Abstract
Introduction
In this study, we aimed to determine the relationship between flow-mediated endothelium-dependent vasodilatation (FMD) and carotid artery intima-media wall thickness (IMT), two surrogate markers of atherosclerosis, in a series of Spanish patients with rheumatoid arthritis (RA) without clinically evident cardiovascular (CV) disease.
Methods
One hundred eighteen patients who fulfilled the 1987 American College of Rheumatology classification criteria for RA, had no history of CV disease and had at least one year of follow-up after disease diagnosis were randomly selected. Brachial and carotid ultrasonography were performed to determine FMD and carotid IMT, respectively.
Results
Carotid IMT values were higher and FMD percentages derived by performing ultrasonography were lower in individuals with a long duration from the time of disease diagnosis. Patients with a disease duration ≤ 7 years had significantly lower carotid IMT (mean ± SD) 0.69 ± 0.17 mm than those with long disease duration (0.81 ± 0.12 mm in patients with ≥ 20 years of follow-up). Also, patients with a long disease duration had severe endothelial dysfunction (FMD 4.0 ± 4.0% in patients with disease duration from 14.5 to 19.7 years) compared with those with shorter disease duration (FMD 7.4 ± 3.8% in patients with disease duration ≤ 7 years). Linear regression analysis revealed that carotid IMT was unrelated to FMD in the whole sample of 118 patients. However, carotid IMT was negatively associated with FMD when the time from disease diagnosis ranged from 7.5 to 19.7 years (P = 0.02).
Conclusions
In patients with RA without CV disease, endothelial dysfunction and carotid IMT increased with the duration of RA. The association between FMD and carotid IMT values was observed only in patients with long disease duration.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease associated with increased incidence of cardiovascular (CV) mortality [1,2]. This is the result of accelerated atherosclerosis [3]. Because of the high incidence of CV events observed in patients with RA, an important step forward might be to identify high-risk individuals who would benefit from active therapy to prevent clinical disease. In this regard, several noninvasive imaging techniques offer clinicians a unique opportunity to study the relationship of surrogate markers to the development of atherosclerosis. Among them, ultrasound techniques based on flow velocity and intimal thickness are considered efficient ways to measure subclinical atherosclerosis. Using brachial artery ultrasonography assessment, we and others have found the presence of endothelial dysfunction expressed by abnormal levels of flow-mediated endothelium-dependent vasodilatation (FMD) in patients without clinically evident CV disease who had either long-standing RA [4] or early-onset RA [5]. Moreover, increased carotid artery intima-media wall thickness (IMT) and increased frequency of carotid plaques have been described in RA patients with or without classic CV risk factors compared to ethnically matched controls [6-11]. Also, besides an association of carotid IMT with markers of inflammation [12,13], the duration of the disease has been associated with an increase in carotid IMT [14] and the presence of carotid plaques [8,11]. This is in accordance with data showing progression of carotid IMT in RA patients with long-standing, severe disease despite treatment with anti-TNF-α therapy [15]. Interestingly, a recent observation disclosed that carotid IMT may predict the development of CV events in patients with RA [16].
Since FMD constitutes a physiologic assessment of endothelial dysfunction and carotid IMT is an anatomic structural measure of subclinical atherosclerosis, it is logical that FMD might be a more useful diagnostic marker than carotid IMT in the early stages of the disease. In contrast, carotid IMT might be considered in the assessment of CV risk among patients with long-standing RA.
No relationship between carotid IMT and brachial artery FMD was found in middle-aged men without a history of CV disease who were considered to be at low or intermediate risk for future CV events based on current risk stratification algorithms [17]. However, because of the increased risk of CV events, this may not be the case for patients with long-standing RA.
Taking into consideration all of these factors together, and based on the experience of our group in the study of subclinical atherosclerosis not only in RA but also in other chronic inflammatory rheumatic diseases using ultrasound techniques [18-20], in the present study we aimed to determine the relationship between FMD and carotid IMT in RA patients without clinically evident CV disease.
Materials and methods
Patients and study protocol
Between January 2008 and December 2009, a series of 118 patients attending the rheumatology outpatient clinic of Hospital Xeral-Calde, Lugo, Spain, who fulfilled the 1987 American College of Rheumatology classification criteria for RA [21] and had no history of CV disease and but had at least one year of follow-up from their disease diagnosis were randomly selected for ultrasonographic assessment. To determine whether endothelial dysfunction was present, FMD was assessed by brachial ultrasonography as previously reported [18,22]. An FMD value < 7% was considered pathologic, indicating the presence of endothelial dysfunction [22]. Intraobserver variability for FMD and NTG was 1.3% and 1.9%, respectively, based on repeat brachial ultrasonography in 32 healthy controls. Assessment of the endothelial function of patients undergoing anti-TNF-α therapy was performed 24 to 48 hours before drug administration. Also, carotid ultrasonography was performed to determine carotid artery IMT. IMT was assessed in the right common carotid artery as previously reported [19,22]. On the basis of a second carotid ultrasonography performed in 20 RA patients and 20 healthy controls within one week after the first assessment, the correlation coefficient for carotid IMT was 0.98. The main epidemiological data of this series of patients are shown in Table 1. The patients' written consent was obtained according to the Declaration of Helsinki, and the design of the work was approved by the Ethics Committee of Galicia (Spain).
Table 1.
Main epidemiologic data for 118 patients with RA who underwent ultrasonographya
| Variable | Mean ± SD or number of patients (%) | Median (IQR) |
|---|---|---|
| Age at the time of the study, years | 58.4 ± 12.9 | 59.5 (49 to 68.5) |
| Disease duration from RA diagnosis, years | 13.8 ± 7.7 | 14 (7 to 19.7) |
| Women | 89 (75.4%) | |
| Rheumatoid factor-positive | 96 (81.4%) |
aRA: rheumatoid arthritis, SD: standard deviation, IQR: interquartile range.
Statistical analysis
Quantitative variables are described using means and standard deviations (SDs) and medians and interquartile ranges (IQRs), and qualitative variables are described as numbers and percentages. The relationship between carotid IMT (as a dependent variable) and FMD (as an independent variable) was explored using linear regression, adjusting for gender, age at the time of RA diagnosis and years from RA disease diagnosis to ultrasonographic assessment. To further explore this relationship, we repeated the regression analysis by stratifying patients into quartiles defined by the time from RA diagnosis to ultrasonographic evaluation.
Results
Patients with RA were stratified into four quartiles according to the time from disease diagnosis to ultrasonographic assessment (Table 2). Following this procedure, we observed that carotid IMT values were greater in individuals with a longer duration from disease diagnosis to ultrasonographic assessment (P < 0.001). In this regard, carotid IMT values were higher if time from RA diagnosis was longer than its median (that is, > 14 years). With respect to this observation, individuals with disease duration from RA diagnosis ≤ 7 years had significantly lower carotid IMT wall thickness (0.69 ± 0.17 mm) than did those with long disease duration (0.81 ± 0.12 mm in individuals with at least 20 years of follow-up from the time of RA diagnosis). Likewise, FMD decreased as time from RA diagnosis increased (P < 0.001). As shown in the carotid artery ultrasonographic evaluation, individuals with longer disease duration from disease diagnosis had severe endothelial dysfunction (FMD 4.0 ± 4.0% in RA patients with disease duration between 14.5 and 19.7 years versus 3.3 ± 4.4% in those with disease duration ≥ 20 years) compared with RA patients who had shorter disease duration (FMD 7.4 ± 3.8% in patients with disease duration ≤ 7 years).
Table 2.
Distribution of carotid IMT and FMD in RA patients stratified into four quartiles according to time from disease diagnosis to ultrasonographya
| Time from RA diagnosis to ultrasonography | Carotid IMT (mm) mean ± SD | FMD (%) mean ± SD |
|---|---|---|
| Quartile 1, one to seven years | 0.69 ± 0.17 | 7.4 ± 3.8 |
| Quartile 2, 7.5 to 14 years | 0.68 ± 0.16 | 6.6 ± 4.6 |
| Quartile 3, 14.5 to 19.7 years | 0.84 ± 0.24 | 4.0 ± 4.0 |
| Quartile 4, 20 to 38 years | 0.81 ± 0.12 | 3.3 ± 4.4 |
| P value | < 0.001 | < 0.001 |
aCarotid IMT: carotid intima-media wall thickness, FMD: flow-mediated endothelium-dependent vasodilatation, RA: rheumatoid arthritis.
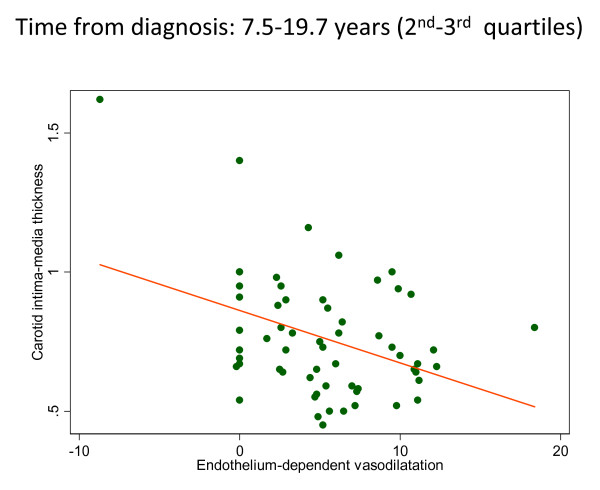
To explore the relationship between FMD and carotid IMT, linear regression analysis was performed. This analysis disclosed that carotid IMT was unrelated to FMD endothelium-dependent vasodilatation in the whole sample of 118 patients (Table 3). However, when patients with RA were stratified according to the time from disease diagnosis until the time of ultrasonography, carotid IMT was negatively associated with FMD when the time from disease diagnosis ranged from 7.5 to 19.7 years (P = 0.02). In patients included in this range of disease duration, the higher FMD percentages were associated with thinner (that is, lower) carotid IMT values (Figure 1). However, in patients with shorter disease duration (one to seven years) or longer disease duration (≥ 20 years), FMD and carotid IMT values remained unrelated (Table 4).
Table 3.
Regression analysis between carotid IMT and FMDa
| Variable | Correlation coefficient (95% confidence interval) | P value |
|---|---|---|
| FMDb | -0.003 (-0.009 to 0.003) | 0.35 |
aCarotid IMT: carotid intima-media wall thickness, FMD: flow-mediated endothelium-dependent vasodilatation, RA: rheumatoid arthritis; bAdjusted for gender, age at the time of disease diagnosis and years from RA diagnosis to ultrasonography assessment.
Figure 1.
Scatterplot illustrating the relationships between FMD and carotid IMT in patients with RA and disease duration ranging between 7 and 20 years derived using linear regression analysis.
Table 4.
Regression analysis between carotid IMT and FMD stratified by disease duration from the time of RA diagnosis until ultrasonography assessmenta
| Time from RA diagnosis until ultrasonography | Correlation coefficient (95% confidence interval) | P value |
|---|---|---|
| Quartile 1, one to seven years | -0.003 (-0.019 to 0.013) | 0.82 |
| Quartiles 2 and 3, 7.5 to 19.7 years | -0.012 (-0.021 to -0.003) | 0.02 |
| Quartile 4, 20 to 38 years | 0.008 (-0.003 to 0.019) | 0.27 |
aCarotid IMT: carotid intima-media wall thickness, FMD: flow-mediated endothelium-dependent vasodilatation, RA: rheumatoid arthritis. Data are adjusted for gender, age at time of diseaseand time from RA diagnosis until ultrasonography.
Discussion
A series of cellular, molecular and pathophysiological events occur during the progression of RA and may also be involved first in endothelial dysfunction and later in atherosclerosis [23]. A healthy endothelium prevents adhesion of mononuclear cells. Inflammation promotes endothelial cell activation, which is characterized by loss of vascular integrity, increased expression of leukocyte adhesion molecules such as selectins, vascular cell adhesion molecule 1 and intercellular adhesion molecule 1 (ICAM-1); change in phenotype from antithrombotic to thrombotic; production of several cytokines; and upregulation of human leukocyte antigen molecules. All of these changes allow endothelial cells to participate in the inflammatory response. In this process, the increased expression of adhesion molecules promotes the adherence and migration of monocytes into the vessel wall. Differentiation of monocytes into macrophages in the intima and activation and further differentiation to form cells characterize the development of early atherosclerotic lesions [24,25]. Continuous endothelial cell activation, manifested by increased levels of the adhesion molecules soluble ICAM-1 and sE-selectin, is present in patients with RA [26]. This endothelial cell activation subsequently leads to endothelial dysfunction, which is an important event in early atherogenesis and also contributes to the development of clinical features in the later stages of the vascular disease, including the progression of atherosclerotic plaque [27]. Interestingly, Kerekes et al. [28] showed that disease duration was associated with impaired FMD and several biomarkers of inflammation. Although in the present study we found no impairment of endothelial function as determined on the basis of brachial FMD in patients with < 7 years' disease duration, longer disease duration, particularly > 14 years, was associated with severe endothelial dysfunction. In keeping with this observation, Södergren et al. [29] found no impairment in endothelial function, measured as FMD, among patients with newly diagnosed RA compared with controls.
With regard to carotid ultrasonography, it is known that a common carotid artery IMT ≥ 0.60 mm is a marker of atherosclerosis [30,31]. In addition, both carotid artery IMT > 0.90 mm and the presence of carotid plaques are considered to be expression of subclinical organ damage and factors influencing CV prognosis in the general population [32]. Interestingly, a recent meta-analysis demonstrated an increased carotid IMT in patients with early RA [33]. Although the increase in carotid IMT in this meta-analysis was much lower than expected in view of the almost doubled CV risk in RA patients [33], carotid IMT was proven to predict the development of CV events in RA patients [16]. Because of that finding, the presence of abnormally high carotid IMT values should be raise clinical suspicions as a sign of the development of CV complications in these patients. In the present study, we observed that individuals with long disease duration (> 14 years) had abnormally high carotid IMT values. With respect to this observation, on the basis of 631 consecutive RA patients, del Rincón et al. [14] showed that IMT increases per unit of age in proportion to RA duration. In del Rincón et al.'s study [14], carotid IMT increased from 0.154 mm/10 years among patients with RA for ≤ 7 years to 0.295 mm/10 years among patients with RA for ≥ 20 years.
In line with the above findings, using electron-beam computed tomography to measure the extent of coronary artery calcification, Chung et al. [34] found that coronary-artery calcification occurred more frequently in patients with established RA than in patients with early RA and controls. A question that needs to be answered is whether carotid ultrasonography and brachial FMD should routinely be performed in all patients with RA to improve CV risk management. With respect to this question, carotid IMT was found to be an independent predictor of vascular events in high-risk individuals without RA in whom risk factors were managed clinically [35]. Since the risk of CV disease is increased in patients with RA, carotid ultrasound might be a potential additional tool for stratifying CV risk in patients with RA [22]. In this regard, a recent study by Evans et al. [36] showed that the presence of carotid plaques in both internal carotid arteries following carotid ultrasonography nearly quadrupled the incidence of new acute coronary syndromes in patients with RA compared with those in RA patients without carotid plaques. On the hand, impaired FMD of the brachial artery due to endothelial dysfunction has been associated with both CV risk factors and future CV morbidity and mortality in the general population [37]. In addition, endothelial dysfunction manifested by impaired FMD was observed in both long-standing RA patients [4] and early-onset RA patients [5] without clinically evident CV disease. These observations support a potential role of FMD in establishing the presence of endothelial dysfunction as a subclinical marker of atherosclerotic disease in RA. They may also provide a basis for the association between RA and atherosclerotic disease.
Of main clinical relevance may be the improvement in endothelial function observed in patients with RA following treatment with TNF-α blockers [38-40] or rituximab [41,42]. However, the beneficial effect of the TNF-α antagonist infliximab on endothelial dysfunction seems to be only temporary [43]. Conflicting results have been described regarding the effects of biologic agents on carotid atherosclerosis [44]. In this regard, while some patients showed significant improvement in carotid IMT following TNF-α blocker therapy [45], others did not experience reduction of IMT following treatment with these drugs [15,40,46].
Previous observations showed no relationship between carotid IMT and brachial artery FMD in middle-aged men at low and intermediate risk of experiencing future CV events [17]. However, the situation might not be the same in patients with long-standing RA. Therefore, we aimed to determine the relationship between both techniques in patients with RA. On the basis of linear regression analysis, we found that carotid IMT was unrelated to FMD in the whole sample of 118 patients. This observation was also in keeping with another study performed in elderly individuals that showed no correlation between brachial FMD and carotid IMT [47]. However, our data suggest that in patients with disease duration ranging between from 7.5 to 19.7 years, carotid IMT is negatively associated with brachial FMD.
Conclusions
In summary, our results reinforce the importance of disease duration in the development of atherosclerosis in patients with RA. Brachial FMD and carotid IMT may indicate distinct and independent stages in the complex pathways leading to accelerated atherosclerosis in patients with RA.
Abbreviations
CV: cardiovascular; IMT: intima-media thickness; IQR: interquartile range; FMD: endothelium-dependent flow-mediated vasodilatation; RA: rheumatoid arthritis; SD: standard deviation.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CGJ performed the ultrasonographic studies, participated in the design of the study and helped to draft the manuscript. JL participated in the design of the study and data analysis and helped to draft the manuscript. MAGG made substantial contributions to the conception and design of the study, the acquisition of data and the coordination of the study. All authors read and approved the final version of the manuscript to be published.
Contributor Information
Carlos González-Juanatey, Email: carlosjuanatey@secardiologia.es.
Javier Llorca, Email: llorcaj@unican.es.
Miguel A González-Gay, Email: miguelaggay@hotmail.com.
Acknowledgements
This study was supported by "Fondo de Investigaciones Sanitarias" grants PI06-0024 and PS09/00748 (Spain). This work was partially supported by the RETICS Program, RD08/0075 (RIER), from "Instituto de Salud Carlos III" (ISCIII).
References
- Goodson N, Marks J, Lunt M, Symmons D. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann Rheum Dis. 2005;64:1595–1601. doi: 10.1136/ard.2004.034777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.CIR.0000054612.26458.B2. [DOI] [PubMed] [Google Scholar]
- González-Gay MA, González-Juanatey C, Martin J. Rheumatoid arthritis: a disease associated with accelerated atherogenesis. Semin Arthritis Rheum. 2005;35:8–17. doi: 10.1016/j.semarthrit.2005.03.004. [DOI] [PubMed] [Google Scholar]
- González-Juanatey C, Testa A, Garcia-Castelo A, Garcia-Porrua C, Llorca J, Vidan J, Hajeer AH, Ollier WE, Mattey DL, González-Gay MA. HLA-DRB1 status affects endothelial function in treated patients with rheumatoid arthritis. Am J Med. 2003;114:647–652. doi: 10.1016/S0002-9343(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Vaudo G, Marchesi S, Gerli R, Allegrucci R, Giordano A, Siepi D, Pirro M, Shoenfeld Y, Schillaci G, Mannarino E. Endothelial dysfunction in young patients with rheumatoid arthritis and low disease activity. Ann Rheum Dis. 2004;63:31–35. doi: 10.1136/ard.2003.007740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumeda Y, Inaba M, Goto H, Nagata M, Henmi Y, Furumitsu Y, Ishimura E, Inui K, Yutani Y, Miki T, Shoji T, Nishizawa Y. Increased thickness of the arterial intima-media detected by ultrasonography in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:1489–1497. doi: 10.1002/art.10269. [DOI] [PubMed] [Google Scholar]
- Park YB, Ahn CW, Choi HK, Lee SH, In BH, Lee HC, Nam CM, Lee SK. Atherosclerosis in rheumatoid arthritis: morphologic evidence obtained by carotid ultrasound. Arthritis Rheum. 2002;46:1714–1719. doi: 10.1002/art.10359. [DOI] [PubMed] [Google Scholar]
- González-Juanatey C, Llorca J, Testa A, Revuelta J, Garcia-Porrua C, González-Gay MA. Increased prevalence of severe subclinical atherosclerotic findings in long-term treated rheumatoid arthritis patients without clinically evident atherosclerotic disease. Medicine (Baltimore) 2003;82:407–413. doi: 10.1097/01.md.0000101572.76273.60. [DOI] [PubMed] [Google Scholar]
- Alkaabi JK, Ho M, Levison R, Pullar T, Belch JJ. Rheumatoid arthritis and macrovascular disease. Rheumatology (Oxford) 2003;42:292–297. doi: 10.1093/rheumatology/keg083. [DOI] [PubMed] [Google Scholar]
- Abu-Shakra M, Polychuck I, Szendro G, Bolotin A, Jonathan BS, Flusser D, Buskila D, Sukenik S. Duplex study of the carotid and femoral arteries of patients with rheumatoid arthritis: a controlled study. Semin Arthritis Rheum. 2005;35:18–23. doi: 10.1016/j.semarthrit.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Roman MJ, Moeller E, Davis A, Paget SA, Crow MK, Lockshin MD, Sammaritano L, Devereux RB, Schwartz JE, Levine DM, Salmon JE. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med. 2006;144:249–256. doi: 10.7326/0003-4819-144-4-200602210-00006. [DOI] [PubMed] [Google Scholar]
- del Rincón I, Williams K, Stern MP, Freeman GL, O'Leary DH, Escalante A. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833–1840. doi: 10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- González-Gay MA, González-Juanatey C, Piñeiro A, Garcia-Porrua C, Testa A, Llorca J. High-grade C-reactive protein elevation correlates with accelerated atherogenesis in patients with rheumatoid arthritis. J Rheumatol. 2005;32:1219–1223. [PubMed] [Google Scholar]
- del Rincón I, O'Leary DH, Freeman GL, Escalante A. Acceleration of atherosclerosis during the course of rheumatoid arthritis. Atherosclerosis. 2007;195:354–360. doi: 10.1016/j.atherosclerosis.2006.09.027. [DOI] [PubMed] [Google Scholar]
- González-Juanatey C, Llorca J, Garcia-Porrua C, Martin J, González-Gay MA. Effect of anti-tumor necrosis factor α therapy on the progression of subclinical atherosclerosis in severe rheumatoid arthritis. Arthritis Rheum. 2006;55:150–153. doi: 10.1002/art.21707. [DOI] [PubMed] [Google Scholar]
- González-Juanatey C, Llorca J, Martin J, González-Gay MA. Carotid intima-media thickness predicts the development of cardiovascular events in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2009;38:366–371. doi: 10.1016/j.semarthrit.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Yan RT, Anderson TJ, Charbonneau F, Title L, Verma S, Lonn E. Relationship between carotid artery intima-media thickness and brachial artery flow-mediated dilation in middle-aged healthy men. J Am Coll Cardiol. 2005;45:1980–1986. doi: 10.1016/j.jacc.2004.12.079. [DOI] [PubMed] [Google Scholar]
- González-Juanatey C, Llorca J, Miranda-Filloy JA, Amigo-Diaz E, Testa A, Garcia-Porrua C, Martin J, González-Gay MA. Endothelial dysfunction in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57:287–293. doi: 10.1002/art.22530. [DOI] [PubMed] [Google Scholar]
- González-Juanatey C, Llorca J, Amigo-Diaz E, Dierssen T, Martin J, González-Gay MA. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57:1074–1080. doi: 10.1002/art.22884. [DOI] [PubMed] [Google Scholar]
- González-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, Dierssen T, Vaqueiro I, Blanco R, Martin J, Llorca J, González-Gay MA. The high prevalence of subclinical atherosclerosis in patients with ankylosing spondylitis without clinically evident cardiovascular disease. Medicine (Baltimore) 2009;88:358–365. doi: 10.1097/MD.0b013e3181c10773. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA Jr, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- González-Gay MA, González-Juanatey C, Vazquez-Rodriguez TR, Martin J, Llorca J. Endothelial dysfunction, carotid intima-media thickness, and accelerated atherosclerosis in rheumatoid arthritis. Semin Arthritis Rheum. 2008;38:67–70. doi: 10.1016/j.semarthrit.2008.02.001. [DOI] [PubMed] [Google Scholar]
- González-Gay MA, González-Juanatey C, Martin J. Inflammation and endothelial dysfunction in rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:115–117. [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Bijl M. Endothelial activation, endothelial dysfunction and premature atherosclerosis in systemic autoimmune diseases. Neth J Med. 2003;61:273–277. [PubMed] [Google Scholar]
- Wållberg-Jonsson S, Cvetkovic JT, Sundqvist KG, Lefvert AK, Rantapää-Dahlqvist S. Activation of the immune system and inflammatory activity in relation to markers of atherothrombotic disease and atherosclerosis in rheumatoid arthritis. J Rheumatol. 2002;29:875–882. [PubMed] [Google Scholar]
- Vita JA, Keaney JF Jr. Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.CIR.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- Kerekes G, Szekanecz Z, Dér H, Sándor Z, Lakos G, Muszbek L, Csipö I, Sipka S, Seres I, Paragh G, Kappelmayer J, Szomják E, Veres K, Szegedi G, Shoenfeld Y, Soltész P. Endothelial dysfunction and atherosclerosis in rheumatoid arthritis: a multiparametric analysis using imaging techniques and laboratory markers of inflammation and autoimmunity. J Rheumatol. 2008;35:398–406. [PubMed] [Google Scholar]
- Södergren A, Karp K, Boman K, Eriksson C, Lundström E, Smedby T, Söderlund L, Rantapää-Dahlqvist S, Wållberg-Jonsson S. Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Res Ther. 2010;12:R158. doi: 10.1186/ar3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veller MG, Fisher CM, Nicolaides AN, Renton S, Geroulakos G, Stafford NJ, Sarker A, Szendro G, Belcaro G. Measurement of the ultrasonic intima-media complex thickness in normal subjects. J Vasc Surg. 1993;17:719–725. doi: 10.1067/mva.1993.41133. [DOI] [PubMed] [Google Scholar]
- Belcaro G, Nicolaides AN, Ramaswami G, Cesarone MR, De Sanctis M, Incandela L, Ferrari P, Geroulakos G, Barsotti A, Griffin M, Dhanjil S, Sabetai M, Bucci M, Martines G. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the CAFES-CAVE study) Atherosclerosis. 2001;156:379–387. doi: 10.1016/S0021-9150(00)00665-1. [DOI] [PubMed] [Google Scholar]
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P. Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology et al. Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. A published erratum appears in J Hypertens 2007, 25:1749. [DOI] [PubMed] [Google Scholar]
- van Sijl AM, Peters MJ, Knol DK, de Vet HC, González-Gay MA, Smulders YM, Dijkmans BA, Nurmohamed MT. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum. 2011;40:389–397. doi: 10.1016/j.semarthrit.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, Pincus T, Avalos I, Stein CM. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52:3045–3053. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Hougaku H, Yamagami H, Hashimoto H, Itoh T, Shimizu Y, Takahashi D, Murata S, Seike Y, Kondo K, Hoshi T, Furukado S, Abe Y, Yagita Y, Sakaguchi M, Tagaya M, Etani H, Fukunaga R, Nagai Y, Matsumoto M, Hori M. OSACA2 Study Group. Carotid intima-media thickness and risk of cardiovascular events in high-risk patients: results of the Osaka Follow-Up Study for Carotid Atherosclerosis 2 (OSACA2 Study) Cerebrovasc Dis. 2007;24:35–42. doi: 10.1159/000103114. [DOI] [PubMed] [Google Scholar]
- Evans MR, Escalante A, Battafarano DF, Freeman GL, O'Leary DH, del Rincón I. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211–1120. doi: 10.1002/art.30265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Avest E, Stalenhoef AF, de Graaf J. What is the role of non-invasive measurements of atherosclerosis in individual cardiovascular risk prediction? Clin Sci (Lond) 2007;112:507–516. doi: 10.1042/CS20060228. [DOI] [PubMed] [Google Scholar]
- Hürlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, Béchir M, Spieker LE, Neidhart M, Michel BA, Gay RE, Lüscher TF, Gay S, Ruschitzka F. Anti-tumor necrosis factor-α treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–2187. doi: 10.1161/01.CIR.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- González-Juanatey C, Llorca J, Sanchez-Andrade A, Garcia-Porrua C, Martin J, González-Gay MA. Short-term adalimumab therapy improves endo-thelial function in patients with rheumatoid arthritis refractory to infliximab. Clin Exp Rheumatol. 2006;24:309–312. [PubMed] [Google Scholar]
- Sidiropoulos PI, Siakka P, Pagonidis K, Raptopoulou A, Kritikos H, Tsetis D, Boumpas DT. Sustained improvement of vascular endothelial function during anti-TNFα treatment in rheumatoid arthritis patients. Scand J Rheumatol. 2009;38:6–10. doi: 10.1080/03009740802363768. [DOI] [PubMed] [Google Scholar]
- González-Juanatey C, Llorca J, Vazquez-Rodriguez TR, Diaz-Varela N, Garcia-Quiroga H, González-Gay MA. Short-term improvement of endothelial function in rituximab-treated rheumatoid arthritis patients refractory to tumor necrosis factor α blocker therapy. Arthritis Rheum. 2008;59:1821–1824. doi: 10.1002/art.24308. [DOI] [PubMed] [Google Scholar]
- Kerekes G, Soltész P, Dér H, Veres K, Szabó Z, Végvári A, Szegedi G, Shoenfeld Y, Szekanecz Z. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin Rheumatol. 2009;28:705–710. doi: 10.1007/s10067-009-1095-1. [DOI] [PubMed] [Google Scholar]
- González-Juanatey C, Testa A, Garcia-Castelo A, Garcia-Porrua C, Llorca J, González-Gay MA. Active but transient improvement of endothelial function in rheumatoid arthritis patients undergoing long-term treatment with anti-tumor necrosis factor α antibody. Arthritis Rheum. 2004;51:447–450. doi: 10.1002/art.20407. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Kerekes G, Soltész P. Vascular effects of biologic agents in RA and spondyloarthropathies. Nat Rev Rheumatol. 2009;5:677–684. doi: 10.1038/nrrheum.2009.219. [DOI] [PubMed] [Google Scholar]
- Del Porto F, Laganà B, Lai S, Nofroni I, Tinti F, Vitale M, Podestà E, Mitterhofer AP, D'Amelio R. Response to anti-tumour necrosis factor α blockade is associated with reduction of carotid intima-media thickness in patients with active rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1111–1115. doi: 10.1093/rheumatology/kem089. [DOI] [PubMed] [Google Scholar]
- Wong M, Oakley SP, Young L, Jiang BY, Wierzbicki A, Panayi G, Chowienczyk P, Kirkham B. Infliximab improves vascular stiffness in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68:1277–1284. doi: 10.1136/ard.2007.086157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeboah J, Burke GL, Crouse JR, Herrington DM. Relationship between brachial flow-mediated dilation and carotid intima-media thickness in an elderly cohort: the Cardiovascular Health Study. Atherosclerosis. 2008;197:840–845. doi: 10.1016/j.atherosclerosis.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]