Abstract
Interstitial lung disease (ILD) and lung fibrosis are characterized by different grades of fibrosis and inflammation. Persistent low-grade inflammation is believed to play a major pathogenic role, leading to an imbalance of cytokines, growth factors, and tissue proteinases. Recruited monocytes and macrophages play a pivotal role through their cytokine expression and possibly differentiation into fibrocytes, pericytes, or myofibroblasts. Atypical bacterial infections can cause ILD, although not usually in the form of usual interstitial pneumonia. On the other hand, bacterial colonization is frequently encountered in patients with chronic fibrotic lung disorders, and patients regularly undergo antibacterial treatment. As demonstrated in patients with diffuse panbronchiolitis and other chronic respiratory disorders, treatment with macrolides can be beneficial. This is partly explained by their antimicrobial effects but, for macrolides, immunomodulatory properties have been identified which might also be beneficial in patients with ILD or lung fibrosis. This article reviews the immunology of lung fibrogenesis and putative implications of macrolides for reinstallation of tolerance.
Keywords: lung fibrosis, inflammation, pneumonia
Introduction
Interstitial lung diseases (ILD) are a heterogeneous group of diffuse parenchyma disorders characterized by different grades of fibrosis and inflammation. Despite varying pathogenesis, they are grouped together because of their similar clinical presentation, chest radiographic appearance, histology, and physiological features.1,2
Environmental, infectious, or self antigens provoke inflammation which, due to either ongoing antigen exposure or lack of resolution, results in fibrosis. Environmental triggers include silica, metals, and hard metal/organic dust exposure, fungi, or animal proteins. Fungal infections, atypical bacterial pneumonias, or viral pneumonias can result in ILD, occurring predominantly in immunocompromised hosts. Drug-induced pneumopathy due to methotrexate or bleomycin therapy can also cause ILD.
Primary ILD is defined as the absence of underlying disease, whilst immunological diseases, such as systemic sclerosis, systemic lupus erythematosus, or rheumatoid arthritis, are generally responsible for secondary disease.3 Idiopathic interstitial pneumonias comprise seven different entities according to their clinical, radiological, and pathological presentation.1 Of these, idiopathic pulmonary fibrosis, which corresponds with the histopathological finding of usual interstitial pneumonia, is the prototype of fibrosing ILD.
Connective tissue diseases, hypersensitivity pneumonitis, and asbestosis can also lead to usual interstitial pneumonia, where abnormal proliferation of mesenchymal cells, especially the occurrence of fibrotic foci, leads to a reticular alteration of lung parenchyma, described as honeycombing.4 However, nonspecific interstitial pneumopathy, which is more typical for secondary ILD, shows a diffuse histopathological picture and generally a more inflammatory, cell-rich infiltrate.5,6
Patients with ILD and lung fibrosis are susceptible to bacterial superinfection. This is due to primary or iatrogenic immunosuppression, reduced mucus clearance, bacterial colonization, or biofilm production. Patients regularly require antibiotic treatment. Long-term treatment strategies similar to those used for cystic fibrosis are rare in ILD, with the exception of chronic aspergillus or nontuberculous mycobacterial infection.7 Antibiotics serve to reduce the bacterial load and virulence, and also, notably for macrolides, have additional beneficial properties. Azithromycin, clarithromycin, and erythromycin also reduce bacterial adherence and biofilm production, and have an immunomodulatory effect.8 These agents can potentially reinstall tolerance on the one hand and protect the patient from ongoing danger signals and damage produced by bacterial colonization or infections on the other. Indeed, positive effects of macrolides have been reported, eg, in patient cohorts with diffuse panbronchiolitis.9 However, the question of whether macrolide treatment is beneficial in ILD or lung fibrosis in general, is elusive. This review gives an overview of the immunology in ILD and the possible implications of the immunomodulatory effects of macrolide antibiotics.
Role of inflammation
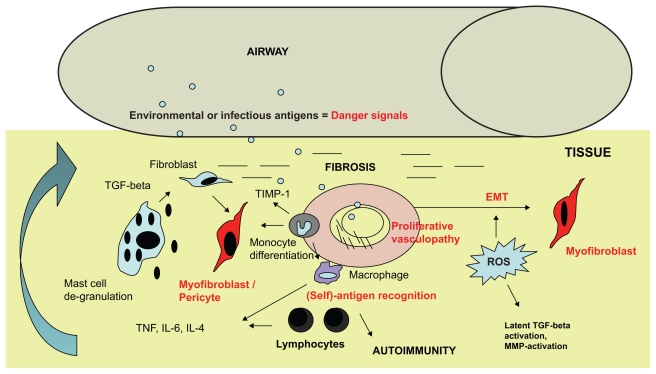
According to our current understanding of lung fibrosis, an injury or “danger signal” leads to parenchymal damage and subsequently triggers lung fibrosis.10 Initial inflammation in the form of alveolitis occurs in all forms of ILD, although there is often less inflammation in usual interstitial pneumonia than in nonspecific interstitial pneumopathy (Wells AU, personal communication). Why inflammation turns into autoinflammation in these patients is unclear. The likely answer is that the normal resolution of inflammation is altered and that tolerance towards the host lung parenchyma is lost. In any case, inflammatory cells induce a Th-1/2 imbalance in favor of profibrotic Th-2 cytokines, such as interleukin (IL)-4 or IL-13, which are regarded as key drivers of fibrosis.11,12 IL-17 produced by Th17+ T cell subsets has been identified as a further orchestrator of the inflammatory cascade and fibrosis in interstitial lung disease.13,14 Once damage has occurred, immune cells of different lineages are activated (Figure 1). Alveolar macrophages, basophils, lymphocytes, and mast cells are described in the inflammatory infiltrate, often with a perivascular distribution.15,16 How they are orchestrated in detail and their effects on stromal cells is not completely understood.
Figure 1.
Transition of inflammation to fibrosis in interstitial lung disease. Toxic or infectious antigens delivered via air or blood supply to lung parenchyma represent a danger signal which triggers an inflammatory response. First, the innate immune system is activated, eg, via mast cells which degranulate and release chemoattractants and growth factors, such as TGF-β. Myofibroblasts which differentiate from fibroblasts, pericytes, or fibrocytes, serve as key effector cells in fibrosis by producing collagen and alpha-smooth muscle actin. They are also derived from epithelial cells by EMT. Lymphocytes recognize antigens which are presented by macrophages. They express mainly Th-2 cytokines, proliferate, and trigger autoimmunity. Proliferative vasculopathy is a result of ongoing low-grade inflammation, and insufficient vascular blood supply further promotes fibrosis.
Abbreviations: EMT, epithelial–mesenchymal transition; IL, interleukin; TGF, transforming growth factor; ROS, reactive oxygen species; MMP, metalloproteinases; TIMP-1, tissue inhibitor of metalloproteinases-1; TNF, tumor necrosis factor.
Macrophages and monocytes, as members of the innate immune system, produce both proinflammatory and profibrotic cytokines.17 Monocytes patrolling on the endothelium are recruited by certain stimuli, such as infection, and then differentiate into macrophages or dendritic cells.18 Our research group has found that monocytes of patients with systemic sclerosis express significantly more tissue inhibitors of metalloproteinases than healthy controls (Huigens et al, submitted for publication). In contrast with neutrophils, monocytes persist in the lung parenchyma for up to two months without persistent influx of blood monocytes.19 Macrophages also promote fibroblast proliferation and collagen deposition.2 Whether monocytes solely express cytokines or differentiate into direct profibrotic cells, such as fibrocytes, pericytes, or myofibroblasts, has not been completely clarified, but it has been reported that, like mesenchymal stem cells, monocytes do have plurigenic properties.20 Mast cells belong to the innate immune system. They are regarded as “tissue sensors” and are also involved in ILD.
Mast cell activation can be IgE-mediated or occur directly via antigens or stimulation of Toll-like receptors.21 Mast cell degranulation has been observed in ILD in several studies.22 Mast cell vesicles contain various profibrotic factors, such as IL-4 and transforming growth factor beta (TGF-β), and mast cells also produce metalloproteinases.23 Our group has recently shown that degranulating mast cells are the main source of TGF-β in the skin of patients with systemic sclerosis.24 Their proximity to fibroblasts and blood vessels may facilitate fibrogenesis. TGF-β is of paramount importance in fibrotic lung disease and also a differentiation factor for Th17 cells.25 Gene expression profiles of ILD lung samples demonstrated a TGF-β fingerprint.26 Similar to parasitic or allergic inflammation, mast cells, as well as basophils, are likely to be involved in producing a Th2-immune response in lung fibrosis.21,27
Lymphocytes belong to the adaptive immune system. They are regularly encountered in the inflammatory infiltrate in ILD samples and play an important role by their cytokine production and capacity to trigger (auto) antigen-specific immunity. Both T cells and B cells are recruited, whereby T effector cells support plasma cells to produce autoantibodies. T cells also downregulate the inflammatory process. It has been shown in bacterial infection that T regulatory cells express IL-10 which protects the tissue from “friendly fire” by reducing cellular interferon-γ expression.28
Fibrocytes
Fibrocytes are circulating, bone marrow-derived CD14+ cells which can produce collagen.29,30 They have features of both monocytes and stromal cells. In wound healing, they differentiate into myofibroblasts, yet remain able to present antigen to lymphocytes.31 In animal models of lung fibrosis, circulating fibrocytes are recruited to injured lungs as an integral component of the pathogenesis of pulmonary fibrosis.32 In patients with systemic sclerosis, there are more fibrocytes in the circulation, expressing higher levels of IL-10 upon proinflammatory stimulation compared with healthy individuals.33 The concept of fibrocytes being involved in ILD is appealing because these cells are derived from an inexhaustible source, the bone marrow, and thus can replenish fibrotic effector cells in the tissue.
Epithelial-mesenchymal transition
Despite inflammation still being regarded as essential for fibrogenesis, there is convincing evidence that inflammation and fibrosis can be dissociated. Ongoing epithelial injury and activation can lead to a transdifferentiation of epithelial cells into mesenchymal cells, a process known as epithelial– mesenchymal transition, which occurs when epithelial cells lose cell–cell attachment, undergo cytoskeletal remodeling, and finally obtain a mesenchymal phenotype. This process is considered to be a key factor for fibrogenesis in general, and epithelial–mesenchymal transition indeed has been observed in ILD.34,35 Induction of epithelial–mesenchymal transition in alveolar epithelial cells is initiated by several factors, notably TGF-β, which certainly is a main stimulatory factor for epithelial–mesenchymal transition in ILD.34,36 However, several other factors, such as fibroblast growth factor, and also endothelin-1 and reactive oxygen species, can be responsible for epithelial–mesenchymal transition.34
Reactive oxygen species
Free radical reactions contribute to fibrogenesis. The source of these free radicals is typically inflammatory stimuli.37 Regardless of the cause, tissue damage leads to oxidative stress.38 In lung inflammation, an increased oxygen burden mainly arises from the accumulation of inflammatory cells, including macrophages and neutrophils, which show an exaggerated release of oxygen radicals.39 Nitric oxide species increase fibroblast proliferation.40 Increased expression of nitric oxide species has been described in unclassified pulmonary fibrosis, and expression of nitric oxide species has been detected also during experimental granuloma formation in vitro and in granulomatous inflammation caused by silica, zymosan, and dextran beads in vivo.41–43
Influence of infection on fibrogenesis
According to current consensus, usual interstitial pneumonia in its classical presentation is generally not caused by infection. Notwithstanding, atypical pneumonias can lead to fibrosing ILD, with a picture similar to cryptogenic organizing pneumonitis.1 In a recent case series, four patients with pulmonary histoplasmosis developed ILD, all of them showing different forms of inflammation. One had bron-chocentric granulomatosis, one had pulmonary alveolar proteinosis, one had diffuse alveolar damage, and, in one biopsy, the clinical manifestations suggested tuberculous primo-infection with systemic dissemination.44 In another study of patients with tuberculosis, atypical mycobacterial infections, or sarcoidosis, it was demonstrated that alpha-smooth muscle actin as a marker of fibrosis, is expressed around both necrotic and non-necrotic granulomas. Alpha-smooth muscle actin expression was higher in the granulomas of patients with mycobacterial infection than in those with sarcoidosis.45 This shows that the etiology of inflammation is important for fibrogenesis, rather than inflammation per se, and it is more likely that ILD occurs in individuals with a genetic predisposition.46 These individuals are more likely to develop autoinflammation and, in some cases, also autoimmunity with identified (self) antigens. One can argue that in certain patients suffering from atypical pneumonia, antibiotic treatment, eg, with macrolides or quinolones, may prevent autoinflammation or autoimmunity.
It remains unclear whether bacterial superinfection ILD directly triggers fibrosis in individuals with underlying ILD. However, cystic fibrosis demonstrates that infections do accelerate deterioration of lung fibrosis, and antibiotic treatment or prophylaxis reduces mortality in these patients.
Macrolide therapy
Macrolides, such as erythromycin, clarithromycin, and azithromycin, are antibiotic compounds which are effective against Gram-positive and Gram-negative bacteria, including streptococci, Haemophilus influenzae, staphylococci, mycoplasma, mycobacteria, rickettsia, and chlamydia. Apart from their established antibacterial effects, there is growing evidence for anti-inflammatory properties of macrolides in chronic respiratory diseases, such as diffuse panbronchiolitis, asthma, cystic fibrosis, or chronic bronchitis.8,9 Macrolides share a macrocyclic lactone ring with other immunosuppressive substances, such as rapamycin.47 Their antimicrobial and possibly immunosuppressive effect is exerted by binding to the 50S ribosomes of prokaryotic and eukaryotic organisms, affecting protein synthesis.48 Interestingly, the immunomodulatory effect is limited to macrolides which share a 14-member ring, and the dose required for immunomodulation is lower compared with the dose necessary for antimicrobial activity. In patients with cystic fibrosis, long-term treatment with azithromycin achieved a better forced expiratory volume in one second and fewer exacerbations than placebo.49 Apart from the simple killing of bacteria, this effect seems to be supported by reducing bacterial adherence, inhibiting biofilm formation, and decreasing microbiological virulence.50
Macrolides not only accumulate in bacteria, but also in host leukocytes.47 They have an inhibitory effect on proinflammatory cytokines, such as tumor necrosis factor-α, IL-1, and nitric oxide. In contrast, macrolides increase the expression of IL-10, one of the most important anti-inflammatory cytokines produced by T or B regulatory cells.51 IL-10 is central in protecting host tissue during an infection by inhibiting the synthesis of interferon gamma by both T cells and natural killer cells and the synthesis of nitric oxide by macrophages (Figure 2).52
Figure 2.
Dual effect of macrolides on bacteria and host immune cells. Macrolides inhibit bacterial protein translation by interfering with the ribosome subunit. They reduce biofilm production and bacterial adherence. An immunomodulatory effect is achieved on host immune cells by reducing the production of proinflammatory cytokines, such as TNF, and increasing IL-10 as an anti-inflammatory cytokine. IL-10 downregulates the cellular interferon-gamma response during infection, and therefore has been described as protecting host tissue from “friendly fire”.
Abbreviations: IL, interleukin; TNF, tumor necrosis factor; NO, nitric oxide.
The exact mechanism by which cellular cytokines are either upregulated or downregulated by macrolides is not clear. It seems that suppression of nuclear factor-κB and an increase in cyclic adenosine monophosphate levels are involved.8,53 Another important observation is that macrolides accelerate apoptosis of neutrophils and other leukocytes and the removal of cell debris by macrophages, respectively.54 Proapoptotic effects has also been postulated for improvement of mucosa-associated lymphoid tissue tumors under long-term macrolide therapy.55 Macrolide treatment has also been shown to reduce superoxide and elastase release from human leukocytes.56
Positive effects of macrolides have been achieved in patients with cryptogenic organizing pneumonia and radiation-related ILD. However, these effects have only been observed in small case studies, and need to be confirmed in controlled trials.
Conclusion
Lung fibrogenesis is a heterogenic complex process associated with destruction of lung parenchyma. Inflammation and cytokine imbalance are pivotal elements in the development of lung fibrosis. Although infection does not cause usual interstitial pneumonia, bacteria can trigger ILD and probably contribute secondarily to the progression of idiopathic interstitial pneumonias in general. Macrolides are safe and effective against a broad range of bacteria, some of which are responsible for ILD. Apart from their antimicrobial effects, macrolides also have immunomodulatory properties. These might protect individuals from ILD progression by interfering with cytokine production, oxygen stress reduction, and host-pathogen interactions. Chronic low-dose macrolide therapy has been successful in patients suffering from diffuse panbronchiolitis and other chronic inflammatory airway diseases, including ILD or lung fibrosis, but there are no randomized, placebo-controlled trials available. All clinical and in vitro observations therefore need confirmation in clinical trials.
Acknowledgment
Sanjay Patel’s critical review of this manuscript has been appreciated.
Footnotes
Disclosure
The author reports no conflict of interest in this work.
References
- 1.American Thoracic Society/European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 2.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 3.Wells AU, Steen V, Valentini G. Pulmonary complications: One of the most challenging complications of systemic sclerosis. Rheumatology. 2009;48:40–44. doi: 10.1093/rheumatology/kep109. [DOI] [PubMed] [Google Scholar]
- 4.Kim EJ, Collard HR, King TE., Jr Rheumatoid arthritis-associated interstitial lung disease: The relevance of histopathologic and radiographic pattern. Chest. 2009;136:1397–1405. doi: 10.1378/chest.09-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bussone G, Mouthon L. Interstitial lung disease in systemic sclerosis. Autoimmun Rev. 2010 September 21; doi: 10.1016/j.autrev.2010.09.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Poletti V, Kitaichi M. Facts and controversies in the classification of idiopathic interstitial pneumonias. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:229–238. [PubMed] [Google Scholar]
- 7.Rogers GB, Stressmann FA, Walker AW, Carroll MP, Bruce KD. Lung infections in cystic fibrosis: Deriving clinical insight from microbial complexity. Expert Rev Mol Diagn. 2010;10:187–196. doi: 10.1586/erm.09.81. [DOI] [PubMed] [Google Scholar]
- 8.Tamaoki J, Kadota J, Takizawa JH. Clinical implications of the immunomodulatory effects of macrolides. Am J Med. 2004;117(Suppl 9A):5S–511S. doi: 10.1016/j.amjmed.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Parnham MJ. Immunomodulatory effects of antimicrobials in the therapy of respiratory tract infections. Curr Opin Infect Dis. 2005;18:125–131. doi: 10.1097/01.qco.0000160901.71813.fe. [DOI] [PubMed] [Google Scholar]
- 10.Snider GL. Interstitial pulmonary fibrosis. Chest. 1986;89:115–121. doi: 10.1378/chest.89.3_supplement.115s. [DOI] [PubMed] [Google Scholar]
- 11.Furuie H, Yamasaki H, Suga M, Ando M. Altered accessory cell function of alveolar macrophages: A possible mechanism for induction of Th2 secretory profile in idiopathic pulmonary fibrosis. Eur Respir J. 1997;10:787–794. [PubMed] [Google Scholar]
- 12.Martinez JA, King TE, Jr, Brown K, et al. Increased expression of the interleukin-10 gene by alveolar macrophages in interstitial lung disease. Am J Physiol. 1997;273:676–683. doi: 10.1152/ajplung.1997.273.3.L676. [DOI] [PubMed] [Google Scholar]
- 13.Simonian PL, Roark CL, Wehrmann F, et al. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol. 2009;182:657–665. [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshizaki A, Yanaba K, Iwata Y, et al. Cell adhesion molecules regulate fibrotic process via Th1/Th2/Th17 cell balance in a bleomycin-induced scleroderma model. J Immunol. 2010;185:2502–2515. doi: 10.4049/jimmunol.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bitterman PB, Rennard SI, Keogh BA, Wewers MD, Adelberg S, Crystal RG. Familial idiopathic pulmonary fibrosis. Evidence of lung inflammation in unaffected family members. N Engl J Med. 1986;314:1343–1347. doi: 10.1056/NEJM198605223142103. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds HY, Fulmer JD, Kazmierowski JA, Roberts WC, Frank MM, Crystal RG. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977;59:165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn TA, Barron L. Macrophages: Master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auffray C, Fogg D, Garfa G, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 19.Doherty DE, Hirose N, Zagarella L, Cherniack RM. Prolonged monocyte accumulation in the lung during bleomycin-induced pulmonary fibrosis. A noninvasive assessment of monocyte kinetics by scintigraphy. Lab Invest. 1992;66:231–242. [PubMed] [Google Scholar]
- 20.Zhao Y, Glesne D, Huberman E. A human peripheral blood monocytederived subset acts as pluripotent stem cells. Proc Natl Acad Sci U S A. 2003;100:2426–2431. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medzhitov R. Inflammation 2010: New adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Brown JM, Swindle EJ, Kushnir-Sukhov NM, Holian A, Metcalfe DD. Silica-directed mast cell activation is enhanced by scavenger receptors. Am J Respir Cell Mol Biol. 2007;36:43–52. doi: 10.1165/rcmb.2006-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards ST, Cruz AC, Donnelly S, et al. c-Kit immunophenotyping and metalloproteinase expression profiles of mast cells in interstitial lung diseases. J Pathol. 2005;206:279–290. doi: 10.1002/path.1780. [DOI] [PubMed] [Google Scholar]
- 24.Hügle T, Hogan V, White K, van Laar JM. Mast cells are a source of transforming growth factor (TGF) beta in systemic sclerosis. Arthritis Rheum. 2010 December 16; doi: 10.1002/art.30190. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Denton CP, Abraham DJ. Transforming growth factor-beta and connective tissue growth factor: Key cytokines in scleroderma pathogenesis. Curr Opin Rheumatol. 2001;13:505–511. doi: 10.1097/00002281-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Konishi K, Gibson KF, Lindell KO, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180:167–175. doi: 10.1164/rccm.200810-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokol CL, Medzhitov R. Role of basophils in the initiation of Th2 responses. Curr Opin Immunol. 2010;22:73–77. doi: 10.1016/j.coi.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jankovic D, Kugler DG, Sher A. IL-10 production by CD4+ effector T cells: A mechanism for self-regulation. Mucosal Immunol. 2010;3:239–246. doi: 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 30.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 31.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathai SK, Gulati M, Peng X, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest. 2010;90:812–823. doi: 10.1038/labinvest.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willis BC, Liebler JM, Luby-Phelp K, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: Potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selman M, Pardo A. The epithelial/fibroblastic pathway in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:S93–97. [PubMed] [Google Scholar]
- 36.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287–305. doi: 10.1016/s0891-5849(96)00327-9. [DOI] [PubMed] [Google Scholar]
- 38.Halliwell B. Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann Rheum Dis. 1995;54:505–510. doi: 10.1136/ard.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallaert B, Lassalle P, Fortin F, et al. Superoxide anion generation by alveolar inflammatory cells in simple pneumoconiosis and in progressive massive fibrosis of nonsmoking coal workers. Am Rev Respir Dis. 1990;141:129–133. doi: 10.1164/ajrccm/141.1.129. [DOI] [PubMed] [Google Scholar]
- 40.Gansauge S, Gansauge F, Nussler AK. Exogenous, but not endogenous, nitric oxide increases proliferation rates in senescent human fibroblasts. FEBS. 1997;410:160–164. doi: 10.1016/s0014-5793(97)00544-9. [DOI] [PubMed] [Google Scholar]
- 41.Saleh D, Barnes PJ, Giaid A. Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:1763–1769. doi: 10.1164/ajrccm.155.5.9154889. [DOI] [PubMed] [Google Scholar]
- 42.Goldman D, Cho Y, Zhao M, Casadevall A, Lee SC. Expression of inducible nitric oxide synthase in rat pulmonary Cryptococcus neoformans granulomas. Am J Pathol. 1996;148:1275–1282. [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuji M, Dimov VB, Yoshida T. In vivo expression of monokine and inducible nitric oxide synthase in experimentally induced pulmonary granulomatous inflammation. Evidence for sequential production of interleukin-1, inducible nitric oxide synthase, and tumor necrosis factor. Am J Pathol. 1995;147:1001–1015. [PMC free article] [PubMed] [Google Scholar]
- 44.Garrido L, Mata-Essayag S, Hartung de Capriles C, Eugenia Landaeta M, Pacheco I, Fuentes Z. Pulmonary histoplasmosis: Unusual histopathologic findings. Pathol Res Pract. 2006;202:373–378. doi: 10.1016/j.prp.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Kaarteenaho-Wiik R, Sademies O, Paakko P, Risteli J, Soini Y. Extracellular matrix proteins and myofibroblasts in granulomas of sarcoidosis, atypical mycobacteriosis, and tuberculosis of the lung. Hum Pathol. 2007;38:147–153. doi: 10.1016/j.humpath.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Frech T, Khanna D, Markewitz B, Mineau G, Pimentel R, Sawitzke A. Heritability of vasculopathy, autoimmune disease, and fibrosis in systemic sclerosis: A population-based study. Arthritis Rheum. 2010;62:2109–2116. doi: 10.1002/art.27469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Labro MT. Cellular and molecular effects of macrolides on leukocyte function. Curr Pharm Des. 2004;10:3067–3080. doi: 10.2174/1381612043383403. [DOI] [PubMed] [Google Scholar]
- 48.Jain R, Danziger LH. The macrolide antibiotics: A pharmacokinetic and pharmacodynamic overview. Curr Pharm Des. 2004;10:3045–3053. doi: 10.2174/1381612043383322. [DOI] [PubMed] [Google Scholar]
- 49.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: A randomized controlled trial. JAMA. 2003;290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 50.Wozniak DJ, Keyser R. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest. 2004;125:62–69. doi: 10.1378/chest.125.2_suppl.62s. [DOI] [PubMed] [Google Scholar]
- 51.Mauri C. Regulation of immunity and autoimmunity by B cells. Curr Opin Immunol. 2010;22:761–767. doi: 10.1016/j.coi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 2002;148:1792–1796. [PubMed] [Google Scholar]
- 53.Kikuchi T, Hagiwara K, Honda Y, et al. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors. J Antimicrob Chemother. 2002;49:745–755. doi: 10.1093/jac/dkf008. [DOI] [PubMed] [Google Scholar]
- 54.Schultz MJ. Macrolide activities beyond their antimicrobial effects: Macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother. 2004;54:21–28. doi: 10.1093/jac/dkh309. [DOI] [PubMed] [Google Scholar]
- 55.Ishimatsu Y, Mukae H, Matsumoto K, et al. Two cases with pulmonary mucosa-associated lymphoid tissue lymphoma successfully treated with clarithromycin. Chest. 2010;138:730–733. doi: 10.1378/chest.09-2358. [DOI] [PubMed] [Google Scholar]
- 56.Villagrasa V, Berto L, Cortijo J, Perpina M, Sanz C, Morcillo EJ. Effects of erythromycin on chemoattractant-activated human polymorphonuclear leukocytes. Gen Pharmacol. 1997;29:605–609. doi: 10.1016/s0306-3623(96)00566-6. [DOI] [PubMed] [Google Scholar]