Abstract
The transient receptor potential vanilloid 1 (TRPV1) is a thermoreceptor that responds to noxious temperatures, as well as to chemical agonists, such as vanilloids and protons. In addition, its channel activity is notably potentiated by proinflammatory mediators released upon tissue damage. The TRPV1 contribution to sensory neuron sensitization by proalgesic agents has signaled this receptor as a prime target for analgesic and anti-inflammatory drug intervention. However, TRPV1 antagonists have notably failed in clinical and preclinical studies because of their unwanted side effects. Recent reports have unveiled previously unrecognized anti-inflammatory and protective functions of TRPV1 in several diseases. For instance, this channel has been suggested to play an anti-inflammatory role in sepsis. Therefore, the use of potent TRPV1 antagonists as a general strategy to treat inflammation must be cautiously considered, given the deleterious effects that may arise from inhibiting the population of channels that have a protective function. The use of TRPV1 antagonists may be limited to treating those pathologies where enhanced receptor activity contributes to the inflamed state. Alternatively, therapeutic paradigms, such as reduction of inflammatory-mediated increase of receptor expression in the cell surface, may be a better strategy to prevent abrogation of the TRPV1 subpopulation involved in anti-inflammatory and protective processes.
Keywords: transient receptor potential, nociceptor, capsaicin, pain, ion channel, analgesia
TRPV1 receptor
Transient receptor potential vanilloid 1 (TRPV1), also known as the capsaicin receptor, was first cloned from rat dorsal root ganglion neurons using an expression-cloning screening strategy.1 This newly cloned cDNA was first named VR1, for vanilloid receptor subtype 1. Because this receptor is a member of the transient receptor potential family of cation channels, it was given the name TRPV1 because it represented the first known member of the transient receptor potential vanilloid subfamily of transient receptor potential channels. To date, TRPV1 orthologs have been identified in eukaryotes, including human, rat, guinea pig, rabbit, mouse, dog, and porcine tissues, but not in prokaryotes. The ability of TRPV1 to respond to noxious stimuli and to be functionally sensitized by proinflammatory mediators has signaled it as a “pathological” receptor, having a significant role in the pain transduction pathway, and in the maintenance of inflammatory conditions in a variety of diseases and injury states.
TRPV1 structure and expression
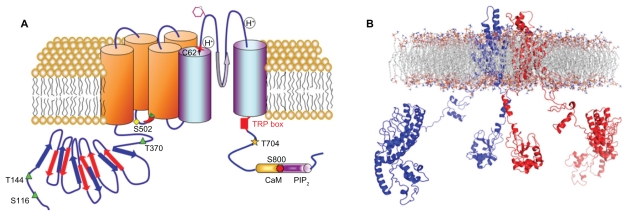
TRPV1 is an 838-amino acid protein with a molecular weight of 95 kDa, consisting of six transmembrane segments, with an amphipathic pore-forming region between the fifth and sixth transmembrane segments, a large N-terminus intracellular domain, and a C-terminal cytosolic region ( Figure 1). Functional TRPV1 channels exist as homomultimers,2 although functional heteroligomers may be formed between TRPV1 and TRPV33 or between TRPV1 and TRPV2,4,5 which may be responsible, at least in part, for the variable responses to agonists and antagonists. The 432-amino acid N-terminus contains at least six ankyrin repeats,6,7 which are essential for channel function8,9 and for orchestrating a plethora of protein–protein interactions that govern the assembly of TRPV1-containing signalplexes.10,11 The 145-amino acid C-terminal contains subdomains involved in distinct channel functions. For instance, adjacent to the channel gate,12 a highly conserved region known as the transient receptor potential domain, is involved in the functional coupling of stimuli sensing and gate opening.13,14 Furthermore, the C-terminus contains the molecular determinants for subunit tetramerization,15,16 two nucleotide-binding Walker-type sites,17 as well as consensus sequences for modulation by phosphoinositides and protein kinases.18,19 More notably, this region has been suggested to hold the temperature sensor of the receptor.20
Figure 1.
A) Putative membrane topology of a transient receptor potential vanilloid 1 subunit displaying the location of residues involved in ligand-binding, proton activation, and post-translational modifications. The transient receptor potential vanilloid 1 domain, and calmodulin- and phosphatidylinositol-4,5-bisphosphate-binding domains are also depicted. B) Side view of the ribbon structural model of two opposite monomers of the transient receptor potential vanilloid 1 channel inserted into the lipid bilayer, after molecular dynamic simulation. The other two monomers are not shown for clarity.
TRPV1 shows a wide tissue distribution. High levels of expression are observed in dorsal root ganglia, trigeminal ganglia, and nodose ganglia.1 TRPV1 is predominantly expressed in small and medium diameter neurons, mainly in the peptidergic ones, that are important in the development of neurogenic pain and inflammation,21 and to a lesser extent in the nonpeptidergic neurons that play a critical role in mediating chronic22 and mechanical pain.23 Although there is still a controversy about the central nervous system distribution of TRPV1,24 several studies have demonstrated the expression of this channel in a wider diversity of brain regions, including the hypothalamus, cerebellum, cerebral cortex, striatum, midbrain, olfactory bulb, medulla, hippocampus, thalamus, and substantia nigra.25 In non-neuronal tissues, TRPV1 expression is detected in keratinocytes26 and melanocytes of the epidermis,27 bladder urothelium,28 smooth muscles,29 glial cells, liver, polymorphonuclear granulocytes,30 mast cells,31 dendritic cells, and macrophages.32
TRPV1 is a nonselective cation channel with near equal selectivity for Na+, K+, Li+, Cs+, and Rb+ ions,1 but moderate selectivity for divalent cations. When activated by capsaicin, the permeability of Mg2+ and Ca2+ relative to Na+ (Px/PNa) is roughly 5 and 10, respectively.1,33,34 Lower Px/PNa values of 3–4 are reported when the channel is activated by heat.35 TRPV1 is also highly permeable to protons and large polyvalent cations, suggesting the existence of a large pore. Several amino acids in the putative pore-forming region between the fifth and sixth transmembrane segment domains are implicated in cation selectivity. Mutation of Glu-648 (E648A) reduces Mg2+ permeability and increases Ca2+ permeability. Mutation of Asp-646 (D646N) reduces Mg2+ permeability and blockade by the cationic dye, ruthenium red.36
The single-channel conductance of capsaicin-activated channels is approximately 90–100 pS at positive potentials. At negative potentials (−60 mV), the conductance is significantly lower, with values of approximately 50 pS.1 TRPV1 currents exhibit significant outward rectification due to a combined effect of voltage on both channel conductance and open probability.37
TRPV1 has different modes of activation
TRPV1 is a polymodal channel, activated by physical and chemical stimuli, including heat, vanilloids, lipids, spider toxins, protons, cations, and voltage.35,38,39 The channel is activated by noxious temperatures with a threshold of approximately 43°C,1 and a temperature-dependent gating characterized by a Q10 ≥ 20 (Q10 is used to estimate the temperature dependence of channel gating).40 The temperature threshold is highly influenced by other ligands that act allosterically and by the receptor phosphorylation state. Thus, when simultaneously activated by other ligands, the threshold may decrease down to 20°C. It has been proposed that temperature regulates TRPV1 by changing the intrinsic voltage sensitivity of the channel.37 The temperature sensitivity of this channel is allosterically linked to chemical and voltage activation.20 Although the mechanisms underlying heat activation remain unclear, a role of the C-terminus and the outer pore region has been proposed.14,41
TRPV1 is activated by capsaicin, the pungent component of hot chili peppers. Capsaicin and related compounds, including resiniferatoxin and olvanil, are highly lipophilic and share a structural similarity to several endogenous fatty acid derivatives that have also been identified as TRPV1 agonists.42,43 These include anandamide (an endocannabinoid), N-arachidonoyl dopamine, oleoyldopamine, 12-hydroperoxyeicosatetraenoic acid (a lipoxygenase product), and 18–20 carbon N-acylethanolamines.44 Vanilloids interact at intracellular regions of TRPV1, as implied by a membrane-impermeable charged capsaicin analog that is only effective when applied cytosolically.45 Consistent with this observation, several intracellular molecular determinants of capsaicin binding have been identified. The amino acid residues, Arg-114 in the N-terminus and Glu-761 in the C-terminal domain, play a key role in ligand binding.46 In addition, Tyr-511 and Ser- 512 located between the second and third transmembrane segments are also critical for vanilloid binding and channel activation,47 and Thr-550 has also been pointed towards as involved in structuring the vanilloid binding site in rat and human TRPV1 channels.48
A pH lower than 5.0 at the extracellular side of the channel activates TRPV1 ion channels. Actually, ligand-, voltage-, and temperature-evoked gating are potentiated by mildly acidic extracellular pH.49 Two glutamate residues located near the extracellular pore-forming region appear critical for proton regulation, ie, Glu-648, at the loop between the fifth and sixth transmembrane segments, is involved in direct activation of the channel by strong pH (pH 4), while Glu-600 located at the end of the fifth transmembrane segment is important for the response of the channel to mildly acidic external conditions (pH 6.5).49 Nevertheless, neutralization of Glu-600 gives rise to a constitutively active channel at 37°C.49
In addition to protons, positively charged compounds are also able to activate TRPV1, suggesting a generalized activation mechanism based primarily on neutralization of Glu-600. External cations tend to enhance agonist-evoked currents, and divalent cations at high (>10 mM)34 or even at physiological concentrations50 gate the channel directly. Polyvalent cations are even more potent channel regulators. For instance, Gd3+ and the polyamine, spermine,51,52 sensitize and activate TRPV1 at micromolar concentrations. These actions may involve interactions at multiple acidic residues, ie, Glu-600, Glu-648, and Asp-646.
TRPV1 also has a voltage-dependent gating. The channel is activated, at least partially, at strong positive potentials and is deactivated at negative potentials. The sensitivity of voltage-dependent activation and deactivation depends on the recording temperature and on the presence of agonists. In the absence of TRPV1 activators, strong membrane depolarization is required to activate the channel (V0.5 of +150 mV at 21°C), whereas in the presence of agonists, much smaller depolarization suffices to gate the channel, namely V0.5 of 0 mV at 37°C, and +10.6 mV at 21°C in the presence of 50 nM capsaicin.37 Thus, the heat or ligand sensitivity of TRPV1 may reflect a shift in its intrinsic voltage dependence. Consequently, the temperature threshold for TRPV1 activation is not constant, but fluctuates depending on the membrane potential. The voltage sensor remains unknown, although the fourth transmembrane segment has been signaled as a putative candidate to hold it. However, unlike voltage-gated channels, TRPV1 and other transient receptor potential channels lack an array of charged residues in their transmembrane segment domains.
From the aforementioned observations, it appears obvious that various activators of TRPV1 potentiate the effect evoked by others, leading to enhanced activity, suggesting a coupling of their receptor sites. This gating cooperativity of various ligands seems synergistic rather than additive20 and, given the polymodal and synergistic modes of activation, implies that the TRPV1 ion channel act as an “integrator” of exogenous stimuli.35 In fact, TRPV1 acts similarly in relation to endogenous agents, which makes it of particular relevance in the context of inflammation, given the wide variety of inflammatory agents generated in inflamed conditions.
In the continuous presence of an activating stimulus, TRPV1 undergoes desensitization. This phenomenon can occur rapidly after a prolonged single application of an agonist, or slowly following repeated agonist applications (also known as tachyphylaxia). Receptor desensitization is believed to occur predominantly via a Ca2+-dependent process because it is largely abolished in the absence of Ca2+. However, it should be noted, that some Ca2+-independent desensitization also occurs, especially with heat activation.53 The Ca2+-dependent mechanism arises because of the high TRPV1 Ca2+ permeability, allowing Ca2+ influx to activate an inhibitory process. Indeed, fast desensitization was significantly reduced in a TRPV1 mutant that possesses markedly reduced Ca2+ permeability.33 Furthermore, desensitization is attenuated by inhibitors of calcineurin, a Ca2+-activated phosphatase, thus linking desensitization to a dephosphorylation event.54 In addition, Ca2+ may signal via calmodulin, which interacts with TRPV1 at the N-terminal and C-terminal regions (positions 189–222 and 767–801). Indeed, disruption of the calmodulin C-terminal region partially inhibits fast desensitization.55
Regulation of TRPV1 channel activity
There is increasing evidence that TRPV1 is subjected to complex regulation manifested at several levels, from gene expression to post-translational modification and formation of receptor heteromers, as well as from subcellular compartmentalization and association with regulatory proteins to many second messengers.11
Limited information is available about what controls TRPV1 transcription in nociceptors. Two functional TRPV1 promoter regions and transcription initiation sites have been identified in the rat, ie, a distal promoter region, P1, and a second more proximal promoter region, P2.56 The P1 region containing a classic TATA box and a downstream transcription initiation site directs the strongest promoter activity within the 233-bp core fragment. The proximal promoter region, P2, which lacks a TATA box, contains an associated transcription initiation site that corresponds to the consensus sequence known as the “initiator” element. Alternate use of dual promoters may represent an important aspect of how TRPV1 gene expression can be dynamically regulated. Nerve growth factor induces activation of the GTPase Ras, which is coupled to the activation of both transcription and translation of TRPV1.57 Nerve growth factor positively regulates transcriptional activity of both rat TRPV1 promoters.
A large body of evidence indicates that post-translational modifications of TRPV1, such as phosphorylation mediated by protein kinase A, protein kinase C, and calmodulin-dependent protein kinase, increase its activity. Phosphorylation at Ser-116 in the N-terminus of TRPV1 is pivotal in protein kinase A-mediated downregulation of TRPV1 desensitization.58 In addition, Thr-144, Thr-370, and Ser-502 are important in protein kinase A-mediated phosphorylation/sensitization of the channel. Moreover, protein kinase C-mediated phosphorylation of TRPV1 not only potentiates capsaicin-evoked or proton-evoked responses, but also reduces its temperature threshold, such that receptors are active under physiological conditions (37°C).59 Two serine residues on TRPV1, Ser-502 and Ser-800, have been recognized to be important in protein kinase C-mediated effects. In addition to this direct effect, protein kinase C can also produce phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis increasing TRPV1 activity, although PIP2 has been proposed to be involved in sensitization of these channels by proinflammatory agents.59 Calmodulin-dependent protein kinase-mediated phosphorylation of TRPV1 at Ser-502 and Thr-704 plays an important role in channel activation in response to capsaicin application.60 In addition, calcineurin-mediated dephosphorylation at the same sites can produce TRPV1 desensitization.61 Similarly, the nonreceptor cellular tyrosine, c-Src kinase, positively regulates TRPV1 channel activity by tyrosine phosphorylation.61
In addition to phosphorylation, the activity of TRPV1 may be regulated by N-glycosylation,62 given that extracellular Asn-604 has been identified as a glycosylation site.63 Similarly, adenosine 5′ triphosphate may allosterically modulate TRPV1 by direct interaction with the nucleotide-binding Walker-type domains and increasing vanilloid-induced channel activity.9 Modulation of the redox state also impacts the physiological activity of TRPV1, possibly involving the Cys-621 amino acid residue located on the extracellular surface.64
Another essential pathway that influences TRPV1 activity is the formation of signalplexes, or the physical assembly of signaling molecules into discrete macromolecular entities. 11 Several signaling proteins have been described as TRPV1-interacting proteins, that could be part of a “TRPV1 receptome” modulating nociceptor activity. As mentioned, TRPV1 associates with intracellular signaling enzymes, including protein kinase A, protein kinase C, Src, inositol 1,4,5-trisphosphate, and calmodulin-dependent protein kinases, and also with calcineurin 2B phosphatase.65 It may also interact with the purinergic P2X3 receptor,66 calmodulin,67 the membrane protein, Pirt,68 the scaffolding protein, AKAP79/150,69 and with cytoskeleton proteins like tubulin.70 Protein kinases modulate channel gating by post-translational modification involving the phosphorylation/dephosphorylation of specific residues that, in turn, lead to a decrease in the temperature threshold of channel activation and a potentiation of its activity, by either destabilizing the closed and desensitized states and/or stabilizing the open state. Other proteins that bind to TRPV1 are snapin and synaptotagmin IX, two components of the SNARE complex that mediates Ca2+-dependent exocytosis.71 Although the precise role of snapin and synaptotagmin IX binding to TRPV1 remains elusive, it could be involved in sorting the receptor into vesicles that will be exocytosed through regulated exocytosis or in promoting channel recruitment to the plasma membrane under inflammatory conditions.
Several proteins that regulate folding (chaperones), protein biosynthesis, surface expression, and channel function have been described to associate with thermotransient receptor potentials. Recently, the γ-aminobutyric A receptor-associated protein, a small cytosolic protein initially described by its ability to interact with the γ subunit of the GABAA receptor,72 was pointed towards as a TRPV1 interacting partner with the cytosolic N-terminal domain of the channel. 73 Noteworthy, in heterologous systems, γ-aminobutyric A receptor-associated protein expression significantly augmented the levels of TRPV1 and its targeting to the plasma membrane, where it appears to favor the formation of receptor clusters. Functionally, γ-aminobutyric A receptor-associated protein appears to induce a decrease in channel activity.73
TRPV1 in inflammation
Inflammation is the physiological response to tissue injury caused by pathogens or harmful agents, and is clinically characterized by swelling, redness, heat, pain, and loss of function of the affected tissue or organ. This response is a complex process perfectly orchestrated by several cell types and chemical mediators, which initiate and regulate the necessary mechanisms to remove injurious agents and repair the affected area. The cellular components include circulating monocytes, macrophages, neutrophils, lymphocytes, and dendritic cells, while the humoral components include cytokines and other chemical substances that destroy pathogens or act as mediators for other cells. When tissue damage occurs, resident immune cells, such as macrophages or dendritic cells, are activated and release mediators in order to initiate the inflammatory response. Usually, during acute inflammation, the magnitude of the inflammatory response is locally adjusted to the injurious condition and finally resolved, maintaining homeostasis. However, an imbalance of the regulatory mechanisms is the cause of inflammation as a pathological process and leads to chronic inflammatory states. Regulatory mechanisms of inflammation include mediators of immune, vascular, or neural origin that maintain the inflammatory process within the physiological range. The role of TRPV1, as a major player in the process of neurogenic inflammation, has been traditionally considered to be neuronal. However, the expression of the channel in immune cells also suggests a contribution to the immune response.30–32,74
Inflammatory mediators are released at the site of injury from immune cells, such as chemokines, cytokines, prostaglandins, bradykinin, or growth factors, as well as from sensory neurons that secrete the neuropeptides, substance P and calcitonin gene-related peptide.75 Some of these mediators are able to activate directly local sensory neurons responsible for transducing the painful sensation that, paradoxically, is necessary to react and to avoid or minimize further damage.76–80 In addition, inflammatory agents are responsible for nociceptor sensitization changing the perception of stimuli, which leads to hyperalgesia (exaggerated response to a mild noxious stimulus) and/or to allodynia (response to a non-noxious stimulus), further minimizing additional damage and facilitating tissue repair. In chronic conditions, this process is exacerbated by synaptic changes at the spinal cord, a process known as central sensitization.81 Neuronal sensitization is believed to play a pivotal role in the development and maintenance of chronic pathological pain conditions.82
Inflammatory regulation of TRPV1
Peripheral sensitization of TRPV1 by proinflammatory agents is mediated by different molecular mechanisms, which include long-term upregulation of TRPV1 expression, but also acute functional modification of the channel (Figure 2). Indeed, increased expression of the channel has been shown in several chronic inflammatory diseases.83–85 This process is also mediated by fast mobilization from a subcellular vesicular reservoir located near the plasma membrane that is recruited by SNARE-dependent exocytosis.11,86
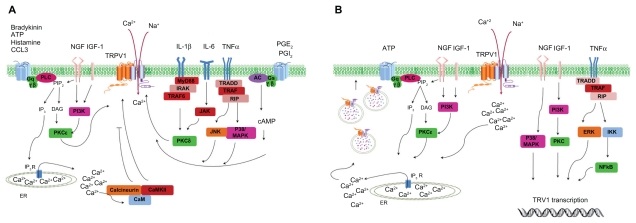
Figure 2.
Regulation of transient receptor potential vanilloid 1 function and expression by proinflammatory mediators. A) Acute post-translation modification of transient receptor potential vanilloid 1 function. Activation of phospholipase C/protein kinase C, protein kinase A, calmodulin-dependent protein kinase, and other intracellular signaling cascades increase transient receptor potential vanilloid 1 activity and cytosolic Ca2+ levels. B) Increase of transient receptor potential vanilloid 1 expression by proinflammatory agents. Rapid receptor translocation to the cell surface from the vesicular reservoir (left side). Long-term upregulation of protein levels by transcription/translation process (right side).
TRPV1 sensitization by nerve growth factor has been well documented, and is a good example of all the inflammatory potentiation strategies described earlier for the modulation of TRPV1 function and expression. Nerve growth factor increases TRPV1 transcription and transport to the peripheral nociceptor terminal, a process mediated by the p38/mitogen-activated protein kinase signaling pathway.87,88 Acute regulation of TRPV1 by nerve growth factor leads to phospholipase C activation and PIP2 hydrolysis. In parallel, nerve growth factor activates phosphatidylinositol 3-kinase-protein kinase C epsilon and calmodulin-dependent protein kinase signaling cascades, increasing TRPV1 opening probability and its translocation to the cell surface from the vesicular pool.89–91 Similar to nerve growth factor, insulin growth factor-1 also enhances TRPV1 membrane currents through phosphatidylinositol 3-kinase and protein kinase C pathways, increasing channel activity and receptor translocation to the cell surface,92 inducing long-term overexpression of TRPV1.93 Both nerve growth factor and insulin growth factor-1 provoke TRPV1 plasma membrane translocation by SNARE-dependent neurosecretion, as was demonstrated by the blockade of Ca2+-dependent neuronal exocytosis with a botulinomimetic peptide which abolished TRPV1 potentiation in dorsal root ganglion neurons.94
Cytokines, such as tumor necrosis factor alpha, interleukin-1β, and interleukin-6, can also regulate TRPV1 function, increasing neuronal excitability.76,95 For instance, the receptors for tumor necrosis factors alpha (ie, TNFR1 and TNFR2) are coexpressed with TRPV1 in sensory neurons, where they can also produce rapid and long-term modification of TRPV1 function. This cytokine increases TRPV1 expression on dorsal root ganglion and trigeminal ganglion neurons via the extracellular signal-regulated kinase pathway.96,97 Additionally, tumor necrosis factor alpha rapidly sensitizes TRPV1 activity and enhances the Ca2+ influx induced by capsaicin. This rapid mechanism seems to be mediated by p38/mitogen-activated protein kinase and the c-jun N-terminal kinase pathway, but not by extracellular signal-regulated kinase.97–99 Although protein kinase C phosphorylation seems also to be implicated, the exact mechanism remains unknown.76,100–102 Tumor necrosis factor alpha can also activate the TRPA1 receptor, which has been implicated in maintaining inflammation-related pain.103 TRPA1 is coexpressed in a subset of TRPV1-expressing nociceptors in trigeminal and dorsal root ganglion neurons104 and functions to detect products of tissue injury, inflammation, and oxidative stress that cause pain and neurogenic inflammation. 105 Under conditions of inflammation or nerve injury, expression of TRPA1 is persistently increased, concurrent with TRPV1.106
Rapid sensitization of TRPV1 currents by interleukin-1β has also been shown to be mediated by protein kinase C activity,107 via a mechanism independent of TRPV1 surface translocation by SNARE-dependent exocytosis.94 Although little is known about the ability of interleukin-6 to sensitize TRPV1,78,108 exposure of dorsal root ganglion cultures to interleukin-6 increases TRPV1 response to heat by a mechanism that involves Janus kinase and protein kinase C.109
Other inflammatory mediators, such as bradykinin, prostaglandin E2, adenosine 5′ triphosphate, and histamine, also sensitize TRPV1. Bradykinin induces excitation and sensitization of TRPV1 to heat via the protein kinase C pathway.110–112 In the same way, TRPV1 potentiation by adenosine 5′ triphosphate113 or by histamine114 is mediated via the phospholipase C/protein kinase C pathway. However, only adenosine 5′ triphosphate has been shown to mobilize TRPV1 to the plasma membrane mediated by the SNARE complex.94 Mechanisms involved in TRPV1 sensitization by prostaglandin E2 and prostaglandin I2 are through phosphorylation by protein kinase A,55 the receptor anchoring for which seems to be mediated by the protein, AKAP150,115 and also by protein kinase C.55
The cellular mechanisms underlying chemokine-induced excitation of sensory neurons include potentiation of TRPV1, in addition to inhibition of K+ conductance.95 CCL3 via the CCR1 receptor enhanced the response of dorsal root ganglion neurons to capsaicin, and decreased the response to hot-plate latency in mice. CCL3-mediated TRPV1 sensitization was reduced by phospholipase C and protein kinase C inhibitors.116 Activation of other chemokine receptors, such as CCR2, also expressed in the dorsal root ganglia, produced sensitization of TRPV1 by phospholipase C/protein kinase C phosphorylation.117
TRPV1 in inflammatory conditions
Besides the direct effect of inflammatory mediators on TRPV1, activation of nociceptors also induces the release of neuropeptides which act both autocrinally on the terminals and paracrinally on target cells, such as mast, immune, and vascular smooth muscle cells.118,119 These peptides contribute to the destruction of the harmful agent and to the repair of damaged tissue. For instance, when the neuropeptides calcitonin gene-related peptide and substance P are released from sensory neurons, their vasodilatory effects facilitate the arrival of more immune cells and proinflammatory mediators at the site of injury, which contributes to plasma extravasation and swelling. In fact, direct activation of sensory nerves is enough to induce an inflammatory response without the presence of pathogens or tissue injury, a process known as neurogenic inflammation or sterile inflammation.120
TRPV1 is expressed in sensory neurons, mainly in peptidergic neurons, found in many tissues close to blood vessels, epithelia, and vascular smooth muscle.121,122 Release of calcitonin gene-related peptide and substance P from sensory neurons is induced by TRPV1 activation via a wide variety of physical and chemical stimuli.123 Sensitization of TRPV1 by inflammatory mediators increases the release of these neuropeptides from a vesicle reservoir.97,124 Due to the proinflammatory effects of these neuropeptides, TRPV1 activation has been long considered as a proinflammatory receptor. However, other neuropeptides with anti-inflammatory properties, such as somatostatin, can also be released as a consequence of Ca2+ influx through the TRPV1 channel.125,126
TRPV1 acts as a transducer of noxious thermal and chemical stimuli in nociceptive sensory neurons, and is vital in mediating enhanced heat sensitivity during inflammation. Preclinical and clinical studies suggest that the TRPV1 receptor is an important component of several disease states, such as pain (inflammatory, visceral, cancer, and neuropathic), airways disease (including chronic cough), inflammatory bowel disease, interstitial cystitis, urinary incontinence, pancreatitis, and migraine.127
Acute and chronic arthritis is characterized by debilitating pain, and by an increment in the levels of neuropeptides in synovial fluid. Due to the role of TRPV1 as an integrator of multiple noxious stimuli as well as its presence in neuropeptide-containing fibers that are present in the knee joint synovium and adjacent bone, this channel has been implicated in the pathological symptoms of acute and chronic arthritis, although the precise mechanism is unclear.128 Keeble et al studied the vascular and hyperalgesic components of joint inflammation in wild-type and TRPV1 knockout mice after intra-articular injection of Freund’s complete adjuvant, and demonstrated that knee swelling and vascular hyperpermeability were significantly lowered in the joints treated with Freund’s complete adjuvant in TRPV1 null mice. Furthermore, intra-articular injection of tumor necrosis factor alpha in these mice produced decreased thermal hyperalgesia and joint swelling, indicating a critical role of tumor necrosis factor alpha and TRPV1 in the pathophysiology of rheumatoid arthritis.129
Cancer pain is a significant clinical problem because it is the first symptom of the disease in approximately 20%–50% of all cancer patients. Bone is the most common site of origin of chronic pain in patients with metastatic lung, prostate, and breast cancers or myeloma.130 There are at least three mechanisms in bone cancer that may contribute to the activation and sensitization of TRPV1 expressed by sensory fibers that innervate the tumor-bearing bone.131 The first is acidosis produced by osteoclasts, the principal bone-resorbing cells, and by lysis of tumor cells that have a lower intracellular pH than normal cells.132 The second is mediated by products released from cancerous tissue, like bradykinin, adenosine 5′ triphosphate, and nerve growth factor, which can modulate TRPV1 function indirectly via activation of second-messenger signaling pathways.130 Because the bone receives a rich sensory innervation by fibers that express TRPV1,133 production of these proalgesic agents may also sensitize TRPV1 channels, thereby generating a state of hyperalgesia and/or allodynia. Finally, the third mechanism is mediated directly by tumor-induced injury to primary afferent neurons.134 In a recent study, it has been shown that activation of TRPV1 was involved in bone cancer pain.135 The investigators found increases in TRPV1 protein levels and in the number of TRPV1-positive neurons in the dorsal root ganglia from a murine model of bone cancer. In support of a role of TPRV1 in bone cancer pain, it has been demonstrated that a receptor antagonist significantly attenuates painful symptoms.130
Itch, a principal symptom in skin diseases, is an important skin manifestation of systemic diseases, and one of the most debilitating symptoms in allergic and atopic dermatitis. 136 It can be triggered by localized, systemic, peripheral, or central stimuli, and there are numerous pruritogenic substances, including neuropeptides, cytokines, proteases, and histamine. 137 Less is known about pathophysiological specificity among the different diseases, but cross-talk between neuron terminals and dermal mast cells is being recognized as an important mechanism involved in pathogenesis. TRPV1-expressing primary afferents generate responses to pruritogenics via multiple mechanisms, like PLCβ3 activation.138 In addition, keratinocytes express a wide range of mediators and receptors involved in itch, and TRPV1 activation by them results in the release of pruritogenic mediators, as well as cellular proliferation, differentiation, and apoptosis.139 Histamine, the best known pruritogenic agent, induces itch by activating PLA2, lipoxygenase and the TRPV1 signaling pathway, as is shown by the decrease in histamine-induced scratching in TRPV1-deficient mice.140
Protective role of TRPV1 against inflammation
Cumulative evidence suggests that TRPV1 may have an anti-inflammatory action in some pathological conditions. Indeed, the number of diseases in which TRPV1 plays a protective role is expanding. For instance, TRPV1 has been shown to have a protective role against inflammatory conditions in the cardiovascular system, and it has been implicated in protecting against ischemia/reperfusion-induced inflammation of the heart.141 A similar action has been reported for liver142 and kidney143 pathologies, thus emphasizing a new emerging anti-inflammatory role for TRPV1.
The TRPV1 receptor also plays a critical modulatory role in contact dermatitis, a chronic allergic condition typified by skin inflammation and itching.144 The genetic disruption of TRPV1 channels or blockade of the TRPV1-dependent sensory neurogenic component by resiniferatoxin increased the inflammatory response in an ear murine model of contact dermatitis. This enhancement suggests that capsaicin-sensitive neurons expressing the TRPV1 channel may act to downregulate the hypersensivity, possibly by influencing the immune state of the skin.
Another protective function of TRPV1 against inflammatory conditions has been reported in the pathological condition of colitis,145 one of the disorders under the collective heading of gastrointestinal disturbances referred to as chronic inflammatory bowel diseases. Immunoreactive TRPV1 fibers have been detected on nerve terminals within the myenteric ganglia and the interganglionic fibers throughout the gastrointestinal tract.146 Using a murine model produced by the infusion of 2,4-dinitrobenzene sulfonic acid through the rectum of mice,147 it was reported that the TRPV1 null mice exhibited higher levels of inflammation than wild-type animals, indicating a protective role of TRPV1 channels in the initiation of this inflammatory condition.
Although this protective action of TRPV1 may be surprising, given the widely known proinflammatory activity of this channel, it should be considered that the main mechanisms involved in the development of chronic inflammation can be drastically different in each inflammatory disease. For instance, TRPV1 may mediate the release of both proinflammatory neuropeptides (substance P and calcitonin gene-related peptide) in some conditions, and of anti-inflammatory (somatostatin) peptides in others, depending on which subpopulation of nociceptors is more abundant in the affected tissue. Likewise, the immune cells and inflammatory mediators involved will depend on the specific inflammatory process and the affected tissue. Moreover, this balance usually changes during the development of pathology according to the time course or state of the disease. Therefore, the contribution of TRPV1 should be analyzed in detail for each inflammatory condition along with the mechanism involved in the specific inflammatory process. This evaluation will become essential because abrogation of the anti-inflammatory role of TRPV1 by potent antagonists may lead to aggravation of the disease or to the appearance of side effects.
Inflammatory process of sepsis
Sepsis is defined as the systemic inflammatory response elicited by an infection. The clinical manifestations of sepsis are abnormality in at least two of the following: body temperature (hyperthermia or hypothermia), heart rate (tachycardia), respiratory rate (tachypnea), and white blood cell count (leukocytopenia, leukocytosis or presence of immature forms greater that 10%). Increasingly grave stages of the systemic inflammatory response to an infection are severe sepsis and septic shock. Sepsis is considered when signs of organ dysfunction are present, while septic shock is defined by the presence of hypotension or hypoperfusion which finally leads to multiple organ failure.148,149 Despite the advances in antibiotics and critical care, severe sepsis remains the leading cause of death in intensive care units, in part because antibiotics cannot control systemic inflammation. In fact, the clinical symptoms of severe sepsis are not exclusively due to infection and can be also triggered by trauma, ischemia, severe injury, burns, or pancreatitis, which participate in the pathogenesis of this systemic inflammatory response syndrome.
The pathogenesis of sepsis is characterized by an excessive production of inflammatory mediators, mainly cytokines, chemokines, lipid mediators, and oxygen radicals. In a normal inflammatory response, these molecules are necessary to enhance leukocyte infiltration at the site of infection, to destroy the pathogen and repair tissue damage. However, in sepsis, this uncontrolled production of inflammatory mediators ends up with excessive vasodilatation, capillary leakage, hypotension, tissue injury, and finally lethal multiple organ failure.150–152 Clinical and experimental studies have emphasized the role of apoptosis in sepsis. Programmed cell death constitutes an active process to control cell removal, but also plays an important role in several pathological states. Activation of intracellular cascades finally leads to DNA degradation, a process regulated by a cascade of caspases, which are critical molecules in programmed cell death.153,154 Accelerated apoptosis of lymphocytes has been observed in animal models of sepsis and in autopsies of patients who died from sepsis.155
Different studies have demonstrated that the immune system is not the only system activated in sepsis. For instance, it is well known that a complex interaction exists between inflammation and coagulation in sepsis. The inflammatory response in sepsis skews the balance to a procoagulant state, promoting thrombus and clot formation. Indeed, patients with increased coagulation factors and reduced anticoagulation factors as a result of sepsis are prone to thrombus formation, compromising tissue perfusion and driving towards organ failure.151,156
In addition to activation of the coagulation cascades, extensive bidirectional communication exists between the immune and nervous systems in all inflammatory processes, which involves a huge diversity of molecular mechanisms.156,157 Release of inflammatory molecules can activate or influence sensory nerve function, which, in turn, can stimulate or inhibit the immune system by the release of neurotransmitters. In patients with sepsis, plasma levels of substance P and calcitonin gene-related peptide are significantly increased, providing evidence that sensory nerves are activated in this pathology.158–160 These bioactive neuronal agents are able to induce inflammation by directly acting on immune cells, mast cells, vascular smooth muscle, or other cell types.118,119 Substance P is known to increase vascular permeability,161 and calcitonin gene-related peptide is a potent vasodilator and hypotensive agent.162 Therefore, both neuropeptides have been mainly considered to be involved in the development of inflammation. However, it has also been shown that calcitonin gene-related peptide can mediate anti-inflammatory and immunosuppressive activities. For instance, it modulates cell adhesion and migration, increases some anti-inflammatory mediators, such as interleukin-10 or prostaglandin I2, and inhibits proinflammatory mediators, such as tumor necrosis factor alpha, among others.163–168 Consistent with these findings, administration of calcitonin gene-related peptide attenuated the development of some inflammatory and organ failure models.169,170 Neuropeptides, such as somatostatin, have also been recently reported to be increased in plasma from septic patients,171 but others, such as endothelin-1 or vasoactive intestinal peptide serum levels, seem to remain unmodified during sepsis.172 However, despite these observations, progress towards understanding the potential involvement of sensory nerves in sepsis and defining the exact role of neuropeptides in the development of this pathology is quite limited.
Is TRPV1 involved in sepsis?
Cumulative evidence associates TRPV1 channel activity with a protective effect in experimental models of sepsis. The role of the TRPV1 channel in sepsis was first evidenced by using its known agonist, capsaicin, and its antagonist, capsazepine. Injection of capsaicin was shown to diminish mortality during abdominal sepsis, suggesting an important role for nociceptive system in the host response to infection.173 A small dose of capsaicin reduced the systemic inflammatory response in septic rats by increasing anti-inflammatory cytokines and attenuating proinflammatory cytokines,174 which was consistent with previous in vitro results in lipopolysaccharide-activated peritoneal macrophages.175 In contrast, TRPV1 blockade with capsazepine reduced channel-mediated protection against endotoxin-induced hypotension and mortality in septic rats.176 Later, similar beneficial effects were also shown in a different rat model of sepsis, where capsaicin significantly attenuated systemic inflammation and multiple organ damage caused by sepsis, and protected against mortality.177 Other TRPV1 agonists and antagonists have also been evaluated in different models of sepsis, all showing a consistent decrease in the development of sepsis or a reduction in some of the pathological symptoms when TRPV1 is activated.178–180
Recent elegant experiments performed in TRPV1 knockout mice lend further support to the protective role of the vanilloid channel in the onset of sepsis. In these animals, there was an enhanced development of the pathological features and biomarkers of the systemic inflammatory response. Early onset, decreased body temperature, and enhanced hypotension were shown, together with an increased level of some inflammatory mediators in peritoneal exudates.181,182 At the same time, protective effects were also shown in another septic model in which TRPV1 null mice showed greater infiltration, more histological lesions, bronchial hyperactivity, and increased myeloperoxidase levels in the lungs.183
The neuropeptides involved in the protective effects of TRPV1 in sepsis remain to be exactly defined. Although it was initially suggested that TRPV1 mediated the effects of sepsis via substance P and the NK1 receptor,176 the regulatory role of the channel in sepsis was shown to be independent of substance P in mice lacking TRPV1.181 Alternatively, because calcitonin gene-related peptide, a potent vasodilator, was shown to be elevated in endotoxin-treated rats,184 it has been considered a critical factor in the development of septic shock.185 Moreover, an essential role has been claimed for somatostatin, because somatostatin receptor blockade aggravates sepsis in the lungs of wild-type mice, while the process is attenuated by injection of this neuropeptide in TRPV1 knockout mice.183
It should be noted that these results have been obtained from different models of sepsis in rats or mice, using several research and pharmacological tools which could explain the differences observed in neuropeptides participating in the effects of TRPV1. Moreover, the tissue parameters and/or biomarkers analyzed in each model are different, which could also contribute to the observed differences in the role of neuropeptides. In addition, it should not be forgotten that endotoxin-induced fever was shown to be initiated via the TRPV1 channel.186,187 However, an overall protective role of TRPV1 in sepsis has been proposed in most of these studies, and the increased levels of some neuropeptides in septic patients could be due to compensatory mechanisms of the organism when trying to control the systemic inflammatory process.
Although understanding of the pathogenesis of inflammation and sepsis has improved, this has not translated into clinical benefit. Therapies for severe sepsis are mainly focused on eradication of infection and on maintenance of systemic perfusion. Despite advances in adjuvant treatments, mortality remains high. In past decades, therapeutic attempts have been focused on inflammatory mediators and processes, but they have failed to translate into efficacy in clinical trials, although animal models have shown promising and successful results. The benefit of corticosteroid therapy in severe sepsis and septic shock remains controversial. Activated protein C, one of the coagulation factors, is the only treatment for sepsis approved by the Food and Drug Administration, which is projected to be useful only in a small subset of patients with severe sepsis. The proposed protective role of TRPV1 implies that antiseptic treatments should preserve its channel activity. Thus, until the role of TRPV1 in sepsis is well understood, potent channel antagonists should be used with caution to treat the septic inflammatory process.
Conclusion
It is becoming clear that TRPV1 contributes to the pathophysiology of inflammatory processes. Intriguingly, this channel may have both a proinflammatory and anti-inflammatory action, depending on the disease. Thus, although it was widely accepted that TRPV1 blockers will become a new generation of anti-inflammatory and analgesic drugs to treat a plethora of human diseases, their clinical use must be reconsidered, because of the newly identified protective roles assigned to TRPV1. We are learning that some of the protective anti-inflammatory effects of TRPV1 were most probably ignored or misinterpreted. Therefore, the better we understand how TRPV1 works and how it contributes to human physiology and pathology, the more challenging it will be to find compounds that target pathological proinflammatory TRPV1 channels, without altering physiologically working and anti-inflammatory subpopulations of channels. Perhaps targeting inflammatory expression and/or recruitment of channels may provide a superior therapeutic paradigm to attenuate inflammation.
Acknowledgments
We are grateful to the members of our laboratory for their continuous support and collaboration. This work was supported by grants from the Ministry of Science and Innovation to AF-M, to JMGR, RP-C, from Consolider-Ingenio to AF-M, JMG-R, and RP-C, from La Marató de TV3 to AF-M and RP-C, and from la Generalitat Valenciana Prometeto to AF-M.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Kedei N, Szabo T, Lile JD, et al. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276(30):28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- 3.Smith GD, Gunthorpe MJ, Kelsell RE, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418(6894):186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 4.Liapi A, Wood JN. Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci. 2005;22(4):825–834. doi: 10.1111/j.1460-9568.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- 5.Rutter AR, Ma QP, Leveridge M, Bonnert TP. Heteromerization and colocalization of TrpV1 and TrpV2 in mammalian cell lines and rat dorsal root ganglia. Neuroreport. 2005;16(16):1735–1739. doi: 10.1097/01.wnr.0000185958.03841.0f. [DOI] [PubMed] [Google Scholar]
- 6.Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch. 2005;451(1):143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- 7.Phelps CB, Procko E, Lishko PV, Wang RR, Gaudet R. Insights into the roles of conserved and divergent residues in the ankyrin repeats of TRPV ion channels. Channels (Austin) 2007;1(3):148–151. doi: 10.4161/chan.4716. [DOI] [PubMed] [Google Scholar]
- 8.Phelps CB, Wang RR, Choo SS, Gaudet R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem. 2010;285(1):731–740. doi: 10.1074/jbc.M109.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54(6):905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Nagy I, Santha P, Jancso G, Urban L. The role of the vanilloid ( capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol. 2004;500(1–3):351–369. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Planells-Cases R, Valente P, Ferrer-Montiel A, Qin F, Szallasi A. Complex regulation of TRPV1 and related thermo-TRPs: Implications for therapeutic intervention. Adv Exp Med Biol. 2011;704:491–515. doi: 10.1007/978-94-007-0265-3_27. [DOI] [PubMed] [Google Scholar]
- 12.Salazar H, Jara-Oseguera A, Hernandez-Garcia E, et al. Structural determinants of gating in the TRPV1 channel. Nat Struct Mol Biol. 2009;16(7):704–710. doi: 10.1038/nsmb.1633. [DOI] [PubMed] [Google Scholar]
- 13.Valente P, Garcia-Sanz N, Gomis A, et al. Identification of molecular determinants of channel gating in the transient receptor potential box of vanilloid receptor I. FASEB J. 2008;22(9):3298–3309. doi: 10.1096/fj.08-107425. [DOI] [PubMed] [Google Scholar]
- 14.Brauchi S, Orio P. Voltage sensing in thermo-TRP channels. Adv Exp Med Biol. 2011;704:517–530. doi: 10.1007/978-94-007-0265-3_28. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Sanz N, Fernandez-Carvajal A, Morenilla-Palao C, et al. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci. 2004;24(23):5307–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang F, Liu S, Yang F, Zheng J, Wang K. Identification of a tetrameric assembly domain in the C-terminus of heat-activated TRPV1 channels. J Biol Chem. 2011;286(17):15308–15316. doi: 10.1074/jbc.M111.223941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak J, Wang MH, Hwang SW, Kim TY, Lee SY, Oh U. Intracellular ATP increases capsaicin-activated channel activity by interacting with nucleotide-binding domains. J Neurosci. 2000;20(22):8298–8304. doi: 10.1523/JNEUROSCI.20-22-08298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voets T, Nilius B. Modulation of TRPs by PIPs. J Physiol. 2007;582(Pt 3):939–944. doi: 10.1113/jphysiol.2007.132522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem. 2004;271(10):1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 20.Latorre R, Vargas G, Orta G, Brauchi S. Voltage and temperature gating in thermoTRP channels. In: Liedtke W, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. London, UK: CRC Taylor and Francis; 2007. [Google Scholar]
- 21.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302(3):839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165(3):896–901. doi: 10.1016/j.neuroscience.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien LY, Cheng JK, Chu D, Cheng CF, Tsaur ML. Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci. 2007;27(37):9855–9865. doi: 10.1523/JNEUROSCI.0604-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavanaugh DJ, Chesler AT, Jackson AC, et al. TRPV1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31(13):5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mezey E, Toth ZE, Cortright DN, et al. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A. 2000;97(7):3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Southall MD, Li T, Gharibova LS, Pei Y, Nicol GD, Travers JB. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J Pharmacol Exp Ther. 2003;304(1):217–222. doi: 10.1124/jpet.102.040675. [DOI] [PubMed] [Google Scholar]
- 27.Choi TY, Park SY, Jo JY, et al. Endogenous expression of TRPV1 channel in cultured human melanocytes. J Dermatol Sci. 2009;56(2):128–130. doi: 10.1016/j.jdermsci.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Lazzeri M, Vannucchi MG, Zardo C, et al. Immunohistochemical evidence of vanilloid receptor 1 in normal human urinary bladder. Eur Urol. 2004;46(6):792–798. doi: 10.1016/j.eururo.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Birder LA, Kanai AJ, de Groat WC, et al. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci U S A. 2001;98(23):13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heiner I, Eisfeld J, Halaszovich CR, et al. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: Evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J. 2003;371(Pt 3):1045–1053. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stander S, Moormann C, Schumacher M, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13(3):129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen CW, Lee ST, Wu WT, Fu WM, Ho FM, Lin WW. Signal transduction for inhibition of inducible nitric oxide synthase and cyclooxygenase-2 induction by capsaicin and related analogs in macrophages. Br J Pharmacol. 2003;140(6):1077–1087. doi: 10.1038/sj.bjp.0705533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohapatra DP, Wang SY, Wang GK, Nau C. A tyrosine residue in TM6 of the vanilloid receptor TRPV1 involved in desensitization and calcium permeability of capsaicin-activated currents. Mol Cell Neurosci. 2003;23(2):314–324. doi: 10.1016/s1044-7431(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 34.Ahern GP, Brooks IM, Miyares RL, Wang XB. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J Neurosci. 2005;25(21):5109–5116. doi: 10.1523/JNEUROSCI.0237-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Martinez C, Morenilla-Palao C, Planells-Cases R, Merino JM, Ferrer-Montiel A. Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J Biol Chem. 2000;275(42):32552–32558. doi: 10.1074/jbc.M002391200. [DOI] [PubMed] [Google Scholar]
- 37.Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: A voltage connection? J Physiol. 2005;567(Pt 1):35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cromer BA, McIntyre P. Painful toxins acting at TRPV1. Toxicon. 2008;51(2):163–173. doi: 10.1016/j.toxicon.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125(2):181–195. doi: 10.1016/j.pharmthera.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Hui K, Qin F. Thermodynamics of heat activation of single capsaicin ion channels VR1. Biophys J. 2003;85(5):2988–3006. doi: 10.1016/S0006-3495(03)74719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grandl J, Kim SE, Uzzell V, et al. Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain. Nat Neurosci. 2010;13(6):708–714. doi: 10.1038/nn.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Petrocellis L, Chu CJ, Moriello AS, Kellner JC, Walker JM, Di Marzo V. Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels. Br J Pharmacol. 2004;143(2):251–256. doi: 10.1038/sj.bjp.0705924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenbaum T, Simon SA. TRPV1 receptors and signal transduction. In: Liedtke W, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. London, UK: CRC Taylor and Francis; 2007. [PubMed] [Google Scholar]
- 44.Movahed P, Jonsson BA, Birnir B, et al. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem. 2005;280(46):38496–38504. doi: 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- 45.Jung J, Hwang SW, Kwak J, et al. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci. 1999;19(2):529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung J, Lee SY, Hwang SW, et al. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem. 2002;277(46):44448–44454. doi: 10.1074/jbc.M207103200. [DOI] [PubMed] [Google Scholar]
- 47.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108(3):421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 48.Gavva NR, Klionsky L, Qu Y, et al. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem. 2004;279(19):20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- 49.Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97(14):8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patil MJ, Jeske NA, Akopian AN. Transient receptor potential V1 regulates activation and modulation of transient receptor potential A1 by Ca2+ Neuroscience. 2010;171(4):1109–1119. doi: 10.1016/j.neuroscience.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tousova K, Vyklicky L, Susankova K, Benedikt J, Vlachova V. Gadolinium activates and sensitizes the vanilloid receptor TRPV1 through the external protonation sites. Mol Cell Neurosci. 2005;30(2):207–217. doi: 10.1016/j.mcn.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Ahern GP, Wang X, Miyares RL. Polyamines are potent ligands for the capsaicin receptor TRPV1. J Biol Chem. 2006;281(13):8991–8995. doi: 10.1074/jbc.M513429200. [DOI] [PubMed] [Google Scholar]
- 53.Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17(10):3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431(6):828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- 55.Moriyama T, Higashi T, Togashi K, et al. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xue Q, Jong B, Chen T, Schumacher MA. Transcription of rat TRPV1 utilizes a dual promoter system that is positively regulated by nerve growth factor. J Neurochem. 2007;101(1):212–222. doi: 10.1111/j.1471-4159.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- 57.Bron R, Klesse LJ, Shah K, Parada LF, Winter J. Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors. Mol Cell Neurosci. 2003;22(1):118–132. doi: 10.1016/s1044-7431(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 58.Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278(50):50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- 59.Rohacs T, Thyagarajan B, Lukacs V. Phospholipase C mediated modulation of TRPV1 channels. Mol Neurobiol. 2008;37(2–3):153–163. doi: 10.1007/s12035-008-8027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123(1):53–62. doi: 10.1085/jgp.200308906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhave G, Gereau RW. Posttranslational mechanisms of peripheral sensitization. J Neurobiol. 2004;61(1):88–106. doi: 10.1002/neu.20083. [DOI] [PubMed] [Google Scholar]
- 62.Wirkner K, Hognestad H, Jahnel R, Hucho F, Illes P. Characterization of rat transient receptor potential vanilloid 1 receptors lacking the N-glycosylation site N604. Neuroreport. 2005;16(9):997–1001. doi: 10.1097/00001756-200506210-00023. [DOI] [PubMed] [Google Scholar]
- 63.Jahnel R, Dreger M, Gillen C, Bender O, Kurreck J, Hucho F. Biochemical characterization of the vanilloid receptor 1 expressed in a dorsal root ganglia derived cell line. Eur J Biochem. 2001;268(21):5489–5496. doi: 10.1046/j.1432-1033.2001.02500.x. [DOI] [PubMed] [Google Scholar]
- 64.Jin X, Morsy N, Winston J, Pasricha PJ, Garrett K, Akbarali HI. Modulation of TRPV1 by nonreceptor tyrosine kinase, c-Src kinase. Am J Physiol Cell Physiol. 2004;287(2):C558–563. doi: 10.1152/ajpcell.00113.2004. [DOI] [PubMed] [Google Scholar]
- 65.Planells-Cases R, Garcia-Sanz N, Morenilla-Palao C, Ferrer-Montiel A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 2005;451(1):151–159. doi: 10.1007/s00424-005-1423-5. [DOI] [PubMed] [Google Scholar]
- 66.Stanchev D, Blosa M, Milius D, et al. Cross-inhibition between native and recombinant TRPV1 and P2X(3) receptors. Pain. 2009;143(1–2):26–36. doi: 10.1016/j.pain.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27(26):7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim AY, Tang Z, Liu Q, et al. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 2008;133(3):475–485. doi: 10.1016/j.cell.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron. 2008;59(3):450–461. doi: 10.1016/j.neuron.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 70.Goswami C, Dreger M, Jahnel R, Bogen O, Gillen C, Hucho F. Identification and characterization of a Ca2+-sensitive interaction of the vanilloid receptor TRPV1 with tubulin. J Neurochem. 2004;91(5):1092–1103. doi: 10.1111/j.1471-4159.2004.02795.x. [DOI] [PubMed] [Google Scholar]
- 71.Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279(24):25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- 72.Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature. 1999;397(6714):69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- 73.Lainez S, Valente P, Ontoria-Oviedo I, et al. GABAA receptor associated protein (GABARAP) modulates TRPV1 expression and channel function and desensitization. FASEB J. 2010;24(6):1958–1970. doi: 10.1096/fj.09-151472. [DOI] [PubMed] [Google Scholar]
- 74.Saunders CI, Fassett RG, Geraghty DP. Up-regulation of TRPV1 in mononuclear cells of end-stage kidney disease patients increases susceptibility to N-arachidonoyl-dopamine (NADA)-induced cell death. Biochim Biophys Acta. 2009;1792(10):1019–1026. doi: 10.1016/j.bbadis.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75(2):125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- 76.Schafers M, Sorkin L. Effect of cytokines on neuronal excitability. Neurosci Lett. 2008;437(3):188–193. doi: 10.1016/j.neulet.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 77.Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60(1):125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schaible HG, von Banchet GS, Boettger MK, et al. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci. 2010;1193:60–69. doi: 10.1111/j.1749-6632.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- 79.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16(11):1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101(1):8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 82.Hill RG. Molecular basis for the perception of pain. Neuroscientist. 2001;7(4):282–292. doi: 10.1177/107385840100700405. [DOI] [PubMed] [Google Scholar]
- 83.Engler A, Aeschlimann A, Simmen BR, et al. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem Biophys Res Commun. 2007;359(4):884–888. doi: 10.1016/j.bbrc.2007.05.178. [DOI] [PubMed] [Google Scholar]
- 84.Cho WG, Valtschanoff JG. Vanilloid receptor TRPV1-positive sensory afferents in the mouse ankle and knee joints. Brain Res. 2008;1219:59–65. doi: 10.1016/j.brainres.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57(7):923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Planells-Cases R, Ferrer-Montiel A. TRP channel trafficking. In: Liedtke W, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. London, UK: CRC Taylor and Francis; 2007. [PubMed] [Google Scholar]
- 87.Pabbidi RM, Cao DS, Parihar A, Pauza ME, Premkumar LS. Direct role of streptozotocin in inducing thermal hyperalgesia by enhanced expression of transient receptor potential vanilloid 1 in sensory neurons. Mol Pharmacol. 2008;73(3):995–1004. doi: 10.1124/mol.107.041707. [DOI] [PubMed] [Google Scholar]
- 88.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36(1):57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 89.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24(24):4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551(Pt 2):433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128(5):509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17. doi: 10.1186/1744-8069-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lilja J, Laulund F, Forsby A. Insulin and insulin-like growth factor type-I up-regulate the vanilloid receptor-1 (TRPV1) in stably TRPV1-expressing SH-SY5Y neuroblastoma cells. J Neurosci Res. 2007;85(7):1413–1419. doi: 10.1002/jnr.21255. [DOI] [PubMed] [Google Scholar]
- 94.Camprubi-Robles M, Planells-Cases R, Ferrer-Montiel A. Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. FASEB J. 2009;23(11):3722–3733. doi: 10.1096/fj.09-134346. [DOI] [PubMed] [Google Scholar]
- 95.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;194:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hensellek S, Brell P, Schaible HG, Brauer R, Segond von BG. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci. 2007;36(3):381–391. doi: 10.1016/j.mcn.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 97.Khan AA, Diogenes A, Jeske NA, Henry MA, Akopian A, Hargreaves KM. Tumor necrosis factor alpha enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience. 2008;155(2):503–509. doi: 10.1016/j.neuroscience.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 98.Hagenacker T, Czeschik JC, Schafers M, Busselberg D. Sensitization of voltage activated calcium channel currents for capsaicin in nociceptive neurons by tumor-necrosis-factor-alpha. Brain Res Bull. 2010;81(1):157–163. doi: 10.1016/j.brainresbull.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 99.Hu Y, Gu Q, Lin RL, Kryscio R, Lee LY. Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor-alpha in rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol. 2010;299(4):L483–492. doi: 10.1152/ajplung.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Constantin CE, Mair N, Sailer CA, et al. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28(19):5072–5081. doi: 10.1523/JNEUROSCI.4476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Russell FA, Fernandes ES, Courade JP, Keeble JE, Brain SD. Tumour necrosis factor alpha mediates transient receptor potential vanilloid 1-dependent bilateral thermal hyperalgesia with distinct peripheral roles of interleukin-1beta, protein kinase C and cyclooxygenase- 2 signalling. Pain. 2009;142(3):264–274. doi: 10.1016/j.pain.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 102.Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33(10):1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garrison SL, Stucky CL. The dynamic TRPA1 channel: A suitable pharmacological pain target. Curr Pharm Biotechnol. 2011 April 5; doi: 10.2174/138920111798357302. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kobayashi K, Fukuoka T, Obata K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493(4):596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 105.Trevisani M, Siemens J, Materazzi S, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104(33):13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Obata K, Katsura H, Mizushima T, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115(9):2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Obreja O, Rathee PK, Lips KS, Distler C, Kress M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: Involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002;16(12):1497–1503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- 108.Obreja O, Biasio W, Andratsch M, et al. Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain. 2005;128(Pt 7):1634–1641. doi: 10.1093/brain/awh490. [DOI] [PubMed] [Google Scholar]
- 109.Andratsch M, Mair N, Constantin CE, et al. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci. 2009;29(43):13473–13483. doi: 10.1523/JNEUROSCI.1822-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci U S A. 1996;93(26):15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mizumura K, Koda H, Kumazawa T. Evidence that protein kinase C activation is involved in the excitatory and facilitatory effects of bradykinin on canine visceral nociceptors in vitro. Neurosci Lett. 1997;237(1):29–32. doi: 10.1016/s0304-3940(97)00793-3. [DOI] [PubMed] [Google Scholar]
- 112.Mizumura K, Sugiura T, Katanosaka K, Banik RK, Kozaki Y. Excitation and sensitization of nociceptors by bradykinin: What do we know? Exp Brain Res. 2009;196(1):53–65. doi: 10.1007/s00221-009-1814-5. [DOI] [PubMed] [Google Scholar]
- 113.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A. 2001;98(12):6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kajihara Y, Murakami M, Imagawa T, Otsuguro K, Ito S, Ohta T. Histamine potentiates acid-induced responses mediating transient receptor potential V1 in mouse primary sensory neurons. Neuroscience. 2010;166(1):292–304. doi: 10.1016/j.neuroscience.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 115.Schnizler K, Shutov LP, Van Kanegan MJ, et al. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci. 2008;28(19):4904–4917. doi: 10.1523/JNEUROSCI.0233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang N, Inan S, Cowan A, et al. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci U S A. 2005;102(12):4536–4541. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104(1):254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 119.Jancsó G. NeuroImmune biology Neruogenic Inflammation in Health and Disease. Amsterdam, The Netherlands: Elsevier; 2009. [Google Scholar]
- 120.Geppetti P, Holzer P. Neurogenic Inflammation. Boca Raton, FL: CRC Press; 1996. [Google Scholar]
- 121.Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol. 1995;45(1):1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- 122.Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19(1):1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- 123.Kichko TI, Reeh PW. TRPV1 controls acid- and heat-induced calcitonin gene-related peptide release and sensitization by bradykinin in the isolated mouse trachea. Eur J Neurosci. 2009;29(9):1896–1904. doi: 10.1111/j.1460-9568.2009.06747.x. [DOI] [PubMed] [Google Scholar]
- 124.Price TJ, Louria MD, Candelario-Soto D, et al. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: Effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci. 2005;6:4. doi: 10.1186/1471-2202-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Than M, Nemeth J, Szilvassy Z, Pinter E, Helyes Z, Szolcsanyi J. Systemic anti-inflammatory effect of somatostatin released from capsaicin-sensitive vagal and sciatic sensory fibres of the rat and guinea-pig. Eur J Pharmacol. 2000;399(2–3):251–258. doi: 10.1016/s0014-2999(00)00341-1. [DOI] [PubMed] [Google Scholar]
- 126.Helyes Z, Szabo A, Nemeth J, et al. Antiinflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund’s adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum. 2004;50(5):1677–1685. doi: 10.1002/art.20184. [DOI] [PubMed] [Google Scholar]
- 127.Adcock JJ. TRPV1 receptors in sensitisation of cough and pain reflexes. Pulm Pharmacol Ther. 2009;22(2):65–70. doi: 10.1016/j.pupt.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 128.Fernandes ES, Russell FA, Spina D, et al. A distinct role for TRPA1, in addition to TRPV1, in TNFalpha-induced inflammatory hyperalgesia and CFA-induced mono-arthritis. Arthritis Rheum. 2011;63(3):819–829. doi: 10.1002/art.30150. [DOI] [PubMed] [Google Scholar]
- 129.Keeble J, Russell F, Curtis B, Starr A, Pinter E, Brain SD. Involvement of transient receptor potential vanilloid 1 in the vascular and hyperalgesic components of joint inflammation. Arthritis Rheum. 2005;52(10):3248–3256. doi: 10.1002/art.21297. [DOI] [PubMed] [Google Scholar]
- 130.Ghilardi JR, Rohrich H, Lindsay TH, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25(12):3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goblirsch MJ, Zwolak P, Clohisy DR. Advances in understanding bone cancer pain. J Cell Biochem. 2005;96(4):682–688. doi: 10.1002/jcb.20589. [DOI] [PubMed] [Google Scholar]
- 132.Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab. 2007;25(2):99–104. doi: 10.1007/s00774-006-0734-8. [DOI] [PubMed] [Google Scholar]
- 133.Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: Sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113(1):155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 134.Peters CM, Ghilardi JR, Keyser CP, et al. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp Neurol. 2005;193(1):85–100. doi: 10.1016/j.expneurol.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 135.Shinoda M, Ogino A, Ozaki N, et al. Involvement of TRPV1 in nociceptive behavior in a rat model of cancer pain. J Pain. 2008;9(8):687–699. doi: 10.1016/j.jpain.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 136.Buddenkotte J, Steinhoff M. Pathophysiology and therapy of pruritus in allergic and atopic diseases. Allergy. 2010;65(7):805–821. doi: 10.1111/j.1398-9995.2010.01995.x. [DOI] [PubMed] [Google Scholar]
- 137.Shim WS, Oh U. Histamine-induced itch and its relationship with pain. Mol Pain. 2008;4:29. doi: 10.1186/1744-8069-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Imamachi N, Park GH, Lee H, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106(27):11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Denda M, Tsutsumi M. Roles of transient receptor potential proteins (TRPs) in epidermal keratinocytes. Adv Exp Med Biol. 2011;704:847–860. doi: 10.1007/978-94-007-0265-3_44. [DOI] [PubMed] [Google Scholar]
- 140.Shim WS, Tak MH, Lee MH, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27(9):2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bolli R, Abdel-Latif A. No pain, no gain: The useful function of angina. Circulation. 2005;112(23):3541–3543. doi: 10.1161/CIRCULATIONAHA.105.581827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Harada N, Okajima K, Yuksel M, Isobe H. Contribution of capsaicin-sensitive sensory neurons to antithrombin-induced reduction of ischemia/reperfusion-induced liver injury in rats. Thromb Haemost. 2005;93(1):48–56. doi: 10.1160/TH04-02-0106. [DOI] [PubMed] [Google Scholar]
- 143.Mizutani A, Okajima K, Murakami K, et al. Activation of sensory neurons reduces ischemia/reperfusion-induced acute renal injury in rats. Anesthesiology. 2009;110(2):361–369. doi: 10.1097/ALN.0b013e3181942f3c. [DOI] [PubMed] [Google Scholar]
- 144.Banvolgyi A, Palinkas L, Berki T, et al. Evidence for a novel protective role of the vanilloid TRPV1 receptor in a cutaneous contact allergic dermatitis model. J Neuroimmunol. 2005;169(1–2):86–96. doi: 10.1016/j.jneuroim.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 145.Sibaev A, Massa F, Yuce B, et al. CB1 and TRPV1 receptors mediate protective effects on colonic electrophysiological properties in mice. J Mol Med. 2006;84(6):513–520. doi: 10.1007/s00109-006-0040-x. [DOI] [PubMed] [Google Scholar]
- 146.Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465(1):121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- 147.Massa F, Sibaev A, Marsicano G, Blaudzun H, Storr M, Lutz B. Vanilloid receptor (TRPV1)-deficient mice show increased susceptibility to dinitrobenzene sulfonic acid induced colitis. J Mol Med. 2006;84(2):142–146. doi: 10.1007/s00109-005-0016-2. [DOI] [PubMed] [Google Scholar]
- 148.Abraham E, Matthay MA, Dinarello C, et al. Consensus conference definitions for sepsis, septic shock, acute lung injury, and acute respiratory distress syndrome: Time for a reevaluation. Crit Care Med. 2000;28(1):232–235. doi: 10.1097/00003246-200001000-00039. [DOI] [PubMed] [Google Scholar]
- 149.Vincent JL, Martinez EO, Silva E. Evolving concepts in sepsis definitions. Crit Care Clin. 2009;25(4):665–675. vii. doi: 10.1016/j.ccc.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 150.Ulloa L, Tracey KJ. The “cytokine profile”: A code for sepsis. Trends Mol Med. 2005;11(2):56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 151.Sriskandan S, Altmann DM. The immunology of sepsis. J Pathol. 2008;214(2):211–223. doi: 10.1002/path.2274. [DOI] [PubMed] [Google Scholar]
- 152.Ulloa L, Brunner M, Ramos L, Deitch EA. Scientific and clinical challenges in sepsis. Curr Pharm Des. 2009;15(16):1918–1935. doi: 10.2174/138161209788453248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Thornberry NA. Caspases: Key mediators of apoptosis. Chem Biol. 1998;5(5):R97–103. doi: 10.1016/s1074-5521(98)90615-9. [DOI] [PubMed] [Google Scholar]
- 154.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6(11):813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 155.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78(2):325–337. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- 156.Shimaoka M, Park EJ. Advances in understanding sepsis. Eur J Anaesthesiol Suppl. 2008;42:146–153. doi: 10.1017/S0265021507003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brogden KA, Guthmiller JM, Salzet M, Zasloff M. The nervous system and innate immunity: The neuropeptide connection. Nat Immunol. 2005;6(6):558–564. doi: 10.1038/ni1209. [DOI] [PubMed] [Google Scholar]
- 158.Joyce CD, Fiscus RR, Wang X, Dries DJ, Morris RC, Prinz RA. Calcitonin gene-related peptide levels are elevated in patients with sepsis. Surgery. 1990;108(6):1097–1101. [PubMed] [Google Scholar]
- 159.Arnalich F, Sanchez JF, Martinez M, et al. Changes in plasma concentrations of vasoactive neuropeptides in patients with sepsis and septic shock. Life Sci. 1995;56(2):75–81. doi: 10.1016/0024-3205(94)00416-p. [DOI] [PubMed] [Google Scholar]
- 160.Beer S, Weighardt H, Emmanuilidis K, et al. Systemic neuropeptide levels as predictive indicators for lethal outcome in patients with postoperative sepsis. Crit Care Med. 2002;30(8):1794–1798. doi: 10.1097/00003246-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 161.Foreman JC, Jordan CC, Oehme P, Renner H. Structure-activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J Physiol. 1983;335:449–465. doi: 10.1113/jphysiol.1983.sp014543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brain SD, Newbold P, Kajekar R. Modulation of the release and activity of neuropeptides in the microcirculation. Can J Physiol Pharmacol. 1995;73(7):995–998. doi: 10.1139/y95-139. [DOI] [PubMed] [Google Scholar]
- 163.Harzenetter MD, Novotny AR, Gais P, Molina CA, Altmayr F, Holzmann B. Negative regulation of TLR responses by the neuropeptide CGRP is mediated by the transcriptional repressor ICER. J Immunol. 2007;179(1):607–615. doi: 10.4049/jimmunol.179.1.607. [DOI] [PubMed] [Google Scholar]
- 164.Numao T, Agrawal DK. Neuropeptides modulate human eosinophil chemotaxis. J Immunol. 1992;149(10):3309–3315. [PubMed] [Google Scholar]
- 165.Levite M, Cahalon L, Hershkoviz R, Steinman L, Lider O. Neuropeptides, via specific receptors, regulate T cell adhesion to fibronectin. J Immunol. 1998;160(2):993–1000. [PubMed] [Google Scholar]
- 166.Fox FE, Kubin M, Cassin M, et al. Calcitonin gene-related peptide inhibits proliferation and antigen presentation by human peripheral blood mononuclear cells: Effects on B7, interleukin 10, and interleukin 12. J Invest Dermatol. 1997;108(1):43–48. doi: 10.1111/1523-1747.ep12285627. [DOI] [PubMed] [Google Scholar]
- 167.Crossman D, McEwan J, MacDermot J, MacIntyre I, Dollery CT. Human calcitonin gene-related peptide activates adenylate cyclase and releases prostacyclin from human umbilical vein endothelial cells. Br J Pharmacol. 1987;92(4):695–701. doi: 10.1111/j.1476-5381.1987.tb11373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Okajima K, Harada N. Regulation of inflammatory responses by sensory neurons: Molecular mechanism(s) and possible therapeutic applications. Curr Med Chem. 2006;13(19):2241–2251. doi: 10.2174/092986706777935131. [DOI] [PubMed] [Google Scholar]
- 169.Gomes RN, Castro-Faria-Neto HC, Bozza PT, et al. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock. 2005;24(6):590–594. doi: 10.1097/01.shk.0000183395.29014.7c. [DOI] [PubMed] [Google Scholar]
- 170.Reinshagen M, Flamig G, Ernst S, et al. Calcitonin gene-related peptide mediates the protective effect of sensory nerves in a model of colonic injury. J Pharmacol Exp Ther. 1998;286(2):657–661. [PubMed] [Google Scholar]
- 171.Suto B, Bagoly T, Borzsei R, et al. Surgery and sepsis increase somatostatin-like immunoreactivity in the human plasma. Peptides. 2010;31(6):1208–1212. doi: 10.1016/j.peptides.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 172.Berg RM, Strauss GI, Tofteng F, et al. Circulating levels of vasoactive peptides in patients with acute bacterial meningitis. Intensive Care Med. 2009;35(9):1604–1608. doi: 10.1007/s00134-009-1515-3. [DOI] [PubMed] [Google Scholar]
- 173.Bryant P, Shumate M, Yumet G, Lang CH, Vary TC, Cooney RN. Capsaicin-sensitive nerves regulate the metabolic response to abdominal sepsis. J Surg Res. 2003;112(2):152–161. doi: 10.1016/s0022-4804(03)00154-9. [DOI] [PubMed] [Google Scholar]
- 174.Demirbilek S, Ersoy MO, Demirbilek S, et al. Small-dose capsaicin reduces systemic inflammatory responses in septic rats. Anesth Analg. 2004;99(5):1501–1507. doi: 10.1213/01.ANE.0000132975.02854.65. [DOI] [PubMed] [Google Scholar]
- 175.Kim CS, Kawada T, Kim BS, et al. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003;15(3):299–306. doi: 10.1016/s0898-6568(02)00086-4. [DOI] [PubMed] [Google Scholar]
- 176.Wang Y, Novotny M, Quaiserova-Mocko V, Swain GM, Wang DH. TRPV1-mediated protection against endotoxin-induced hypotension and mortality in rats. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1517–1523. doi: 10.1152/ajpregu.00005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ang SF, Moochhala SM, Bhatia M. Hydrogen sulfide promotes transient receptor potential vanilloid 1-mediated neurogenic inflammation in polymicrobial sepsis. Crit Care Med. 2010;38(2):619–628. doi: 10.1097/CCM.0b013e3181c0df00. [DOI] [PubMed] [Google Scholar]
- 178.De Winter BY, Bredenoord AJ, Van NL, et al. Involvement of afferent neurons in the pathogenesis of endotoxin-induced ileus in mice: Role of CGRP and TRPV1 receptors. Eur J Pharmacol. 2009;615(1–3):177–184. doi: 10.1016/j.ejphar.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 179.Murai M, Tsuji F, Nose M, et al. SA13353 (1-[2-(1-Adamantyl)ethyl]-1-pentyl-3-[3-(4-pyridyl)propyl]urea) inhibits TNF-alpha production through the activation of capsaicin-sensitive afferent neurons mediated via transient receptor potential vanilloid 1 in vivo. Eur J Pharmacol. 2008;588(2–3):309–315. doi: 10.1016/j.ejphar.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 180.Tsuji F, Murai M, Oki K, et al. Effects of SA13353, a transient receptor potential vanilloid 1 agonist, on leukocyte infiltration in lipopolysaccharide- induced acute lung injury and ovalbumin-induced allergic airway inflammation. J Pharmacol Sci. 2010;112(4):487–490. doi: 10.1254/jphs.09295sc. [DOI] [PubMed] [Google Scholar]
- 181.Clark N, Keeble J, Fernandes ES, et al. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J. 2007;21(13):3747–3755. doi: 10.1096/fj.06-7460com. [DOI] [PubMed] [Google Scholar]
- 182.Guptill V, Cui X, Khaibullina A, et al. Disruption of the transient receptor potential vanilloid 1 can affect survival, bacterial clearance, and cytokine gene expression during murine sepsis. Anesthesiology. 2011 March 4; doi: 10.1097/ALN.0b013e318212515b. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Helyes Z, Pozsgai G, Borzsei R, et al. Inhibitory effect of PACAP-38 on acute neurogenic and non-neurogenic inflammatory processes in the rat. Peptides. 2007;28(9):1847–1855. doi: 10.1016/j.peptides.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 184.Orliac ML, Peroni RN, Abramoff T, Neuman I, Podesta EJ, Adler-Graschinsky E. Increases in vanilloid TRPV1 receptor protein and CGRP content during endotoxemia in rats. Eur J Pharmacol. 2007;566(1–3):145–152. doi: 10.1016/j.ejphar.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 185.Huttemeier PC, Ritter EF, Benveniste H. Calcitonin gene-related peptide mediates hypotension and tachycardia in endotoxic rats. Am J Physiol. 1993;265(2 Pt 2):H767–769. doi: 10.1152/ajpheart.1993.265.2.H767. [DOI] [PubMed] [Google Scholar]
- 186.Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M. Attenuated fever response in mice lacking TRPV1. Neurosci Lett. 2005;378(1):28–33. doi: 10.1016/j.neulet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 187.Dogan MD, Patel S, Rudaya AY, Steiner AA, Szekely M, Romanovsky AA. Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor. Br J Pharmacol. 2004;143(8):1023–1032. doi: 10.1038/sj.bjp.0705977. [DOI] [PMC free article] [PubMed] [Google Scholar]