Abstract
Background:
Homeotic genes are key developmental regulators that are highly conserved throughout evolution. Their encoded homeoproteins function as transcription factors to control a wide range of developmental processes. Although much is known about homeodomain-DNA interactions, only a small number of genes acting downstream of homeoproteins have been identified. Here we use a functional genomic approach to identify candidate target genes of the Drosophila homeodomain transcription factor Labial.
Results:
High-density oligonucleotide arrays with probe sets representing 1,513 identified and sequenced genes were used to analyze differential gene expression following labial overexpression in Drosophila embryos. We find significant expression level changes for 96 genes belonging to all functional classes represented on the array. In accordance with our experimental procedure, we expect that these genes are either direct or indirect targets of labial gene action. Among these genes, 48 were upregulated and 48 were downregulated following labial overexpression. This corresponds to 6.3% of the genes represented on the array. For a selection of these genes, we show that the data obtained with the oligonucleotide arrays are consistent with data obtained using quantitative RT-PCR.
Conclusions:
Our results identify a number of novel candidate downstream target genes for Labial, suggesting that this homeoprotein differentially regulates a limited and distinct set of embryonically expressed Drosophila genes.
Background
The homeotic/Hox genes encode a network of evolutionarily conserved homeodomain transcription factors that are involved in the specification of segmental identity along the anterior-posterior body axis of animals as diverse as insects and vertebrates [1, 2, 3, 4, 5, 6]. In Drosophila, these genes are arranged on the chromosome in two gene clusters known as the Antennapedia and Bithorax complexes. There is a correlation between the relative position of the Hox genes within the cluster and their spatial and temporal expression pattern in the body in that genes located towards the 3' end are expressed more anterior and earlier than genes located towards the 5' end (spatial and temporal colinearity) [7, 8, 9, 10, 11].
Given their central role in developmental processes, it has been proposed that the homeoproteins do not act directly to specify morphological differences but rather control a battery of subordinate genes encoding cellular functions directly required in differentiation [12, 13]. In search of these subordinate genes, various strategies such as enhancer trapping, immunoprecipitation of chromatin fragments, subtractive hybridization, selection for binding sites in yeast, and heat-shock-induced overexpression have been used [9, 14, 15, 16, 17, 18, 19, 20, 21].Only a small number of target genes of homeoproteins have been identified to date, however; most of these encode either transcription factors or cell-signaling molecules [9]. In contrast to these results, recent studies suggest that homeoproteins may bind at significant levels to the majority of genes in the Drosophila embryo and regulate a large number of downstream genes [22, 23].
Here we focus on the homeotic gene labial (lab) in the Drosophila embryo. It is the most proximal gene within the Drosophila Antennapedia complex; it encodes an Antennapedia-like Q50 homeodomain transcription factor and is one of the most anteriorly expressed homeotic genes along the anterior-posterior body axis [24, 25, 26, 27]. Genetic studies have demonstrated that lab is required for proper head formation [28] and for the specification of cellular identity in the midgut [29] as well as in the embryonic brain [30]. The lab gene and its vertebrate Hox1 orthologs are among the best-characterized examples of evolutionary conservation of structure, expression and function of Hox genes in animal development [31, 32, 33, 34, 35].
To address the question of which and how many downstream genes are under control of lab, we used a combination of in vivo overexpression techniques and quantitative transcript imaging with oligonucleotide arrays. By using transgenic flies carrying the lab gene under the control of a heat-inducible promoter, we ubiquitously overexpressed lab following heat-shock treatment in Drosophila embryos. We then used high-density oligonucleotide arrays representing 1,513 identified Drosophila genes for large-scale detection and quantification of induced gene expression [36, 37, 38, 39]. We find significant changes in gene expression for 96 identified genes following lab overexpression. Quantitative reverse-transcriptase PCR on a selection of these genes verified the differential expression levels in response to heat-shock-induced overexpression of lab. Our findings identify a number of novel candidate downstream genes for lab and thus show that oligonucleotide arrays are powerful tools for analyzing, at a genome-wide level, the number, identity and quantitative expression level of genes in the Drosophila embryo.
Results
In this study, transgenic fly strains carrying the lab coding sequence under control of the heat-inducible Hsp70 promotor were used [40]. Stage 10-17 embryos were given a 25 minute heat pulse to overexpress lab, and allowed to recover for 25 minutes (see Materials and methods for heat-shock protocol). Ubiquitous overexpression of lab was verified by whole mount in situ hybridization with a lab-specific antisense RNA probe. Ubiquitous overexpression of Labial protein (Lab) was verified by immunocytochemistry with an anti-Lab antibody. These experiments demonstrated that both lab RNA and Lab protein were strongly overexpressed 50 minutes after the onset of heat shock in these strains (Figure 1). Wild-type control flies were subjected to an identical heat-shock regime.
Figure 1.
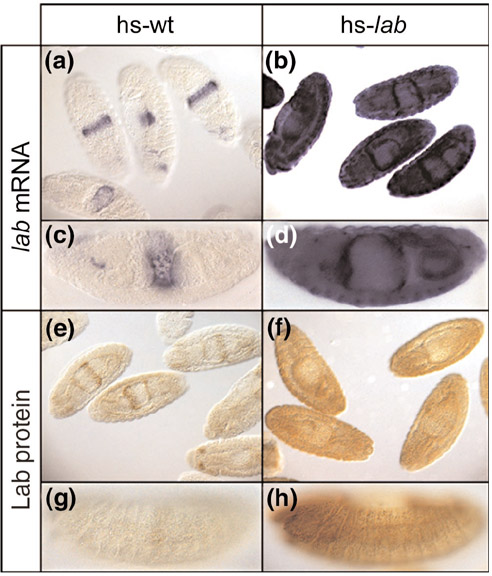
Heat-shock-driven ubiquitous overexpression of lab monitored by in situ hybridization and immunocytochemistry. (a-d) RNA in situ hybridization; (e-h) immunocytochemical staining. Expression of lab is shown in heat-shocked wild-type embryos (a,c,e,g) and in heat-shocked embryos carrying a hs-lab construct (b,d,f,h). (a,b,e,f) Overview of stage 10-17 embryos. (c,d) Higher magnification of a single stage 15 embryo and (g,h) a single stage 13 embryo; lateral view, and anterior to the left. Embryos were exposed to a heat shock at 36°C for 25 min and were allowed to recover for another 25 min before fixation.
Following ubiquitous overexpression of lab, transcript profiles were analyzed using a high-density oligonucleotide array and compared to the transcript profiles of heat-shocked wild-type control embryos. For each of the two experimental conditions ('hs-wt'and 'hs-lab'), four replicate experiments were performed and the data set was analyzed with an unpaired t-test (see [39] and Materials and methods). The genes represented on the oligonucleotide array correspond to probe sets that are complementary to 1,513 identified and sequenced Drosophila genes. Most of these genes can be grouped into 14 functional categories according to the nature of the encoded protein [39].
At a significance level of p ≤ 0.01, a total of 96 genes were found to be differentially regulated following lab overexpression compared with heat-shocked wild-type control embryos. This corresponds to 6.3% of the genes represented on the array. At a significance level of p ≤ 0.05, 205 genes were found to be differentially regulated following lab overexpression compared with heat-shocked wild-type control embryos (data not shown). This corresponds to 13.5% of the genes represented on the array. The relative distribution of lab-regulated genes in particular functional classes, as well as the percentage of genes regulated within a given functional class, were comparable between the p ≤ 0.01 group and the p ≤ 0.05 group. Only genes that were differentially expressed at a significance level of p ≤ 0.01 are considered further. We propose these genes to be potential direct or indirect downstream targets for the homeodomain transcription factor Labial.
When ubiquitously expressed in the embryo, lab caused a significant transcriptional response among a wide variety of genes belonging to all functional classes represented on the array (Table 1). The functional class with the highest absolute number of differentially regulated genes was 'transcriptional regulation' (n = 20). Other functional classes with high numbers of differentially regulated genes were 'metabolism' (n = 13), 'proteolytic systems/apoptosis' (n = 12), 'cell-surface receptors/cell adhesion molecules (CAMs)/ion channels' (n = 12), and 'RNA binding' (n = 7). Relative to the number of genes represented on the array within a given functional class, the highest relative percentage of differentially regulated genes was found in the functional classes 'proteolytic systems/apoptosis' (19.4%), 'cell cycle' (13.5%), 'transposable elements' (11.4%), 'chromatin structure' (11.1%), 'RNA binding' (11.9%), and 'transcriptional regulation' (7.6%).
Table 1.
Genes differentially expressed in response to lab overexpression
| Functional class | Genes on the array (N) | Differentially expressed transcripts (n) | n/N × 100 (%) | Down-regulated | Up-regulated |
| Signal transduction | 107 | 5 | 4.7 | 2 | 3 |
| Transcriptional regulation | 263 | 20 | 7.6 | 14 | 6 |
| Cell cycle | 37 | 5 | 13.5 | 0 | 5 |
| Cytoskeleton/structural proteins | 149 | 5 | 3.4 | 4 | 1 |
| Metabolism | 315 | 13 | 4.1 | 6 | 7 |
| Translation | 59 | 1 | 1.7 | 1 | 0 |
| Heat-shock proteins | 18 | * | * | * | * |
| Transcription/replication/repair | 73 | 4 | 5.5 | 0 | 4 |
| Proteolytic systems/apoptosis | 62 | 12 | 19.4 | 1 | 11 |
| Cell surface receptors/CAMs/ion channels | 181 | 12 | 6.6 | 10 | 2 |
| Transposable elements | 35 | 4 | 11.4 | 3 | 1 |
| Chromatin structure | 36 | 4 | 11.1 | 2 | 2 |
| RNA binding | 59 | 7 | 11.9 | 2 | 5 |
| Secreted proteins | 34 | 2 | 5.9 | 2 | 0 |
| Unknown function | 85 | 2 | 2.4 | 1 | 1 |
| ΣN = 1513 | Σn = 96 | 48 | 48 |
Genes that are differentially expressed following heat-induced ubiquitous overexpression of lab in stage 10-17 hs-lab embryos, grouped according to functional classses. *The functional class 'heat-shock proteins' was excluded from the analysis (see Materials and methods). N, number of genes within a functional group present on the chip; n, number of genes differentially expressed within a functional group following lab overexpression; n/N × 100, number of differentially expressed genes within a functional class following lab overexpression, given as a percentage of the total number of genes in this class present on the array; downregulated, total number of genes within each functional class differentially downregulated following lab overexpression; upregulated, total number of genes within each functional class differentially upregulated following lab overexpression.
Figure 2 shows the lab-regulated genes and presents a quantitative representation of the change in expression levels for these genes. Of the 96 genes that were differentially regulated, 48 showed increased expression levels and 48 showed decreased expression levels. The gene with the highest increase in expression level (26-fold) was lab itself, in accordance with our experimental procedure. Increases in expression levels above 10-fold were also observed for Bicaudal C (BicC), swallow (swa) and oskar (osk), all encoding proteins involved in RNA binding, as well as for the wings apart-like (wapl) gene belonging to the functional class 'chromatin structure'. The increased expression levels in BicC, swa, and osk are surprising, as all these genes are known to function as maternal control genes during early embryogenesis [41, 42]. As lab activity is normally only observed from gastrulation onwards [26], this suggests that high levels of widespread ectopic lab expression are able to activate genes which under wild-type conditions show spatio-temporal expression domains that do not overlap with that of lab.
Figure 2.
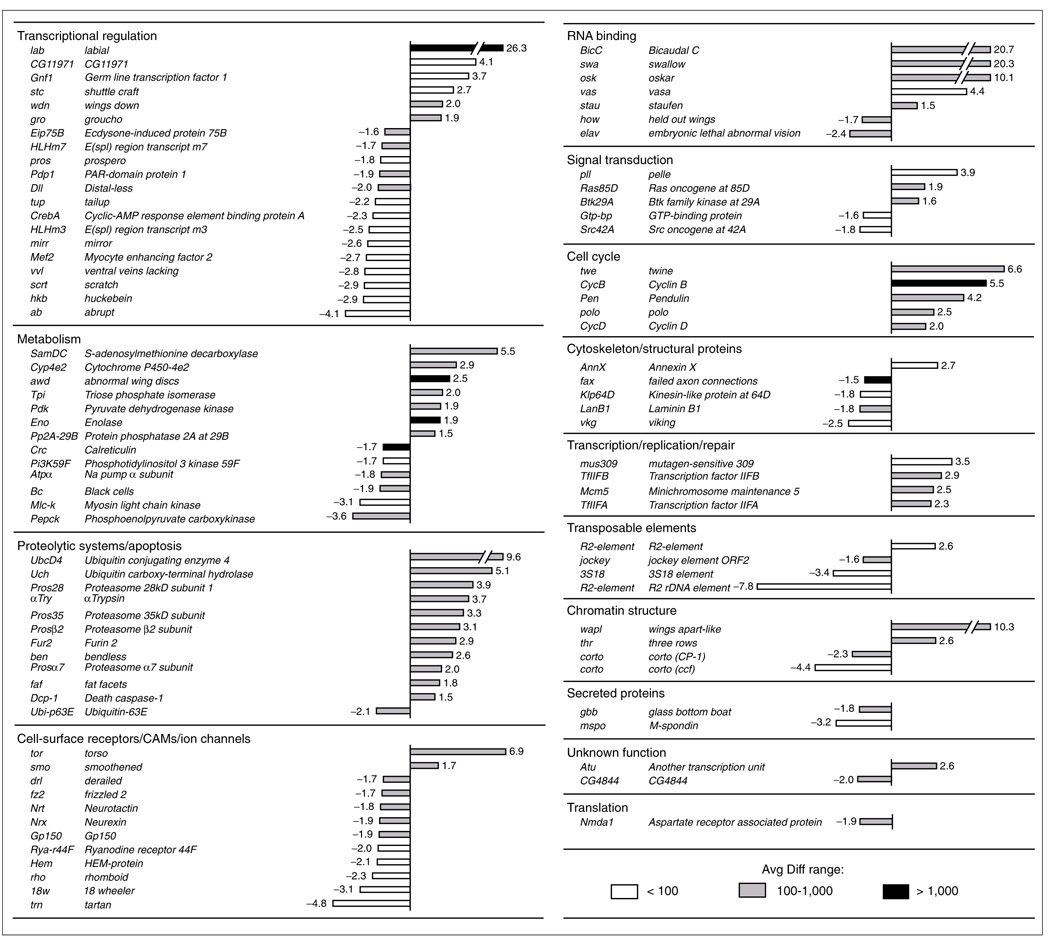
Genes differentially expressed in response to heat-shock-induced overexpression of lab, grouped according to functional classes. Bars represent the fold change between differentially expressed genes in heat-shocked wild-type embryos and heat-shocked hs-lab embryos. Positive values indicate that the relative expression level of a gene is increased (upregulated) following lab overexpression and negative values indicate a decrease (downregulated). Absolute average difference (Avg Diff; see Materials and methods) values are given for the lab overexpression condition as follows: white bars represent Avg Diff <100, gray bars represent Avg Diff ranging from 100-1,000, and black bars represent Avg Diff >1,000.
Increases in the 5-10-fold range were seen for six genes. One encodes the enzyme ubiquitin carboxy-terminal hydrolase, whose mammalian homolog has also been found to be differentially upregulated by ectopic overexpression of the lab ortholog Hoxa1 [43]. Increased expression levels in the 1.5-5-fold range were prominent in several functional classes. For example, in the functional class 'proteolytic systems/apoptosis', 12 of 13 differentially regulated genes were upregulated and most of these showed increased expression levels ranging between 1.5 and 5. Strikingly, in the functional classes 'cell cycle' and 'transcription/replication/repair' all the differentially regulated genes were upregulated. Thus, differentially expressed genes such as twine (twe), Cyclin B (CycB) and Cyclin D (CycD), belonging to the functional category 'cell cycle', were all upregulated following lab overexpression. It is notable in this respect that recent experiments carried out on mammalian cell lines showed that ectopic overexpression of the lab ortholog Hoxa1 also causes differential upregulation of cell-cycle regulatory proteins [43].
Decreases in expression levels in the 10-fold and above range were not observed, and decreases in the 5-10-fold range were only seen for the transposable R2 rDNA element gene. Decreased expression levels in the 1.5-5-fold range were, however, prominent in the functional class 'transcriptional regulation' and in the functional class 'cell-surface receptors/CAMs/ion channels'. Thus, almost three-quarters of the differentially regulated genes encoding transcription factors showed significant decreases in expression levels following lab overexpression. For example, the genes prospero (pros), Distal-less (Dll), tailup/islet (tup), mirror (mirr), huckebein (hkb) and abrupt (ab) were all downregulated. Interestingly, it has been shown that Distal-less is a direct target of homeotic gene control [9], and recent genetic studies demonstrated that tailup/islet expression in the lab-specific territory of the embryonic Drosophila brain is dependent on lab gene action [30]. As with the functional class 'transcriptional regulation', 10 out of 12 genes representing the functional category 'cell-surface receptors/CAMs/ion channels' were downregulated, including the genes derailed (drl), frizzled 2 (fz2), Neurotactin (Nrt), Neurexin (Nrx), rhomboid (rho) and 18 wheeler (18w). As is the case for tailup/islet, Neurotactin expression in the lab-specific territory of the embryonic Drosophila brain is dependent on lab gene action [30]. The 18w locus has been identified as a binding site of the homeotic protein Ubx in polytene chromosomes [18].
To verify the differences in gene expression level after heat-shock-induced overexpression of lab as compared to heat-shocked wild-type embryos, quantitative RT-PCR was performed on selected candidate target genes. Changes in expression levels were determined for eight genes that were differentially regulated following lab overexpression, namely lab, swa, Ubiquitin conjugating enzyme 4 (UbcD4), twe, cycB, Ubiquitin carboxy-terminal hydrolase (Uch), scratch (scrt) and phosphoenolpyruvate carboxykinase (Pepck). The gene squid (sqd), whose expression level remained unchanged under both experimental conditions, served as a control. As indicated in Table 2, these experiments showed that the changes in relative expression level, as measured by RT-PCR, are consistent with the data obtained with the oligonucleotide arrays.
Table 2.
Comparison of fold change between oligonucleotide arrays and RT-PCR
| Avg Diff (array) | Fold change | |||
| Gene | hs-wt | hs-lab | Array | RT-PCR |
| lab | 41 | 1078 | 26.3 | 55.7 |
| swa | 20 | 406 | 20.3 | 18.4 |
| UbcD4 | 44 | 423 | 9.6 | 6.5 |
| twe | 20 | 132 | 6.6 | 4.9 |
| cycB | 243 | 1344 | 5.5 | 4.6 |
| Uch | 61 | 312 | 5.1 | 12.1 |
| sqd | 373 | 370 | 1.0 | 1.1 |
| scrt | 225 | 79 | -2.9 | -3.7 |
| Pepck | 610 | 171 | -3.6 | -4.6 |
RT-PCR was performed on cDNA derived from heat-shocked wild-type embryos and heat-shocked hs-lab embryos. Fold changes determined by RT-PCR are represented as the mean values of eight independent replicates, derived from two different cDNA preparations. Avg Diff, absolute average difference value (see Materials and methods).
Discussion
We have used a novel combination of manipulative genetics and functional genomics to gain further insight into homeotic gene action in Drosophila from a genomic perspective. Using inducible overexpression and quantitative transcript imaging through oligonucleotide arrays, we have identified 96 genes (only 6.3% of the 1,513 identified genes represented on the oligonucleotide array) whose expression levels change significantly following lab overexpression.
These findings suggest that Lab regulates a limited and distinct set of candidate downstream genes. This appears to contrast with previous reports indicating that in late embryogenesis the majority of Drosophila genes are under control of homeoproteins [23, 44]. It should be stressed, however, that a number of features of our functional genomic analysis prevent a direct comparison with these reports, which are based on DNA-binding studies. First, although our analysis can quantify gene expression accurately and simultaneously for many identified genes, the temporal and spatial resolution of our analysis is low. This is because our experimental design averages gene expression throughout the embryo and during several embryonic stages. In consequence, our analysis may fail to detect genes that are only expressed in a small subset of cells or during a very restricted time period in embryogenesis. Second, our overexpression protocol makes it difficult to control the level of Lab protein as well as the temporal dynamics and stability of this protein. As different levels of a given homeoprotein can have different functional consequences in terms of developmental specificity [29, 45], the high level of Lab protein may bias the set of candidate downstream target genes identified. Third, in our studies lab overexpression is not accompanied by concomitant overexpression of cofactors, which are thought to act together with homeotic proteins to determine their in vivo target specificity [34, 46]. It should be noted that the gene mirror, which has been proposed to be an additional cofactor for homeoprotein specificity [47], was detected as downregulated following lab overexpression.
Although the question of the total number of target genes that are regulated by homeoproteins in vivo must await further analysis, our genomic perspective of lab gene targets does reveal several specific features of homeoprotein action. First, our results demonstrate that the homeodomain transcription factor Lab acts on numerous candidate target genes that also encode transcription factors. The category 'transcriptional regulation' comprises one of the largest sets of differentially regulated genes following lab overexpression. This is consistent with the idea that homeobox genes establish developmental patterns by acting through a cascade of transcription factors which regulate the expression of their own subset of downstream genes [1, 2, 9, 15]. Second, our data indicate that upregulation of gene expression is prominent in several functional classes. Thus, virtually all of the lab-regulated genes in the functional classes 'cell cycle', 'transcription/replication/repair', and 'proteolytic systems/apoptosis' show increased expression. Third, our results show that lab overexpression causes not only widespread activation but also widespread repression of gene expression. Thus, of the 96 genes that are potential targets of lab, half are downregulated by overexpression of this homeobox gene. This widespread repression is especially pronounced in the functional classes of 'transcriptional regulation' and 'cell-surface receptors/CAMs/ion channels'. For example, following lab overexpression, over 80% of the differentially regulated genes encoding cell-surface receptors/CAMs/ion channels showed decreased expression.
Conclusions
Taken together, our results identify a large number of novel candidate downstream genes of the homeodomain transcription factor Lab. To our knowledge, most of these 96 identified and sequenced genes have not been previously shown to be lab targets. At present, we do not know which genes are direct targets (regulated directly by Lab protein binding to DNA regulatory sequences) or indirect targets of lab gene action. Furthermore, our results demonstrate that oligonucleotide arrays are useful tools for analyzing, at a genome-wide level, the number, identity and quantitative expression levels of candidate downstream genes differentially regulated in vivo by developmental control genes. This confirms the general utility of microarrays for studying diverse molecular and cellular processes in Drosophila [48, 49, 50]. Considering the evolutionary conservation of gene structure, expression and function [1, 35], we propose that these results obtained in Drosophila will also be valid for lab orthologs in other animals, including vertebrates. It will now be important to determine which of the detected candidate downstream genes in Drosophila are direct targets and how they exert the developmental genetic programs imposed by lab gene action.
Materials and methods
Fly strains, embryo collections and heat-shock regime
The wild type was Drosophila melanogaster Oregon-R. For ectopic overexpression of lab, we used the line p(w+hs-lab) with a heat-shock lab construct homozygous on the X chromosome [40]. All fly stocks were kept on standard cornmeal/yeast/agar medium at 25°C. Embryos were collected overnight for 12 h on grape juice plates, further kept for 4 h at 25°C and then subjected to a 36°C heat shock for 25 min, followed by a recovery period of 25 min at 25°C before RNA isolation. Therefore, at the time of RNA isolation these embryos were at embryonic stages 10-17 (stages according to [51]). Embryos younger than embryonic stage 10 were not used, as heat shock in these earlier stages results in lethality [52].
Whole-mount in situ hybridization and immunocytochemistry
For in situ hybridization, digoxigenin-labeled sense and antisense lab RNA probes were generated in vitro, with a DIG labeling kit (Roche Diagnostics) and hybridized to whole-mount embryos following standard procedures [53]. Hybridized transcripts were detected with an alkaline phosphatase-conjugated anti-digoxigenin Fab fragment (Roche Diagnostics) using Nitro blue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) (Sigma) as chromogenic substrates. For immunocytochemistry, embryos were dechorionated, fixed and labeled according to [54]. The primary antibody was rabbit anti-LAB [55] used 1:100. The histochemical staining was performed using the Vectastain Elite ABC Kit (Vector Laboratories). Embryos were mounted in Canada balsam (Serva) and photographed with a Prog/Res/3008 digital camera (Kontron Electronic) on a Zeiss Axioskop microscope with differential interference contrast optics. Photographs were arranged and labeled using Microsoft PowerPoint, 97.
High-density oligonucleotide arrays
Gene expression analysis was performed as described [36], using a custom-designed Drosophila oligonucleotide array (ROEZ003A; Affymetrix). The genes represented on the array and considered in this study correspond to 1,513 sequenced Drosophila genes encoding open reading frames deposited in SWISS-PROT/TrEMBL databases as of spring 1998. For a complete list of these genes see the supplementary data of [39]. Each gene is represented on the array by a set of 20 oligonucleotide probes (25mers) matching the gene sequence. To control the specificity of hybridization, the same set of probes, containing a single nucleotide mismatch in a central position, is represented on the array. The difference between the perfect match hybridization signal and the mismatch signal is proportional to the abundance of a given transcript and calculated as its average difference value (Avg Diff) [37]. Drosophila genes that were not unambiguously represented by a probe set of 20 probe pairs on the array were excluded from further analysis (29 probe sets were not used in this study).
RNA sample preparation and hybridization
Initial experiments designed to determine the sensitivity and reproducibility of hybridization showed that the use of total RNA versus poly(A)+ RNA as a template for cDNA synthesis and subsequent amplification (synthesis of cRNA) gave comparable results, despite the fact that we consistently detected 5S RNA and histone genes present on the array with cRNA derived from total RNA. On the basis of these findings, all experiments were carried out using a total RNA protocol [56].
Total RNA was isolated from 200 mg of embryonic tissue, using guanidinium isothiocyanate in combination with acidic phenol (pH 4.0) (fast RNA tube green kit from BIO101) in a fast prep homogenizer FP120 (BIO 101). After precipitation, the RNA was dissolved in DEPC-treated water (Ambion) and spectrophotometrically quantified using a GeneQuant RNA/DNA calculator (Pharmacia Biotech). cDNA was synthesized upon total RNA as a template, using the SuperScript Choice System for cDNA synthesis (Gibco/BRL) with a T7-(T)24 DNA primer.
This primer (5'-GGCCAGTGAATTGTAATACGACTCACTATAGGGAGGCGG-(T)24VN-3') was purified by PAGE. For first-strand cDNA synthesis, a typical 40 μl reaction contained 25 μg RNA, 200 pmol T7-(T)24 primer, 500 μM of each dNTP and 800 units reverse transcriptase (AMV Superscript II). The reaction was incubated for 1 h at 42°C. Second-strand cDNA synthesis was carried out at 18°C for 2 h in a total volume of 340 μl, using 20 units Escherichia coli DNA ligase, 80 units E. coli DNA polymerase I and 4 units RNase H in the presence of 250 μM of each dNTP. After second-strand cDNA synthesis, 0.5 μl RNase A (100 mg/ml) (Qiagen) was added and the samples were incubated at 37°C for 30 min. Thereafter, 7.5 μl proteinase K (10 mg/ml) (Sigma) was added and the samples were further incubated at 37°C for another 30 min. After cDNA synthesis was completed, samples were phenol-chloroform extracted, using Phase Lock Gel (5 Prime-3 Prime) and ethanol precipitated. Biotinylated antisense cRNA was synthesized from the dsDNA template, using T7 RNA polymerase (MEGAscript T7 Kit: Ambion.). A 20 μl reaction volume contained between 0.3-1.5 μg cDNA, 7.5 mM of both ATP and GTP, 5.6 mM of both UTP and CTP and 1.8 mM of both biotinylated Bio-16-UTP and Bio-11-CTP (ENZO diagnostics) and 2 μl 10x T7 enzyme mix. The reaction was incubated at 37°C for 8 h. Thereafter, the unincorporated NTPs were removed by running the sample over an RNeasy spin column (Qiagen). Samples were precipitated, taken up in 20 μl DEPC-treated water and spectrophotometrically quantified. Thereafter, 40 μg of the biotinylated antisense cRNA was fragmented by heating the sample to 95°C for 35 min in a volume of 25 μl, containing 40 mM tris-acetate (pH 8.1), 100 mM potassium acetate, 30 mM magnesium acetate. After the fragmentation, the samples were placed on ice.
Gene Chips were pre-hybridized with 220 μl hybridization buffer (1x MES (pH 6.7), 1 M NaCl, 0.01% Triton, 0.5 μg/μl acetylated BSA, 0.5 μg/μl sonicated herring sperm DNA) for 15 min at 45°C on a rotisserie (Heidolph) at 60 rpm. Hybridization was done in a final volume of 220 μl hybridization buffer, containing 40 μg fragmented biotinylated cRNA. The samples were heated to 95°C for 5 min and briefly spun down. Hybridizations were carried out for 16 h at 45°C with mixing on a rotisserie at 60 rpm. After hybridization, the arrays were briefly rinsed with 6x SSPE-T (0.9 M NaCl, 0.06 M NaH2PO4, 6 mM EDTA, 0.01% Triton) and washed on a Fluidics station (Affymetrix). Hybridized arrays were stained with 220 μl detection solution (1x MES buffer, containing 2.5 μl streptavidin-R phycoerythrin conjugate (1 mg/ml) (Molecular Probes)) and 2.0 mg/ml acetylated BSA (Sigma) at 40°C for 15 min and washed again.
Data analysis
Pixel intensities were measured with a commercial confocal laser scanner (Hewlett Packard) and expression signals were analyzed with commercial software (Genechip 3.1; Affymetrix). Detailed data analysis was carried out using RACE-A (Roche), Access 97 and Excel 97 (Microsoft) software. For quantification of relative transcript abundance the normalized average difference value (Avg Diff) was used. For each of the three experimental conditions (wt, hs-wt, hs-lab), four replicates were carried out (for the experimental conditions wt and hs-wt see [39], including the supplementary data). For the difference of the means of the Avg Diff values over the four replicates between condition 1 (hs-wt) and condition 2 (hs-lab), a t-test was performed. Moreover, for downregulation, the mean Avg Diff value of a gene had to be above or equal to 50 in condition 1; for upregulation, the mean Avg Diff value of a gene had to be above or equal to 50 in condition 2. Genes which had a normalized Avg Diff below 20 obtained automatically an Avg Diff of 20 (RACE-A protocol). To obtain a comprehensive analysis of the number and identity of genes differentially regulated by lab, candidates that were already differentially expressed in heat-shocked wild-type embryos compared to non-heat-shocked wild-type controls, were excluded from further analysis ([39] and data not shown). Previously, we have used quantiative RT-PCR to confirm that relative expression level changes in the 1.5-fold and above range, as detected on this array, accurately reflect differences in mRNA abundance in vivo in Drosophila embryos [39]. In consequence, in this report only relative expression level changes in the 1.5-fold and above range are presented.
Reverse transcriptase PCR (RT-PCR)
Three hundred nanograms of poly(A)+ RNA, isolated from heat-shocked wild-type embryos and heat-shocked hs-lab embryos (mRNA isolation kit; Roche Diagnostics), was reverse transcribed with AMV-RT and random hexamers (first-strand cDNA synthesis kit for RT-PCR; Roche Diagnostics). PCR was performed with 100 pg of template DNA and gene-specific primers (designed, using SEQ WEB, Wisconsin Package Version 10.0; GCG) on a light cycler (LightCycler; Roche Diagnostics). Continuous fluorescence observation of amplifying DNA was done using SYBR Green I (LightCycler - FastStart DNA master SYBR GreenI; Roche Diagnostics). After cycling, a melting curve was produced by slow denaturation of the PCR end-products to check the specificity of amplification. To compare the relative amounts of PCR products, we monitored the amplification profile on a graph, displaying the log of the fluorescence against the number of cycles. Relative fold changes for a given gene under both conditions (heat shock wt versus heat shock hs-lab) were calculated using the fit point method (LightCycler operator's manual, version 3.0; Roche Diagnostics).
Functional classification
The genes represented on the high-density oligonucleotide array were grouped into 14 functional classes according to the function of the gene product and currently available genetic data [39]. For this, notations in Flybase, Interactive Fly and SWISS-PROT/TrEMBL databases were used. A comprehensive presentation of all the genes represented on the oligonucleotide array as well as their attribution to functional classes is given as supplementary data to [39].
Acknowledgments
Acknowledgements
We thank Jan Mous, Adrian Roth, Michel Tessier, Monika Seiler and Reto Brem for essential contributions and helpful advice. We thank Clemens Broger, Martin Strahm and Martin Neeb (F. Hoffman-La Roche) for allowing us to use their RACE-A CHIP analysis software and Volker Schmid and Natalie Yanze for help with the light cycler. This research was supported by grants from the SNSF and the EU-BIOTECH program (to H.R.) and by F. Hoffmann-La Roche.
References
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Manak JR, Scott MP. A class act: conservation of homeodomain protein functions. Development. 1994;Suppl.:61–71. [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Carroll SB. Homeotic genes and the evolution of arthropods and chordates. Nature. 1995;376:479–485. doi: 10.1038/376479a0. [DOI] [PubMed] [Google Scholar]
- Maconochie M, Nonchev S, Morrison A, Krumlauf R. Paralogous Hox genes: function and regulation. Annu Rev Genet. 1996;30:529–556. doi: 10.1146/annurev.genet.30.1.529. [DOI] [PubMed] [Google Scholar]
- Gellon G, McGinnis W. Shaping animal body plans in development and evolution by modulation of Hox expression patterns. BioEssays. 1998;20:116–125. doi: 10.1002/(SICI)1521-1878(199802)20:2<116::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Morata G. Homeobox genes: their function in Drosophila segmentation and pattern formation. Cell. 1994;78:181–189. doi: 10.1016/0092-8674(94)90289-5. [DOI] [PubMed] [Google Scholar]
- Duboule D, Morata G. Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet. 1994;10:358–364. doi: 10.1016/0168-9525(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Graba Y, Aragnol D, Pradel J. Drosophila Hox complex downstream targets and the function of homeotic genes. BioEssays. 1997;19:379–388. doi: 10.1002/bies.950190505. [DOI] [PubMed] [Google Scholar]
- Morata G, Sanchez-Herrero E. Patterning mechanisms in the body trunk and the appendages of Drosophila. Development. 1999;126:2823–2828. doi: 10.1242/dev.126.13.2823. [DOI] [PubMed] [Google Scholar]
- Mann RS, Morata G. The developmental and molecular biology of genes that subdivide the body of Drosophila. Annu Rev Cell Dev Biol. 2000;16:243–271. doi: 10.1146/annurev.cellbio.16.1.243. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A. Genetic control of wing disk development in Drosophila. Ciba Foundation Symp. 1975;29:161–182. doi: 10.1002/9780470720110.ch8. [DOI] [PubMed] [Google Scholar]
- Pradel J, White RAH. From selectors to realizators. Int J Dev Biol. 1998;42:417–421. [PubMed] [Google Scholar]
- Andrew DJ, Scott MP. Downstream of the homeotic genes. The New Biologist. 1992;4:5–15. [PubMed] [Google Scholar]
- Morata G. Homeotic genes of Drosophila. Curr Opin Genet Dev. 1993;3:606–614. doi: 10.1016/0959-437x(93)90096-8. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Qian YQ, Billeter M, Furukubo-Tokunaga K, Schier AF, Resendez-Perez D, Affolter M, Otting G, Wüthrich K. Homeodomain-DNA recognition. Cell. 1994;78:211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Mastick GS, McKay R, Oligino T, Donovan K, Lopez AJ. Identification of target genes regulated by homeotic proteins in Drosophila melanogaster through genetic selection of Ultrabithorax protein binding sites in yeast. Genetics. 1995;139:349–363. doi: 10.1093/genetics/139.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botas J, Auwers L. Chromosomal binding sites of Ultrabithorax homeotic proteins. Mech Dev. 1996;56:129–138. doi: 10.1016/0925-4773(96)00519-9. [DOI] [PubMed] [Google Scholar]
- Mannervik M. Target genes of homeodomain proteins. BioEssays. 1999;21:267–270. doi: 10.1002/(SICI)1521-1878(199904)21:4<267::AID-BIES1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Nasiadka A, Krause HM. Kinetic analysis of segmentation gene interactions in Drosophila embryos. Development. 1999;126:1515–1526. doi: 10.1242/dev.126.7.1515. [DOI] [PubMed] [Google Scholar]
- Nasiadka A, Grill A, Krause HM. Mechanisms regulating target gene selection by the homeodomain-containing protein Fushi tarazu. Development. 2000;127:2965–2976. doi: 10.1242/dev.127.13.2965. [DOI] [PubMed] [Google Scholar]
- Biggin MD, McGinnis W. Regulation of segmentation and segmental identity by Drosophila homeoproteins: the role of DNA binding in functional activity and specificity. Development. 1997;124:4425–4433. doi: 10.1242/dev.124.22.4425. [DOI] [PubMed] [Google Scholar]
- Liang Z, Biggin MD. Eve and ftz regulate a wide array of genes in blastoderm embryos: the selector homeoproteins directly or indirectly regulate most genes in Drosophila. Development. 1998;125:4471–4482. doi: 10.1242/dev.125.22.4471. [DOI] [PubMed] [Google Scholar]
- Mlodzik M, Fjose A, Gehring WJ. Molecular structure and spatial expression of a homeobox gene from the labial region of the Antennapedia-complex. EMBO J. 1988;7:2569–2578. doi: 10.1002/j.1460-2075.1988.tb03106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich RJ, Merrill VKL, Pultz MA, Kaufman TC. Isolation, structure and expression of labial, a homeotic gene of the Antennapedia complex involved in Drosophila head development. Genes Dev. 1989;3:399–414. doi: 10.1101/gad.3.3.399. [DOI] [PubMed] [Google Scholar]
- Kaufman TC, Seeger MA, Olsen G. Molecular and genetic organization of the antennapedia gene complex of Drosophila melanogaster. Adv Genet. 1990;27:309–362. doi: 10.1016/s0065-2660(08)60029-2. [DOI] [PubMed] [Google Scholar]
- Duboule D. Guidebook to the Homeobox Genes Oxford: Oxford University Press, 1994.
- Merrill VKL, Diederich RJ, Turner FR, Kaufman TC. A genetic and developmental analysis of mutations in labial, a gene necessary for proper head formation in Drosophila melanogaster. Dev Biol. 1989;135:376–391. doi: 10.1016/0012-1606(89)90187-5. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Bienz M. Specification of a single cell type by a Drosophila homeotic gene. Cell. 1994;76:689–702. doi: 10.1016/0092-8674(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Hirth F, Hartmann B, Reichert H. Homeotic gene action in embryonic brain development of Drosophila. Development. 1998;125:1579–1589. doi: 10.1242/dev.125.9.1579. [DOI] [PubMed] [Google Scholar]
- Pöpperl H, Bienz M, Studer M, Chan S-K, Aparicio S, Brenner S, Mann RS, Krumlauf R. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell. 1995;81:1031–1042. doi: 10.1016/s0092-8674(05)80008-x. [DOI] [PubMed] [Google Scholar]
- Lutz B, Lu H-C, Eichele G, Miller D, Kaufman TC. Rescue of Drosophila labial null mutant by chicken ortholog Hoxb-1 demonstrates that the function of Hox genes is phylogenetically conserved. Genes Dev. 1996;10:176–184. doi: 10.1101/gad.10.2.176. [DOI] [PubMed] [Google Scholar]
- Chan S-K, Pöpperl H, Krumlauf R, Mann RS. An extradenticle-induced conformational change in a HOX protein overcomes an inhibitory function of the conserved hexapeptide motif. EMBO J. 1996;15:2476–2487. [PMC free article] [PubMed] [Google Scholar]
- Mann RS, Chan S-K. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996;12:258–262. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- Hirth F, Reichert H. Conserved genetic programs in insect and mammalian brain development. BioEssays. 1999;21:677–684. doi: 10.1002/(SICI)1521-1878(199908)21:8<677::AID-BIES7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Lockhart DJ, Dong H, Byrne MC, Follettie MT, Gallo MV, Chee MS, Mittmann M, Wang C, Kobayashi M, Horton H, Brown EL. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- Lipshutz RJ, Fodor SP, Gingeras TR, Lockhart DJ. High density synthetic oligonucleotide arrays. Nat Genet. 1999;21:20–24. doi: 10.1038/4447. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Yandell MD, Wortman JR, Gabor Miklos GL, Nelson CR, Hariharan IK, Fortini ME, Li PW, Apweiler R, Fleischmann W, et al. Comparative genomics of the eukaryotes. Science. 2000;287:2204–2215. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans R, Egger B, Loop T, Kammermeier L, He H, Hartmann B, Certa U, Hirth F, Reichert H. Quantitative transcript imaging in normal and heat shocked Drosophila embryos by using high-density oligonucleotide arrays. Proc Natl Acad Sci USA. 2000;97:12138–12143. doi: 10.1073/pnas.210066997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer JG, Kaufman TC. Homeotic genes have specific functional roles in the establisment of the Drosophila embryonic peripheral nervous system. Development. 1992;115:35–47. doi: 10.1242/dev.115.1.35. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Nusslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992;68:201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Micklem DR. mRNA localisation during development. Dev Biol. 1995;172:377–395. doi: 10.1006/dbio.1995.8048. [DOI] [PubMed] [Google Scholar]
- Shen J, Wu H, Gudas LJ. Molecular cloning and analysis of a group of genes differentially expressed in cells which overexpress the Hoxa1 homeobox gene. Exp Cell Res. 2000;259:274–283. doi: 10.1006/excr.2000.4963. [DOI] [PubMed] [Google Scholar]
- Carr A, Biggin MD. A comparison of in vivo and in vitro DNA-binding specificities suggests a new model for homeoprotein DNA binding in Drosophila embryos. EMBO J. 1999;18:1598–1608. doi: 10.1093/emboj/18.6.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DL, Benassayag C, Randazzo FM, Kaufman TC. Levels of homeotic protein function can determine developmental identity: evidence from low-level expression of the Drosophila homeotic gene proboscipedia under Hsp70 control. EMBO J. 1995;14:767–778. doi: 10.1002/j.1460-2075.1995.tb07055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann RS, Affolter M. Hox proteins meet more partners. Curr Opin Genet Dev. 1998;8:423–429. doi: 10.1016/s0959-437x(98)80113-5. [DOI] [PubMed] [Google Scholar]
- Mann RS. The specificity of homeotic gene function. BioEssays. 1995;17:855–863. doi: 10.1002/bies.950171007. [DOI] [PubMed] [Google Scholar]
- Bryant Z, Subrahmanyan L, Tworoger M, LaTray L, Liu C-R, Li M-J, Van den Engh G, Ruohola-Baker H. Characterization of differentially expressed genes in purified Drosophila follicle cells: Towards a general strategy for cell type-specific developmental analysis. Proc Natl Acad Sci USA. 1999;96:5559–5564. doi: 10.1073/pnas.96.10.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KP, Rifkin SA, Hurban P, Hogness DS. Microarray analysis of Drosophila development during metamorphosis. Science. 1999;286:2179–2184. doi: 10.1126/science.286.5447.2179. [DOI] [PubMed] [Google Scholar]
- Andrews J, Bouffard GG, Cheadle C, Lu J, Becker KG, Oliver B. Gene discovery using computational and microarray analysis of transcription in the Drosophila melanogaster testis. Genome Res. 2000;10:2030–2043. doi: 10.1101/gr.10.12.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega J, Hartenstein V. The Embryonic Development of Drosophila melanogaster Heidelberg: Springer; 1997.
- Walter MF, Petersen NS, Biessmann H. Heat shock causes the collapse of the intermediate filament cytoskeleton in Drosophila embryos. Dev Genet. 1990;11:270–279. doi: 10.1002/dvg.1020110405. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Therianos S, Leuzinger S, Hirth F, Goodman CS, Reichert H. Embryonic development of the Drosophila brain: formation of commissural and descending pathways. Development. 1995;121:3849–3860. doi: 10.1242/dev.121.11.3849. [DOI] [PubMed] [Google Scholar]
- Grieder NC, Marty T, Ryoo H-D, Mann RS, Affolter M. Synergistic activation of a Drosophila enhacer by HOM/EXD and DPP signaling. EMBO J. 1997;16:7402–7410. doi: 10.1093/emboj/16.24.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevappa M, Warrington JA. A high-density probe array sample preparation method using 10- to 100-fold fewer cells. Nat Biotechnol. 1999;17:1134–1136. doi: 10.1038/15124. [DOI] [PubMed] [Google Scholar]


