluorescent antibodies against F4/80, CD11b, and CD11c, as well as with the relevant isotype controls. Levels of surface marker expression were detected using a FACSCalibur immunocytometry system. (D) Macrophage populations in spleens of WT and Lnk KO mice. Total cells were extracted from spleens of WT and Lnk KO mice and stained with fluorescent antibodies against F4/80, CD11b, and CD11c (upper diagram). Total spleen cell numbers are shown in the lower diagram.
Lnk does not regulate macrophage differentiation
By real-time PCR, we observed that during M-CSF-driven differentiation of macrophages from bone marrow progenitors, Lnk expression correlated with the appearance of c-Fms expression by the cells (Fig. 2B). We, therefore, assessed whether Lnk regulates macrophage differentiation. The speed of macrophage differentiation was not altered in Lnk KO cultures, and the morphology of the macrophages was normal (data not shown). Expression of the myeloid surface markers F4/80, CD11b, and CD11c was similar in bone marrow- and peritoneum-derived WT and Lnk KO macrophages (Fig. 2C). Thus, Lnk does not appear to affect the fidelity of macrophage differentiation. Furthermore, we investigated macrophage populations in spleens of WT and Lnk KO mice and could not detect significant differences in the percentage of F4/80-, CD11b-, and CD11c-positive spleen cells. Total spleen cell numbers turned out to be similar as well (Fig. 2D). CD11c expression on macrophages is a common but not obligatory feature, yet it is considered as a classical dendritic cell marker [33, 34].
Lnk influences downstream signaling of c-Fms
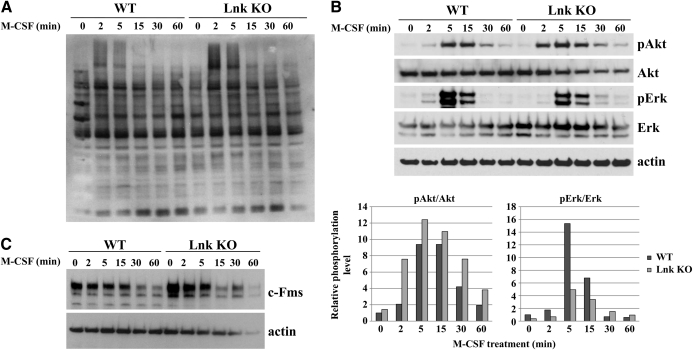
Having shown that Lnk and c-Fms interact, we investigated the influence of Lnk on the downstream signaling of c-Fms. Lnk has been reported to inhibit the MAPK, STAT, and the PI3K/Akt pathways of diverse cytokine receptors after stimulation with their specific ligands. We therefore assayed total protein phosphorylation as well as Akt and Erk phosphorylation upon M-CSF stimulation of serum-starved bone marrow-derived macrophages from Lnk KO and WT mice at the indicated time-points. Except for two protein bands with high molecular weight, total protein phosphorylation was not changed in Lnk KO as compared with WT macrophages (Fig. 3A). Similarly, in the absence of Lnk, M-CSF stimulation led to an earlier and markedly increased phosphorylation of Akt compared with WT macrophages (Fig. 3B). In contrast, levels of phosphorylated Erk were reduced in Lnk KO cells (Fig. 3B). The extents of Akt and Erk activation were measured by densitometry and are shown in the lower diagrams of Fig. 3B. Levels of c-Fms were elevated in the Lnk KO macrophages in the serum-starved culture conditions (Fig. 3C) compared with WT macrophages. This observation could be confirmed at the mRNA level (data not shown). Therefore, the difference in c-Fms expression seems to be at least partially regulated at the transcriptional level.
Figure 3. Lnk inhibits M-CSF-induced signaling in macrophages.
WT and Lnk KO bone marrow-derived macrophages were serum-starved for 6 h and treated with M-CSF, and lysates were harvested, Western blotted, and probed for phosporylation of total cell lysates (A) and levels of phosphorylated Akt (pAkt), Akt, phosphorylated Erk (pErk), Erk, and β-actin at the indicated time-points (B). In a similar experiment, the blot was probed for c-Fms and β-actin (C).
ROS production is increased in Lnk KO macrophages
We next investigated whether functional differences occurred between WT and Lnk KO bone marrow-derived macrophages. We first assessed the ability of the macrophages to phagocytose yeast cell wall particles (zymosan). Zymosan internalization was not affected by Lnk deletion (Fig. 4A). We next measured induction of ROS following detection of zymosan by the β-glucan receptor Dectin-1 [35]. In the presence of M-CSF, ROS induction was elevated dramatically in Lnk KO macrophages (Fig. 4B). In the absence of M-CSF, no difference existed between WT and Lnk KO macrophages (Fig. 4B). Similarly, overnight pretreatment of macrophages with IFN-γ, a potent primer of ROS induction, overcame the effect of Lnk on M-CSF signaling (data not shown). Taken together, the data demonstrate that Lnk does not restrict the ability of the macrophages to produce ROS in response to Dectin-1 ligation but rather, limits the augmentation of Dectin-1-induced ROS by c-Fms signals.
Figure 4. ROS production is increased in Lnk KO macrophages.
(A) Zymosan internalization of bone marrow-derived macrophages from WT and Lnk KO mice. Cells were fed with FITC-labeled zymosan particles for 30 min. Fluorescence of internalized zymosan was assessed by flow cytometry in comparison with unlabeled macrophages. (B) Bone marrow-derived macrophages from Lnk KO and WT mice were plated overnight in factor-free culture media or media containing M-CSF. After stimulation with zymosan, ROS production was measured by luminol-enhanced chemiluminescence [relative light units (RLU)] at the indicated time-points. Diagram shows results of unprimed and M-CSF-primed macrophages, with or without zymosan stimulation.
KO of Lnk has no impact on cytokine production in macrophages
As another macrophage function that might be influenced by Lnk, we explored the induction of TNF-α and IL-6 after exposure of the cells to various microbial stimuli. Lnk KO and WT macrophages derived from bone marrow were plated in medium containing M-CSF and stimulated with zymosan, LPS, CpG, and Pam3CSK4 for 20 h. Cytokine production was assayed by ELISA. No reproducibly significant differences in levels of TNF-α (Fig. 5A) and IL-6 (Fig. 5B) induction occurred between the WT and Lnk KO macrophages upon stimulation with any of these microbial ligands.
Figure 5. Lnk does not influence cytokine production in macrophages.
Lnk KO and WT macrophages were plated in medium supplemented with M-CSF and induced with zymosan, LPS, CpG, and Pam3CSK4, respectively. After 18 h, supernatants were collected, and levels of (A) TNF-α or (B) IL-6 were measured by ELISA. Each figure shows 1 representative of 4 independent experiments measured in triplicates.
Lnk inhibits M-CSF-induced migration of macrophages
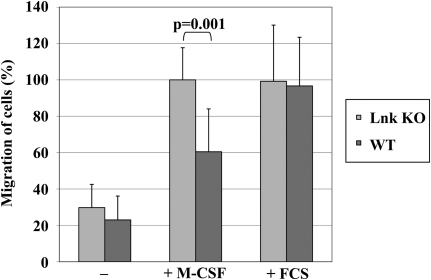
M-CSF is known to be a chemoattractive substance that can induce migration of macrophages toward the source of M-CSF [36]. Therefore, we examined if Lnk can retard M-CSF-triggered migration of macrophages. Thioglycollate-triggered, peritoneum-derived macrophages were harvested from WT and Lnk KO mice and cultured overnight. After 24 h, these adherent macrophages were plated in equal numbers in the upper chamber of a transwell in serum-free medium. The lower chamber contained serum-free medium with or without 100 ng/mL M-CSF or medium supplemented with 10% FCS. Undirected migration without the chemoattractive M-CSF and migration toward the unspecific stimulant FCS were equal by Lnk KO and Lnk WT macrophages (Fig. 6). In contrast, M-CSF triggered significantly higher rates of migrating Lnk KO compared with WT macrophages, indicating that Lnk plays a role in regulating c-Fms-directed macrophage migration.
Figure 6. Lnk inhibits M-CSF-triggered migration of macrophages.
Peritoneum-derived macrophages from Lnk KO and WT mice were plated in serum-free medium in the upper chamber of a transwell. The lower chamber contained serum-free medium with (+) or without (–) M-CSF or medium supplemented with 10% FCS. Migration capacity was measured by counting cells adhering to the lower side of the membrane after a 20-h incubation period. The graph shows means and sd of 3 independent experiments. The percentage of migrated macrophages was normalized to the number of migrated Lnk KO cells toward M-CSF.
DISCUSSION
In this study, we show that Lnk interacts directly with c-Fms when the receptor becomes activated by its ligand M-CSF. This adds to the growing list of cytokine receptors, to which Lnk binds, including c-Kit and Mpl. Activated c-Fms is necessary for proliferation of macrophage progenitor cells. We show that deletion of Lnk results in increased clonogenic growth of macrophage progenitors. Furthermore, we found that up-regulation and activation of c-Fms by its ligand (M-CSF) are associated with increased expression of Lnk. In addition, Lnk has been shown previously to influence hematopoietic cells, especially lymphopoiesis, erythropoiesis, and thrombopoiesis. Studies have shown that Lnk KO mice have elevated monocyte counts in their peripheral blood [4]. Taken together, the data suggest that Lnk may prevent excessive proliferation of cells differentiating down the macrophage pathway.
To date, how Lnk inhibits signaling pathways downstream of target cytokine receptors is unknown. We have shown that Lnk binds to a select tyrosine residue in c-Kit [12]; perhaps its inhibitory activity might be a result of binding competition with activating adaptors. Lnk could be the connection between the activated receptor and the ubiquitin ligase c-Cbl, for which Lnk possesses a putative binding site. Furthermore, Lnk conceivably could interact directly, not just with activated receptors but additionally, with downstream targets or other activation-related proteins such as Jak2 [37]. Our data indicate that Lnk does not influence TLR or Dectin-1 signaling directly but demonstrate that it can influence some of the responses initiated upon detection of microbial components by these receptors through modulation of c-Fms signaling.
We found that M-CSF markedly activated Akt in Lnk KO macrophages. This highlights the importance of the Akt pathway in macrophage activity, and Lnk acts as an inhibitory protein to restrict its activity. We and others showed previously that Lnk inhibited the Akt pathway after stimulation of EpoR and Mpl [7, 8, 38].
Macrophages need to migrate to the site of inflammation. We [39] and others [40] showed previously that bacterial LPS can stimulate stromal cells to produce TNF, which can stimulate M-CSF. We show here that Lnk KO macrophages have increased M-CSF-stimulated migration. We envision that Lnk may play a role in retaining macrophages at the site of infection or preventing excessive numbers of macrophages from entering an inflammation site. Macrophages produce ROS and a variety of additional cytokines at the site of inflammation. We show that Lnk KO macrophages have enhanced ROS production when they are activated by zymosan in the presence of M-CSF. Interestingly, FoxO3 KO hematopoietic progenitors have a reduced expression of Lnk, and this hypersensitizes the cells to ROS, generated by cytokine signaling, leading to myeloproliferation [41]. FoxO3 is inhibited by Akt, and Lnk KO mice display increased Akt activation.
M-CSF signaling is known to regulate ROS production, which may be elevated in Lnk-deficient macrophages as a result of enhanced c-Fms signaling. Alternatively, M-CSF signals may control the expression of components of the phagocyte oxidase complex. Either way, Lnk may regulate the ability of macrophages to mount effective antimicrobial responses. Also, data suggest that ROS activates Akt downstream of c-Fms, which in turn, regulates cell survival and differentiation [42]. Therefore, Lnk is possibly part of a regulatory mechanism that affects levels of ROS generation and the sensitivity of cells to ROS. More generally, these results suggest that Lnk has a role in oxidative metabolism, which is implicated in many physiological processes and pathological conditions, including hematopoietic malignancies. Future studies are needed to address these possibilities and the role of Lnk in regulating these responses.
Taken together, we show that Lnk is an integral inhibitor of the c-Fms pathway. Lnk binds to c-Fms upon M-CSF activation of the receptor and blunts its activity, including proliferation of macrophage progenitor cells, M-CSF-stimulated migration, and generation of ROS. Thus, Lnk represents an important negative-feedback mechanism to control inflammation. Studying the progeny of Lnk deletional mice mated with mice afflicted with diseases of abnormal inflammation or infection can suggest novel therapeutic approaches.
ACKNOWLEDGMENTS
S.G. was supported by the Deutsche Krebshilfe and the Tower Cancer Research Foundation. We thank Carol Stocking and Bernhard Bruene for providing reagents. H.P.K. is a member of the UCLA Jonsson Comprehensive Cancer Center, holds the endowed Mark Goodson Chair of Oncology Research at Cedars-Sinai Medical Center/UCLA School of Medicine, and has an A*STAR Investigators grant.
- Akt
- serine/threonine protein kinase
- c-Fms
- M-CSFR
- c-Kit
- stem cell factor receptor
- EpoR
- erythropoietin receptor
- FoxO3
- forkhead box O3
- HEK
- human embryonic kidney
- KO
- knock-out
- Lnk
- lymphocyte adaptor protein
- M-CFU
- macrophage-CFU
- Mpl
- thrombopoietin receptor
- Pam3CSK4
- palmitoyl-3-cysteine-serine-lysine-4
- PH
- pleckstrin homology
- SH2
- Src homology 2
DISCLOSURE
The authors declare no competing financial interests.
AUTHORSHIP
S.G. designed and performed the research and wrote the paper. H.S.G. helped design the experiments and provided advice. B.N. and H.X. took care of breeding and genotyping of the mice. M.K-M. performed experiments. H.S. helped write the manuscript. D.M.U. contributed technical equipment, vital reagents, and consideration. C.H.B. supervised the research and constructively discussed results. H.P.K. reviewed the data and made suggestions of additional studies.
REFERENCES
- 1. Ema H., Sudo K., Seita J., Matsubara A., Morita Y., Osawa M., Takatsu S., Takaki S., Nakauchi H. (2005) Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Dev. Cell 8, 907–914 [DOI] [PubMed] [Google Scholar]
- 2. Nobuhisa I., Takizawa M., Takaki S., Inoue H., Okita K., Ueno M., Takatsu K., Taga T. (2003) Regulation of hematopoietic development in the aorta-gonad-mesonephros region mediated by Lnk adaptor protein. Mol. Cell. Biol. 23, 8486–8494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takaki S., Morita H., Tezuka Y., Takatsu K. (2002) Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor protein. J. Exp. Med. 195, 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Velazquez L., Cheng A. M., Fleming H. E., Furlonger C., Vesely S., Bernstein A., Paige C. J., Pawson T. (2002) Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J. Exp. Med. 195, 1599–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takaki S., Sauer K., Iritani B. M., Chien S., Ebihara Y., Tsuji K., Takatsu K., Perlmutter R. M. (2000) Control of B cell production by the adaptor protein Lnk. Definition of a conserved family of signal-modulating proteins. Immunity 13, 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takaki S., Tezuka Y., Sauer K., Kubo C., Kwon S. M., Armstead E., Nakao K., Katsuki M., Perlmutter R. M., Takatsu K. (2003) Impaired lymphopoiesis and altered B cell subpopulations in mice overexpressing Lnk adaptor protein. J. Immunol. 170, 703–710 [DOI] [PubMed] [Google Scholar]
- 7. Tong W., Lodish H. F. (2004) Lnk inhibits Tpo-Mpl signaling and Tpo-mediated megakaryocytopoiesis. J. Exp. Med. 200, 569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tong W., Zhang J., Lodish H. F. (2005) Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood 105, 4604–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y., He X., Schembri-King J., Jakes S., Hayashi J. (2000) Cloning and characterization of human Lnk, an adaptor protein with pleckstrin homology and Src homology 2 domains that can inhibit T cell activation. J. Immunol. 164, 5199–5206 [DOI] [PubMed] [Google Scholar]
- 10. Takaki S. (2002) Lnk, an adaptor protein regulate production of B cells and hematopoietic stem cells. Tanpakushitsu Kakusan Koso 47, 2188–2193 [PubMed] [Google Scholar]
- 11. Boulday G., Coulon F., Fraser C. C., Soulillou J. P., Charreau B. (2002) Transcriptional up-regulation of the signaling regulatory protein LNK in activated endothelial cells. Transplantation 74, 1352–1354 [DOI] [PubMed] [Google Scholar]
- 12. Gueller S., Gery S., Nowak V., Liu L., Serve H., Koeffler H. P. (2008) Adaptor protein Lnk associates with Tyr(568) in c-Kit. Biochem. J. 415, 241–245 [DOI] [PubMed] [Google Scholar]
- 13. Guilbert L. J., Stanley E. R. (1980) Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J. Cell Biol. 85, 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stoy N. (2001) Macrophage biology and pathobiology in the evolution of immune responses: a functional analysis. Pathobiology 69, 179–211 [DOI] [PubMed] [Google Scholar]
- 15. Adams D. O., Hamilton T. A. (1984) The cell biology of macrophage activation. Annu. Rev. Immunol. 2, 283–318 [DOI] [PubMed] [Google Scholar]
- 16. Bourette R. P., Rohrschneider L. R. (2000) Early events in M-CSF receptor signaling. Growth Factors 17, 155–166 [DOI] [PubMed] [Google Scholar]
- 17. Cecchini M. G., Dominguez M. G., Mocci S., Wetterwald A., Felix R., Fleisch H., Chisholm O., Hofstetter W., Pollard J. W., Stanley E. R. (1994) Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 120, 1357–1372 [DOI] [PubMed] [Google Scholar]
- 18. Stanley E. R., Chen D. M., Lin H. S. (1978) Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature 274, 168–170 [DOI] [PubMed] [Google Scholar]
- 19. Roth P., Stanley E. R. (1992) The biology of CSF-1 and its receptor. Curr. Top. Microbiol. Immunol. 181, 141–167 [DOI] [PubMed] [Google Scholar]
- 20. Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. (1985) The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell 41, 665–676 [DOI] [PubMed] [Google Scholar]
- 21. Cheng M., Wang D., Roussel M. F. (1999) Expression of c-Myc in response to colony-stimulating factor-1 requires mitogen-activated protein kinase kinase-1. J. Biol. Chem. 274, 6553–6558 [DOI] [PubMed] [Google Scholar]
- 22. Valledor A. F., Comalada M., Xaus J., Celada A. (2000) The differential time-course of extracellular-regulated kinase activity correlates with the macrophage response toward proliferation or activation. J. Biol. Chem. 275, 7403–7409 [DOI] [PubMed] [Google Scholar]
- 23. Jones G. E., Prigmore E., Calvez R., Hogan C., Dunn G. A., Hirsch E., Wymann M. P., Ridley A. J. (2003) Requirement for PI 3-kinase γ in macrophage migration to MCP-1 and CSF-1. Exp. Cell Res. 290, 120–131 [DOI] [PubMed] [Google Scholar]
- 24. Kelley T. W., Graham M. M., Doseff A. I., Pomerantz R. W., Lau S. M., Ostrowski M. C., Franke T. F., Marsh C. B. (1999) Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J. Biol. Chem. 274, 26393–26398 [DOI] [PubMed] [Google Scholar]
- 25. Wheeler A. P., Smith S. D., Ridley A. J. (2006) CSF-1 and PI 3-kinase regulate podosome distribution and assembly in macrophages. Cell Motil. Cytoskeleton 63, 132–140 [DOI] [PubMed] [Google Scholar]
- 26. Yu W., Chen J., Xiong Y., Pixley F. J., Dai X. M., Yeung Y. G., Stanley E. R. (2008) CSF-1 receptor structure/function in MacCsf1r−/− macrophages: regulation of proliferation, differentiation, and morphology. J. Leukoc. Biol. 84, 852–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ozinsky A., Underhill D. M., Fontenot J. D., Hajjar A. M., Smith K. D., Wilson C. B., Schroeder L., Aderem A. (2000) The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97, 13766–13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown G. D., Taylor P. R., Reid D. M., Willment J. A., Williams D. L., Martinez-Pomares L., Wong S. Y., Gordon S. (2002) Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 196, 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takeda K., Kaisho T., Akira S. (2003) Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 [DOI] [PubMed] [Google Scholar]
- 30. Sester D. P., Beasley S. J., Sweet M. J., Fowles L. F., Cronau S. L., Stacey K. J., Hume D. A. (1999) Bacterial/CpG DNA down-modulates colony stimulating factor-1 receptor surface expression on murine bone marrow-derived macrophages with concomitant growth arrest and factor-independent survival. J. Immunol. 163, 6541–6550 [PubMed] [Google Scholar]
- 31. Goyal A., Wang Y., Graham M. M., Doseff A. I., Bhatt N. Y., Marsh C. B. (2002) Monocyte survival factors induce Akt activation and suppress caspase-3. Am. J. Respir. Cell Mol. Biol. 26, 224–230 [DOI] [PubMed] [Google Scholar]
- 32. Sweet M. J., Campbell C. C., Sester D. P., Xu D., McDonald R. C., Stacey K. J., Hume D. A., Liew F. Y. (2002) Colony-stimulating factor-1 suppresses responses to CpG DNA and expression of Toll-like receptor 9 but enhances responses to lipopolysaccharide in murine macrophages. J. Immunol. 168, 392–399 [DOI] [PubMed] [Google Scholar]
- 33. Alatery A., Basta S. (2008) An efficient culture method for generating large quantities of mature mouse splenic macrophages. J. Immunol. Methods 338, 47–57 [DOI] [PubMed] [Google Scholar]
- 34. Moon K. A., Kim S. Y., Kim T. B., Yun E. S., Park C. S., Cho Y. S., Moon H. B., Lee K. Y. (2007) Allergen-induced CD11b+ CD11c(int) CCR3+ macrophages in the lung promote eosinophilic airway inflammation in a mouse asthma model. Int. Immunol. 19, 1371–1381 [DOI] [PubMed] [Google Scholar]
- 35. Underhill D. M., Rossnagle E., Lowell C. A., Simmons R. M. (2005) Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106, 2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Webb S. E., Pollard J. W., Jones G. E. (1996) Direct observation and quantification of macrophage chemoattraction to the growth factor CSF-1. J. Cell Sci. 109, 793–803 [DOI] [PubMed] [Google Scholar]
- 37. Bersenev A., Wu C., Balcerek J., Tong W. (2008) Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J. Clin. Invest. 118, 2832–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gery S., Gueller S., Chumakova K., Kawamata N., Liu L., Koeffler H. P. (2007) Adaptor protein Lnk negatively regulates the mutant MPL, MPLW515L, associated with myeloproliferative disorders. Blood 110, 3360–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamato K., el-Hajjaoui Z., Simon K., Koeffler H. P. (1990) Modulation of interleukin-1 β RNA in monocytic cells infected with human immunodeficiency virus-1. J. Clin. Invest. 86, 1109–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schlick E., Hartung K., Chirigos M. A. (1984) Role of prostaglandin E and interferon in secretion of colony-stimulating factor by murine macrophages after in vitro treatment with biological response modifiers. Int. J. Immunopharmacol. 6, 407–418 [DOI] [PubMed] [Google Scholar]
- 41. Yalcin S., Mungamuri S. K., Marinkovic D., Zhang X., Tong W., Cullen D., Ghaffari S. (2008) Oxidative stress-mediated activation of AKT/mTOR signaling pathway leads to myeloproliferative syndrome in FoxO3 null mice: a role for Lnk adaptor protein. Blood 112, 509 (ASH Annual Meeting Abstracts) [Google Scholar]
- 42. Wang Y., Zeigler M. M., Lam G. K., Hunter M. G., Eubank T. D., Khramtsov V. V., Tridandapani S., Sen C. K., Marsh C. B. (2007) The role of the NADPH oxidase complex, p38 MAPK, and Akt in regulating human monocyte/macrophage survival. Am. J. Respir. Cell Mol. Biol. 36, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]