Abstract
Background
The development of highly vascularized and inflammatory periprosthetic tissue characterizes the progress of aseptic loosening, a major complication of joint arthroplasty. Vascular endothelial growth factor (VEGF) is an important cell signaling protein involved in angiogenesis. The purpose of this study was to investigate whether R2/Fc (a VEGF neutralizing antibody) and SU5416 (a VEGF receptor II [Flk-1] inhibitor) could ameliorate particle-induced inflammatory osteolysis in a mouse model.
Methods
Ultrahigh molecular weight polyethylene (UHMWPE) particles were introduced into established air pouches in BALB/c mice, followed by implantation of calvaria bone from syngeneic littermates. Drug treatment was started 2 weeks after bone implantation, and mice without drug treatment were included as controls. Pouch tissues were harvested 4 weeks after bone implantation for molecular and histological analysis, and implanted bone degradation was analyzed by microcomputed tomography.
Results
Exposure to UHMWPE particles induced inflammatory osteolysis, which was associated with increased expression of VEGF/Flt-1 proteins. Treatment with R2/Fc significantly improved UHMWPE particle-induced inflammatory osteolysis, and reduced the expression of VEGF/Flt-1 proteins. However, SU5416 treatment showed no effect on UHMWPE particle-induced inflammatory osteolysis.
Conclusion
Our findings indicate that VEGF signaling exerts a regulatory effect on the development of UHMWPE-induced inflammatory osteolysis, through its unique Flt-1, rather than Flk-1, receptor located on monocyte/macrophage cell lineages. These data provide a biological rationale for a VEGF/Flt-1-targeted treatment strategy, especially during the early stages of the wear debris-induced inflammatory response.
Keywords: vascular endothelial growth factor, Flt -1, osteolysis, osteoclastogenesis, R2/Fc, SU5416, animal model, wear debris
Introduction
It is estimated that more than 500,000 total joint arthroplasties are performed in the US annually. Aseptic loosening due to wear debris-induced osteolysis is the most common cause of implant failure.1,2 Aseptic loosening is characterized by the formation of a chronic inflammatory response to wear debris from the implant, leading to bone resorption (osteolysis) and loss of fixation.3 The periprosthetic tissue at the bone-implant interface shows a high degree of vascularization.4 A number of factors contribute to angiogenesis, and the major signaling protein, vascular endothelial growth factor (VEGF) is produced by multiple cell types, including macrophages and osteoblasts.5,6 VEGF exerts its biological activity by binding to two receptors, VEGF receptor-1 (VEGFR-1; Flt-1) and VEGFR-2 (Flk-1/KDR).7 VEGF is actively involved in the process of inflammation,8 osteoclastogenesis,9,10 and bone resorption.10 However, the role of VEGF in wear debris-induced inflammatory osteolysis has not been well determined.
VEGF R2/Fc chimera is a 110 kDa peptide containing the extracellular domain of a VEGF receptor linked to the heavy chain of the IgG molecule, which binds and inactivates free VEGF. Efforts toward the discovery of receptor tyrosine kinase inhibitors with specific activity against VEGF receptors have led to the synthesis and characterization of a number of novel compounds. One of these SU5416,11 a cell-permeable indolinone compound that acts as a selective adenosine triphosphate (ATP)-competitive inhibitor of VEGF-R2 (KDR/Flk-1), with slightly weaker inhibition of VEGF-R1 (Flt-1). However, it appears to be specific for VEGF receptors because it has no effect on other kinases, such as the epidermal growth factor receptor, in concentrations up to 10 μM. Using a well established mouse osteolysis model,12,13 we report that exposure to ultrahigh-molecular-weight polyethylene (UHMWPE) particles increased VEGF expression at both the mRNA and protein levels in pouch tissues, and increased VEGF gene expression is closely associated with pouch tissue inflammation status, suggesting that VEGF has a role in the regulation of implant wear-induced tissue inflammation and osteoclastogenesis.14 Furthermore, we also found that VEGF inhibitor treatment significantly prevented the development of UHMWPE particle-induced inflammation and osteoclastic bone resorption using this mouse model.13 The aim of this study was to investigate the therapeutic response of either VEGF R2/Fc or SU5416 in a mouse model of UHMWPE particle-induced inflammatory osteolysis.
Materials and methods
Materials
Female BALB/c mice (aged 8–10 weeks) were purchased from Jackson Laboratory (Bar Harbor, ME) and quarantined within Wayne State University facility prior to experimentation. Recombinant human VEGF 165 (293-VE), a 42 kDa peptide with 97% homology to rabbit and mouse VEGF, was purchased from R & D Systems (Minneapolis, MN). VEGF R2/Fc was purchased from R & D Systems. SU5416 was purchased from Calbiochem (San Diego, CA). Rabbit-anti-VEGF-A (sc-152) and goat polyclonal antibodies against interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), RANKL, and CD68 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Ultra high molecular weight polyethylene particles
UHMWPE15 particles were gifted by John Cuckler from the University of Alabama, Birmingham, AL. Scanning electron microscopy analysis demonstrated that 90% of the UHMWPE particles were less than 5.5 μm in diameter, with a mean size of 2.6 μm, and diameters ranging from less than 0.6 μm to 21 μm (standard deviation [SD] 2.4 μm). UHMWPE particles were washed in 70% ethanol solution briefly and resuspended in sterile phosphate-buffered saline for injection. The particle suspension was determined to be endotoxin-free by the Limulus assay (Endosafe, Charles Rivers, Charlestown, SC).
Mouse osteolysis model and drug treatment
Institutional approval was obtained for all animal procedures. The use of the bone-implanted inflammatory air pouch as a model of debris-induced osteolysis has been described previously.12 Air pouches were established in female BALB/c mice as described elsewhere.15 Six days later, mice with established air pouches were anesthetized by intraperitoneal injection of pentobarbital 50 mg/kg (Fisher Scientific, Pittsburgh, PA). A 0.5 cm incision overlying the pouch was made, and a section of calvaria bone (approximately 0.4 × 0.25 cm) from a genetically identical donor mouse was inserted into the pouch. The pouch layers and the skin incision were then closed using 4-0 Prolene sutures. On the following day, pouches were injected with 0.5 mL saline containing either 1% UHMWPE or saline alone (control). Seven days after bone implantation, pouches were rechallenged with either 0.5 mL of UHMWPE particles (1%) to maintain persistent inflammation or with 0.5 mL of saline for the control groups. Fourteen days after particle implantation, while UHMWPE particles induced inflammatory osteolysis, 0.9% saline was injected intraperitoneally in the absence (untreated) or presence of recombinant human VEGF 165 5 μg/kg/day, VEGF R2/Fc chimera 5 μg/kg/day, or SU5416 20 mg/kg/day. Drug treatment was continued for 14 days until the mice were sacrificed. The pouches injected with saline alone were included as a control. Each experimental group included eight mice. The experimental design and grouping are outlined in Table 1. Mice were sacrificed in a carbon dioxide chamber at 14 days after drug treatment. The pouch membranes containing implanted bone were harvested for morphological analysis.
Table 1.
Mice groups and treatment protocol
| Group | n | Treatment |
|---|---|---|
| 1 | 8 | PBS (control) |
| 2 | 8 | UHMWPE (0.5 mg per pouch) |
| 3 | 8 | UHMWPE + VEGF (5 μg/kg/day, intraperitoneal injection daily) |
| 4 | 8 | UHMWPE + VEGF R2/Fc chimera protein (5 μg/kg/day, intraperitoneal injection daily) |
| 5 | 8 | UHMWPE + SU 5416 (20 mg/kg/day, intraperitoneal injection daily) |
Abbreviations: PBS, phosphate-buffered saline; VEGF, vascular endothelial growth factor; UHMWPE, ultrahigh-molecular-weight polyethylene.
Histological evaluation and image analysis
The histological parameters of inflammatory osteolysis were measured using techniques described elsewhere.15 Tissue samples were fixed in 10% formalin, followed by decalcification in 10% ethylenediamine tetra-acetic acid, and were paraffin-embedded. Tissue sections (6 μm) were stained with hematoxylin and eosin to evaluate pouch membrane inflammation and implant bone erosion. Four separate sections per specimen were evaluated in a blinded fashion. Digital images were acquired using a Zeiss light microscope equipped with a Toshiba CCD, and these images were analyzed using the Image-Pro software package (Media Cybernetics, Silver Spring, MD). Pouch membrane thickness was determined at six points on each section, with an even distribution of measurement on the proximal side, distal side, and transition curve of the pouch. The total number of cells (based upon nucleus count) was analyzed as described previously.15
Evaluation of osteoclastogenesis
Osteoclast-like cells were identified by tartrate-resistant acid phosphatase (TRAP, EC3.1.3.2) staining in paraffin tissue sections using a commercial kit (Sigma, St. Louis, MO), as described previously.16 The presence of dark purple-staining granules in the cytoplasm was considered to be the specific criterion for TRAP+ cells. Positive TRAP localization was quantified by pixel area count and reported as a percentage of the total implanted bone area in the pouch tissue using the Image-Pro software package (Image-Pro Plus, Media Cybernetics).
Immunohistological stains for VEGF, FLT-1, IL-1, TNF, CD 68, and RANKL
An immunohistochemical technique was performed in tissue paraffin sections. Briefly, tissue sections were deparaffinized and blocked with 1.5% normal goat serum. Rabbit or goat polyclonal antimouse VEGF, FLT-1, IL-1, TNF, CD68, and RANKL (Santa Cruz Inc) antibodies at a concentration of 2 μg/mL were applied to sections and incubated overnight in a moisturized chamber at 4°C. Biotin-conjugated secondary antibody (goat antirabbit IgG) was incubated for 30 minutes after extensive washing. Avidin biotin enzyme reagent was then applied onto sections for 30 minutes. The color was developed by adding 3.3′-diaminobezidine tetrahydrochloride. In negative control sections, the primary antibody was replaced with 1.5% normal goat serum. Digital images of representative fields of view were captured and analyzed using the Image-Pro software package. The level of protein expression and localization were evaluated in six different fields and expressed as pixel density. Computerized image analysis was used to analyze stained sections to obtain the percentage of cells positive to the target protein. Using a 400× microscope objective, eight randomly selected fields were analyzed for each section. Reproducibility of multiple sections from the same site was checked through the analysis of duplicates in a blind manner.
Determination of osteolysis
Mice were scanned on the day of bone implantation and four weeks after bone implantation (immediately before termination) using an RS-9 In Vivo MicroCT Scanner (General Electric Systems, London, Canada). The scan was performed at a resolution of 0.093 mm (cubic voxel length) using the manufacturer’s defined settings. After data acquisition, two-dimensional projection data were reconstructed into three-dimensional volumes, which were then reoriented in the appropriate dimensions to place the data sets in consistent orientation for image analysis. Erosion surface as a fraction of total bone surface was determined. Bone density of the calvaria was further analyzed with the proprietary microcomputed tomography (micro CT) software.
Statistical analysis
Mice were randomly assigned to three experimental groups with eight mice in each group. Statistical analysis among groups was performed by the analysis of variance test with the Schafer formula for post hoc multiple comparisons, using the SPSS software package (version 7.5; SPSS Inc, Chicago, IL). Data were expressed as mean ± standard error of the mean. A P value of less than 0.05 was considered significant.
Results
Animal health
The mice used in this study tolerated both the surgery and the drug treatments well. No mice were excluded from this study due to weight loss, drug toxicity, or pouch infection, as determined by clinical observation and histological analysis.
Therapeutic effects of drugs on UHMWPE-induced tissue inflammation
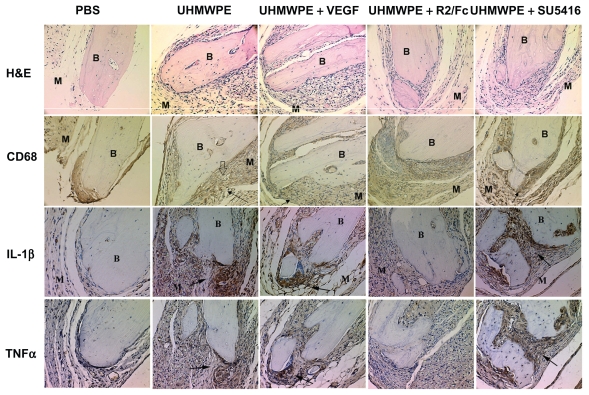
To investigate the potential therapeutic effects of VEGF inhibitors on UHMWPE particle-induced inflammation, drug treatment was started two weeks after bone implantation, when UHMWPE particle stimulation had induced significant tissue inflammation and bone damage. As shown in Figure 1, image analysis of tissue sections stained with hematoxylin and eosin showed that UHMWPE particle-induced tissue inflammatory responses were characterized by increased cellular infiltration and membrane proliferation, compared with saline controls. It was observed that in mice challenged with UMHWPE particles, VEGF treatment slightly increased cellular infiltration and membrane proliferation as compared with untreated mice, although this increase did not reach statistical significance. Quantitative image analysis, as shown in Table 2, revealed that UHMWPE particles significantly increased pouch membrane thickness and the number of infiltrating cells as compared with saline-injected controls. Treatment with F2/Rc protein significantly decreased UHMWPE particle-induced membrane thickness and cellular infiltration (P < 0.05). However, treatment with SU5416 showed no therapeutic effects.
Figure 1.
Therapeutic effects of VEGF inhibitors on UHMWPE particles- induced tissue inflammation. Representative tissues histology of hematoxylin and eosin (H&E) stain and immunohistochemical stains of CD68, IL-1b and TNFa in mice pouch membranes. (Original magnification × 200). B, Implanted bone; M, pouch membrane. Positive staining was indicated by arrowhead, and UHMWPE particle deposit spot was indicated by hollow arrowhead. Data of quantitative image analysis was shown in Table 2
Abbreviations: RANKL, Receptor activator of nuclear factor kappa B ligand; TRAP, tartrate-resistant acid phosphatase; VEGF, vascular endothelial growth Factor; PBS, phosphate-buffered saline; UHMWPE, ultra high-molecular weight polyethylene.
Table 2.
Quantitative assessment of the pouch membrane histology profiles by Image-Pro software analysis. Measurement of total cell counts in pouch tissues.
| PBS | UHMWPE | UHMWPE + VEGF | UHMWPE + R2/Fc | UHMWPE + SU5416 | |
|---|---|---|---|---|---|
| Membrane thickness (mm) | 0.015 ± 0.003 | 0.042 ± 0.009* | 0.046 ± 0.011* | 0.015 ± 0.009** | 0.043 ± 0.014* |
| Cellular infiltration (mm2) | 3590 ± 863 | 8331 ± 1123* | 8680 ± 811* | 4406 ± 951** | 7496 ± 480* |
| CD68 positive-stained cells (%) | 7.5 ± 5.2 | 25 ± 7.5* | 22.5 ± 6.8* | 13.8 ± 2.8** | 25 ± 3.3* |
| TNFα positive-stained cells (%) | 5 ± 2.9 | 20 ± 5.7* | 19 ± 4.0* | 6.6 ± 3.3** | 21 ± 3.3* |
| IL-1β positive-stained cells (%) | 8.3 ± 3.0 | 23.3 ± 6.8* | 20 ± 6.1* | 13 ± 2.8** | 22 ± 3.3* |
p<0.05, vs. PBS;
p<0.05, vs. UHMWPE, UHMWPE+VEGF, and UHMWPE+SU5416.
Abbreviations: TNF, tumor necrosis factor; IL, interleukin; PBS, phosphate-buffered saline; VEGF, vascular endothelial growth factor; UHMWPE, ultrahigh-molecularweight polyethylene.
The aggregation of macrophages in response to UHMWPE stimulation was analyzed by immunostaining of CD68, a macrophage cell surface marker. As shown in Figure 1 and Table 2, CD68+ cells were significantly increased in response to UHM-WPE particle stimulation, and were especially concentrated in areas of particle accumulation. VEGF treatment resulted in a slight increase of CD68+ cells in UHMWPE-containing pouch tissues. F2/Rc treatment reduced the number of CD68+ cells in the infiltrate, whereas SU5416 treatment had no effects on the aggregation of CD68+ cells in pouch tissues. These data indicate that UHMWPE-induced inflammatory cellular infiltration was mainly composed of macrophages, and the therapeutic effects of F2/Rc on UHMWPE particle-induced inflammation was mainly through reduction of inflammatory macrophage recruitment.
Particle-induced tissue inflammation was invariably accompanied by local accumulation of proinflammatory cytokines, such as IL-1β and TNFα. Immunohistochemical study showed that exposure to UHMWPE particles significantly increased both IL-1β and TNFα protein staining in the pouch tissue (Figure 1) compared with controls. As expected, F2/Rc treatment significantly reduced both IL-1β and TNFα protein expression, while SU5416 treatment demonstrated little effect on cytokine production. Taken together, these data verified that F2/Rc treatment reduced UHMWPE particle-induced inflammatory cytokine production and significantly ameliorated tissue inflammatory morphology. Pouch tissue inflammation, assessed by the production of IL-1β and TNFα in tissues of F2/Rc-treated mice, was significantly lower than in the tissues of untreated mice. However, no significant difference in production of these cytokines was found between mice with and without VEGF treatment in the presence of UHMWPE particle stimulation. Furthermore, SU5416 treatment showed no therapeutic effects in improving pouch membrane inflammation.
Therapeutic effects of drugs on UHMWPE-induced VEGF and Flt-1 protein production
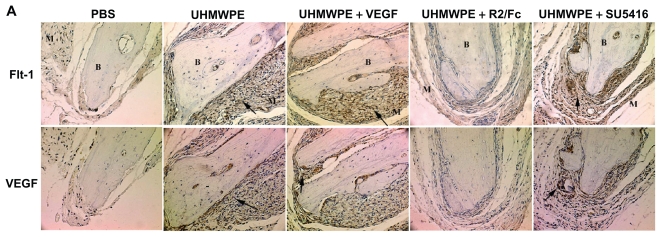
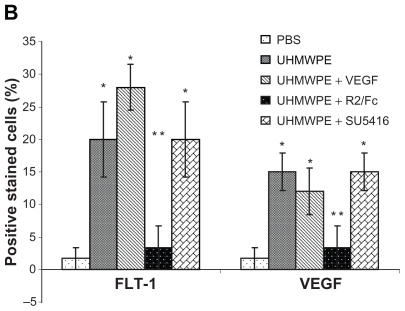
Immunostaining was used to measure VEGF and Flt-1 protein expression and location in pouch membranes. As shown in Figure 2A, UHMWPE particles induced strong VEGF staining, especially in the UHMWPE deposit foci surrounded by the aggregation of inflammatory cells that were mainly composed of macrophages as previously described.14 R2/Fc treatment resulted in a significant reduction of VEGF staining as compared with untreated mice. SU5416 treatment showed no effects on expression of VEGF protein. As shown in Figure 2B, R2/Fc treatment significantly reduced the percentage of VEGF+-stained cells (P < 0.05), suggesting that UHMWPE-induced VEGF expression was efficiently suppressed by R2/Fc treatment. Flt-1 staining was significantly increased by UHM-WPE particle stimulation, as compared with control pouches which had received phosphate-buffered saline injections. R2/Fc treatment significantly reduced the staining intensity of Flt-1 protein, but SU5416 treatment showed no change of Flt-1 staining intensity, as shown in Figure 2B.
Figure 2A.
Immunohistochemical detection of Flt-1 and VEGF in mice pouch membranes. (Original magnification × 200.) B, Implanted bone; M, pouch membrane. Positive staining was indicated by arrowhead
Abbreviations: RANKL, Receptor activator of nuclear factor kappa B ligand; TRAP, tartrate-resistant acid phosphatase; VEGF, vascular endothelial growth Factor; PBS, phosphate-buffered saline; UHMWPE, ultra high-molecular weight polyethylene
Figure 2B.
Positive stained cells was quantified by Image-Pro software as described in Materials and Methods. The value represents percentage of positive stained cells. *p<0.05, vs. PBS; **p<0.05, vs. UHMWPE, UHMWPE+VEGF, and UHMWPE+SU5416
Abbreviations: RANKL, Receptor activator of nuclear factor kappa B ligand; TRAP, tartrate-resistant acid phosphatase; VEG F, vascular endothelial growth Factor; PBS, phosphate-buffered saline; UHMWPE, ultra high-molecular weight polyethylene
Therapeutic effects of drugs on UHMWPE-induced osteoclastic bone resorption
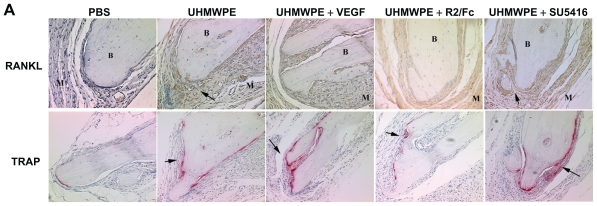
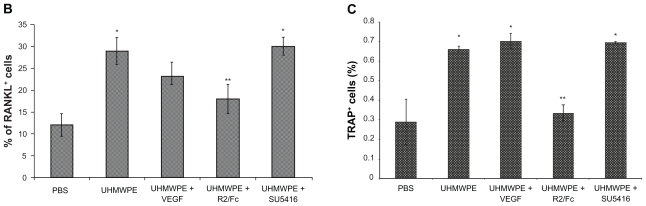
Enhanced osteoclastogenesis has been recognized as a hallmark of various forms of osteoporosis, including the bone loss that occurs in aseptic loosening.17 RANKL/RANK signaling is essential for the development of osteoclastogenesis. As depicted in Figure 3, the results of immunohistochemistry showed increased RANKL staining in UHMWPE-containing pouches, compared with that seen in phosphate-buffered saline controls. RANKL staining was predominantly found at the interface between pouch membrane and implanted bones, and in the cytoplasm of cells present within aggregates of inflammatory cells. As seen in Figure 3, R2/Fc treatment significantly reduced RANKL staining, while SU5416 treatment showed no significant difference when compared with untreated mice (Figure 3B).
Figure 3A.
Therapeutic Effects of VEGF inhibitors on UHMWPE particles- induced inflammatory osteoclastogenesis. (A) Representative immunohistochemical staining of RANKL and histochemical TRAP staining in mice pouch membranes. (Original magnification × 200) B, Implanted bone; M, pouch membrane. Positive stained cells were indicated by arrowheads
Abbreviations: RANKL, Receptor activator of nuclear factor kappa B ligand; TRAP, tartrate-resistant acid phosphatase; VEGF, vascular endothelial growth Factor; PBS, phosphate-buffered saline; UHMWPE, ultra high-molecular weight polyethylene
Figure 3.
Therapeutic Effects of VEGF inhibitors on UHMWPE particles- induced inflammatory osteoclastogenesis. (B) RANKL+ cells were quantified by Image-Pro software. The value represents percentage of RANKL+ cells. (C) TRAP+ cells were quantified as percentage of total implanted bone area, as described in Materials and Methods. * p<0.05, vs. PBS; **p<0.05, vs. UHMWPE, UHMWPE+VEGF and UHMWPE+SU5416
Abbreviations: RANKL, Receptor activator of nuclear factor kappa B ligand; TRAP, tartrate-resistant acid phosphatase; VEGF, vascular endothelial growth Factor; PBS, phosphate-buffered saline; UHMWPE, ultra high-molecular weight polyethylene
Histochemical TRAP staining was used to measure the number of osteoclasts in pouch tissue. Figure 3 illustrates discrete foci of TRAP+ cells at the interface between implanted calvaria and pouch membrane in pouches with phosphate-buffered saline injection. The bone morphology remained intact, and no resorption lacunae were observed. Intense TRAP staining was observed in pouches stimulated with UHMWPE, and TRAP+ cells extended into adjoining areas. Regions where TRAP+ cells localized were often pitted, suggesting active osteoclastic bone resorption. R2/Fc treatment caused a significant reduction in the number of TRAP+ cells, and the bone resorption lacunae were remarkably reduced (P < 0.05). However, SU5416 treatment did not affect UHMWPE particle-induced increase of TRAP+ cells. We also observed that VEGF treatment slightly increased TRAP+ cells in pouches with or without UHMWPE particle stimulation, suggesting that VEGF may have a regulatory role in the development of osteoclastogenesis.
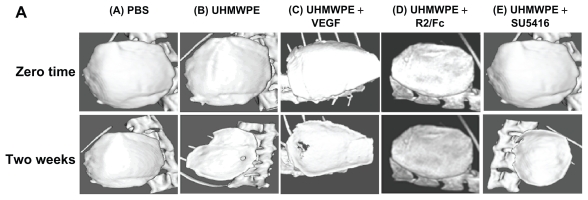
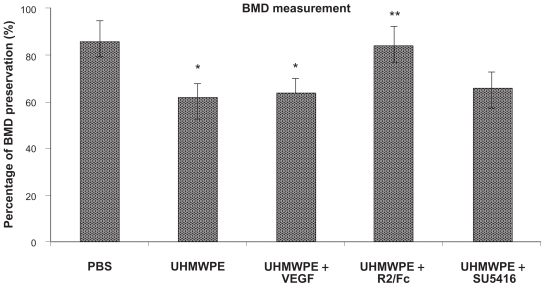
Consistent with the histological profiles, UHMWPE particle stimulation dramatically increased the implanted bone degradation in comparison with saline controls. As shown in Figure 4A, the micro CT imaging analysis showed a significant alteration of plateau surface contour in pouches with UHMWPE particle stimulation, as compared with the control pouches, suggesting progressive implanted bone degradation. R2/Fc treatment dramatically lessened bone degradation caused by UHMWPE particles, while SU5416 treatment did not show therapeutic effect on implanted bone reservation. The effects of R2/Fc treatment were further confirmed by quantitative bone mineral density measurement (P < 0.05). In summary, the data indicate that R2/Fc treatment has a therapeutic effect by interfering with UHMWPE particle-induced inflammatory osteolysis.
Figure 4A.
Therapeutic effects of VEGF inhibitors on UHMWPE particle-induced inflammatory osteolysis. (microCT image analysis) (A) Representative 3D isosurface analysis
Abbreviations: Micro-CT, microcomputed tomography; PBS, phosphate-buffered saline; UHMWPE, ultra high-molecular weight polyethylene; VEGF, vascular endothelial growth Factor
Discussion
This study revealed that R2/Fc protein treatment significantly inhibits UHMWPE particle-induced pouch tissue inflammation, tissue RANKL production, and TRAP+ cell formation, thus improving established UHMWPE particle-induced inflammatory osteolysis in a mouse model. R2/Fc chimera protein binds and inactivates circulating VEGF through its VEGF receptor domain, without triggering VEGF signaling. However, SU5416 (a specific Flk-1 inhibitor) treatment demonstrates no effect on UHMWPE particle-induced inflammatory osteolysis. A proangiogenic status4 in the loosening periprosthetic tissue augments the inflammatory response to wear debris.4,18 VEGF is one of the most powerful angiogenic agents known.5 Saffar et al19 found that VEGF was expressed in all periprosthetic tissues, with intense VEGF staining found on both macrophages and multinucleated giant cells within the implant lining layer associated with the deposit of wear debris. Goodman et al18 reported recently that VEGF is overexpressed in periprosthetic tissues around loose implants, and demonstrated that the VEGF staining was colocalized with CD11b-positive macrophages. Furthermore, these authors found that the supernatants from titanium particle-challenged monocyte/macrophages significantly increased macrophage chemotactic activity, which could be inhibited by a neutralizing antibody against VEGF. We showed here that VEGF expression is significantly increased after UHMWPE particle stimulation, suggesting that VEGF plays a critical role in the pathogenesis of aseptic loosening.
Multiple lines of evidence indicate that VEGF is critically involved in the initiation and persistence of inflammation.8,20–25 Although IL-1 and TNF are among the major cytokines detected in periprosthetic tissue,26 and are known to be potent mediators of bone resorption associated with aseptic loosening,27,28 the regulatory mechanisms of VEGF by these cytokines in the progression of aseptic loosening are still unclear. We have shown that R2/Fc protein treatment suppresses UHMWPE particle-induced tissue inflammation and reduces the level of IL-1β and TNFα in UHMWPE-stimulated pouch tissues. Inhibition of inflammation mediated by VEGF within the pouch tissue is an attractive approach to aseptic loosening treatment because it can prevent the formation of chronic inflammation provoked by wear debris in the periprosthetic tissue, especially at the early stage of aseptic loosening development. Relationships between VEGF activity and osteoclastogenesis have been reported. These include the stimulation of endothelial cells to produce receptor activation of nuclear factor κB (RANK), a critical protein in initiating osteoclastogenesis,29 the sharing of similar biological activity of macrophage colony-stimulating factor30,31 in the support of osteoclastogenesis, and the stimulation of osteoclast recruitment,30 differentiation,32 activation, and survival.9 The wear debris-induced increase of VEGF production reported previously,4,18 and found in this study, could exacerbate bone degradation that ultimately leads to the development of aseptic loosening after total joint replacement. Although R2/Fc treatment significantly diminished UHMWPE particle-induced inflammatory osteolysis, the detailed cellular mechanisms of its effects are still unknown. We proposed that the therapeutic response of R2/Fc on inflammatory osteolysis acts mainly through inhibiting recruitment of inflammatory cells (mainly macrophages) to inflamed tissues,18,33 macrophage activation,34 and osteoclastogenesis.29 Because other inflammatory cytokines, such as IL-1β and TNFα are known to be potent mediators of bone resorption associated with aseptic loosening,27,28 it may be more effective if anti-VEGF treatment is combined with other therapeutics, such as TNFα inhibitors. Although it is important to determine the precise mechanism of action, the significant suppression of UHMWPE particle-induced inflammatory osteolysis by R2/Fc is an attractive approach for this debilitating disease.
The receptors for VEGF belong to the tyrosine kinase receptor family, and two specific VEGF receptors have been identified to date, ie, VEGF receptor-1 (VEGFR-1; fms-like tyrosine kinase, Flt-1), and VEGFR-2 (Flk-1/KDR).7 VEGF appears to exert inflammatory effects through binding to its receptors, especially Flt-1. In addition to endothelial cells, Flt-1 is also expressed on cells of the monocyte-macrophage lineages and is involved in macrophage activation,33 migration,35,36 and differentiation.9,30 The effectiveness of anti-Flt-1 antibody in collagen arthritis appears specific, because anti-Flk-1 antibody treatment proved ineffective,25 and similar results were reported by De Bandt’s group in the transgenic K/BxN mouse RA model.21 The failure of anti-Flk-1 antibody to block arthritis suggests that suppression of inflammation (rather than angiogenesis) is primarily responsible for the clinical improvement. We have noticed a positive association between tissue inflammation and the levels of VEGF and Flt-1 gene transcripts in mouse pouch tissues containing UHMWPE particles.14 SU5416 is a specific inhibitor of Flk-1 tyrosine kinase activity.37 In this study, we found that SU5416 treatment has no significant therapeutic effect on UHMWPE particle-induced tissue inflammation and osteoclastogenic bone resorption. In addition, SU5416 shows little effect on inflammatory cytokine production and osteoclast formation. These data provide insight into the critical role of VEGF/Flt-1 signaling in UHMWPE particle-induced inflammatory osteolysis, and may have important implications for the future treatment of aseptic loosening conditions. It is obvious that VEGF/Flk-1 signaling plays little, if any, role in the pathogenesis of aseptic loosening development. Therefore, developing specific inhibitors targeted against VEGF/Flt-1 signaling will be promising in the prevention and treatment of aseptic loosening, especially in the early stages.
The interactive network of the VEGF/Flt-1 and RANKL/RANK pathways may play important roles in the initiation, progression, and resolution of aseptic loosening. Our data confirmed that R2/Fc treatment, through trapping VEGF in the milieu, inhibits UHMWPE particle-enhanced VEGF/Flt-1 signaling. As a result, a remarkable reduction of UHMWPE particle-induced pouch inflammatory osteolysis was observed. However, SU5416 treatment demonstrates no effects on UHMWPE particle-induced tissue damage. These data suggest that VEGF may be actively involved in the regulation of RANK/RANKL gene expression, and that it exerts a regulatory effect on the development of UHMWPE particle-induced inflammatory osteoclastogenesis through its unique Flt-1, rather than Flk-1, receptor located on monocyte/macrophage cell lineages. Taken together, our findings provide the biological rationale for a VEGF/Flt-1-targeted treatment strategy, especially in the early stages of wear debris-induced inflammatory response.
Figure 4B.
Therapeutic effects of VEGF inhibitors on UHMWPE particle- induced inflammatory osteolysis. ( microCT image analysis) ( B) Measurement of bone mineral density (BMD).
* p<0.05, vs. PBS; ** p<0.05, vs. UHMWPE and UHMWPE+VEGF
Abbreviations: BMD, bone mineral density; PBS, phosphate-buffered saline; UHMWPE, ultra high-molecular weight polyethylene; VEGF, vascular endothelial growth Factor
Acknowledgments
This study was supported by Stryker Company and the Department of Biomedical Engineering Fund for Medical Research and Education.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Berry DJ, Harmsen WS, Cabanela ME, Morrey BF. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: Factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84(2):171–177. doi: 10.2106/00004623-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Keener JD, Callaghan JJ, Goetz DD, Pederson DR, Sullivan PM, Johnston RC. Twenty-five-year results after Charnley total hip arthroplasty in patients less than fifty years old: A concise follow-up of a previous report. J Bone Joint Surg Am. 2003;85-A(6):1066–1072. doi: 10.2106/00004623-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Bauer TW. Particles and peri-implant bone resorption. Clin Orthop Relat Res. 2002;(405):138–143. doi: 10.1097/00003086-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Al Saffar N, Mah JT, Kadoya Y, Revell PA. Neovascularisation and the induction of cell adhesion molecules in response to degradation products from orthopaedic implants. Ann Rheum Dis. 1995;54(3):201–208. doi: 10.1136/ard.54.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodsell DS. The molecular perspective: VEGF and angiogenesis. Oncologist. 2002;7(6):569–570. doi: 10.1634/theoncologist.7-6-569. [DOI] [PubMed] [Google Scholar]
- 6.Harry LE, Paleolog EM. From the cradle to the clinic: VEGF in developmental, physiological, and pathological angiogenesis. Birth Defects Res Part C Embryo Today. 2003;69(4):363–374. doi: 10.1002/bdrc.10024. [DOI] [PubMed] [Google Scholar]
- 7.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269(43):26988–26995. [PubMed] [Google Scholar]
- 8.Kunstfeld R, Hirakawa S, Hong YK, et al. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104(4):1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa M, Kaneda T, Arakawa T, et al. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS Lett. 2000;473(2):161–164. doi: 10.1016/s0014-5793(00)01520-9. [DOI] [PubMed] [Google Scholar]
- 10.Henriksen K, Karsdal M, Delaisse JM, Engsig MT. RANKL and vascular endothelial growth factor (VEGF) induce osteoclast chemotaxis through an ERK1/2-dependent mechanism. J Biol Chem. 2003;278(49):48745–48753. doi: 10.1074/jbc.M309193200. [DOI] [PubMed] [Google Scholar]
- 11.Giles FJ, Stopeck AT, Silverman LR, et al. SU5416, a small molecule tyrosine kinase receptor inhibitor, has biologic activity in patients with refractory acute myeloid leukemia or myelodysplastic syndromes. Blood. 2003;102(3):795–801. doi: 10.1182/blood-2002-10-3023. [DOI] [PubMed] [Google Scholar]
- 12.Ren WP, Yang S, Wooley PH. A novel murine model of orthopaedic wear debris-associated osteolysis. Scand J Rheumatology. 2004;33(5):349–357. doi: 10.1080/03009740410005944. [DOI] [PubMed] [Google Scholar]
- 13.Ren W, Zhang R, Markel DC, et al. Blockade of vascular endothelial growth factor activity suppresses wear debris-induced inflammatory osteolysis. J Rheumatol. 2007;34(1):27–35. [PubMed] [Google Scholar]
- 14.Ren WP, Markel DC, Zhang R, et al. Association between UHMWPE particle-induced inflammatory osteoclastogenesis and expression of RANKL, VEGF, and Flt-1 in vivo. Biomaterials. 2006;27(30):5161–5169. doi: 10.1016/j.biomaterials.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Wooley PH, Morren R, Andary J, et al. Inflammatory responses to orthopaedic biomaterials in the murine air pouch. Biomaterials. 2002;23(2):517–526. doi: 10.1016/s0142-9612(01)00134-x. [DOI] [PubMed] [Google Scholar]
- 16.Ren WP, Wu B, Mayton L, Wooley PH. Polyethylene and methyl methacrylate particle-stimulated inflammatory tissue and macrophages up-regulate bone resorption in a murine neonatal bone resorption in a murine neonatal calvaria in vitro organ system. J Orthop Res. 2002;20(5):1031–1037. doi: 10.1016/S0736-0266(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 17.Wooley PH, Schwarz EM. Aseptic loosening. Gene Ther. 2004;11(4):402–407. doi: 10.1038/sj.gt.3302202. [DOI] [PubMed] [Google Scholar]
- 18.Miyanishi K, Trindade MC, Ma T, Goodman SB, Schurman DJ, Smith RL. Periprosthetic osteolysis: Induction of vascular endothelial growth factor from human monocyte/macrophages by orthopaedic biomaterial particles. J Bone Miner Res. 2003;18(9):1573–1583. doi: 10.1359/jbmr.2003.18.9.1573. [DOI] [PubMed] [Google Scholar]
- 19.Jell GMR, Al-Saffar N. Does a pro-angiogenic state exist in the bone-implant interface of aseptically loosened joint prosthesis. J Mat Sci Mater Med. 2001;12(10–12):1069–1073. doi: 10.1023/a:1012858426382. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda M, Hosoda Y, Hirose S, Okada Y, Ikeda E. Expression of vascular endothelial growth factor isoforms and their receptors Flt-1, KDR, and neuropilin-1 in synovial tissues of rheumatoid arthritis. J Pathol. 2000;191(4):426–433. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH649>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 21.De Bandt M, Ben Mahdi MH, Ollivier V, et al. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J Immunol. 2003;171(9):4853–4859. doi: 10.4049/jimmunol.171.9.4853. [DOI] [PubMed] [Google Scholar]
- 22.Fava RA, Olsen NJ, Spencer-Green G, et al. Vascular permeability factor/endothelial growth factor (VPF/VEGF): Accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med. 1994;180(1):341–346. doi: 10.1084/jem.180.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haywood L, McWilliams DF, Pearson CI, et al. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48(8):2173–2177. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- 24.Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102(1):161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 25.Luttun A, Tjwa M, Carmeliet P. Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1): Novel therapeutic targets for angiogenic disorders. Ann N Y Acad Sci. 2002;979:80–93. doi: 10.1111/j.1749-6632.2002.tb04870.x. [DOI] [PubMed] [Google Scholar]
- 26.Stea S, Visentin M, Granchi D, et al. Cytokines and osteolysis around total hip prostheses. Cytokine. 2000;12(10):1575–1579. doi: 10.1006/cyto.2000.0753. [DOI] [PubMed] [Google Scholar]
- 27.Childs LM, Goater JJ, O’Keefe RJ, Schwarz EM. Efficacy of etanercept for wear debris-induced osteolysis. J Bone Miner Res. 2001;16(2):338–347. doi: 10.1359/jbmr.2001.16.2.338. [DOI] [PubMed] [Google Scholar]
- 28.Jones LC, Frondoza C, Hungerford DS. Immunohistochemical evaluation of interface membranes from failed cemented and uncemented acetabular components. J Biomed Mater Res. 1999;48(6):889–898. doi: 10.1002/(sici)1097-4636(1999)48:6<889::aid-jbm19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Min JK, Kim YM, Kim YM, et al. Vascular endothelial growth factor up-regulates expression of receptor activator of NF-kappa B (RANK) in endothelial cells. Concomitant increase of angiogenic responses to RANK ligand. J Biol Chem. 2003;278(41):39548–39557. doi: 10.1074/jbc.M300539200. [DOI] [PubMed] [Google Scholar]
- 30.Niida S, Kaku M, Amano H, et al. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. J Exp Med. 1999;190(2):293–298. doi: 10.1084/jem.190.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kodama I, Niida S, Sanada M, et al. Estrogen regulates the production of VEGF for osteoclast formation and activity in op/op mice. J Bone Miner Res. 2004;19(2):200–206. doi: 10.1359/JBMR.0301229. [DOI] [PubMed] [Google Scholar]
- 32.Kaku M, Kohno S, Kawata T, et al. Effects of vascular endothelial growth factor on osteoclast induction during tooth movement in mice. J Dent Res. 2001;80(10):1880–1883. doi: 10.1177/00220345010800100401. [DOI] [PubMed] [Google Scholar]
- 33.Sawano A, Iwai S, Sakurai Y, et al. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97(3):785–791. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- 34.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101(1):40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87(8):3336–3343. [PubMed] [Google Scholar]
- 36.Matsumoto Y, Tanaka K, Hirata G, et al. Possible involvement of the vascular endothelial growth factor-Flt-1-focal adhesion kinase pathway in chemotaxis and the cell proliferation of osteoclast precursor cells in arthritic joints. J Immunol. 2002;168(11):5824–5831. doi: 10.4049/jimmunol.168.11.5824. [DOI] [PubMed] [Google Scholar]
- 37.Sukbuntherng J, Cropp G, Hannah A, Wagner GS, Shawver LK, Antonian L. Pharmacokinetics and interspecies scaling of a novel VEGF receptor inhibitor, SU5416. J Pharm Pharmacol. 2001;53(12):1629–1636. doi: 10.1211/0022357011778232. [DOI] [PubMed] [Google Scholar]