Abstract
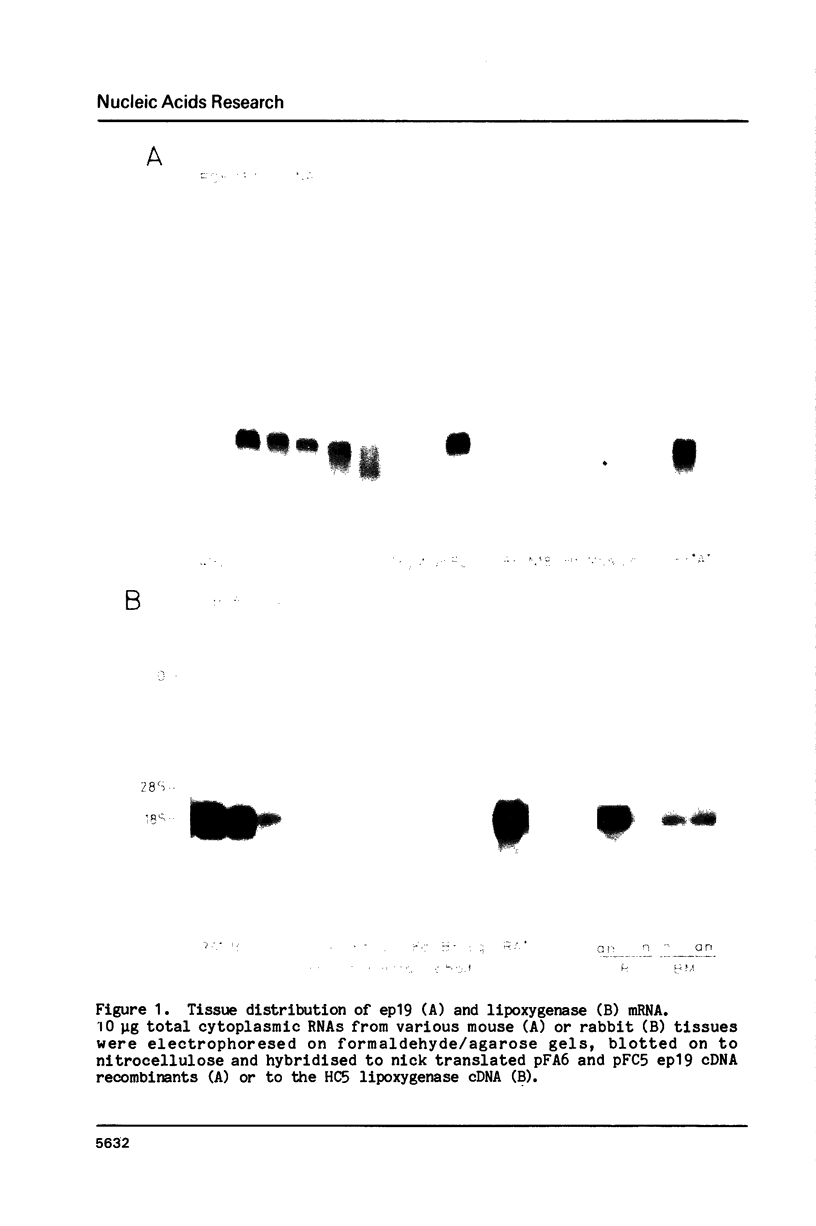
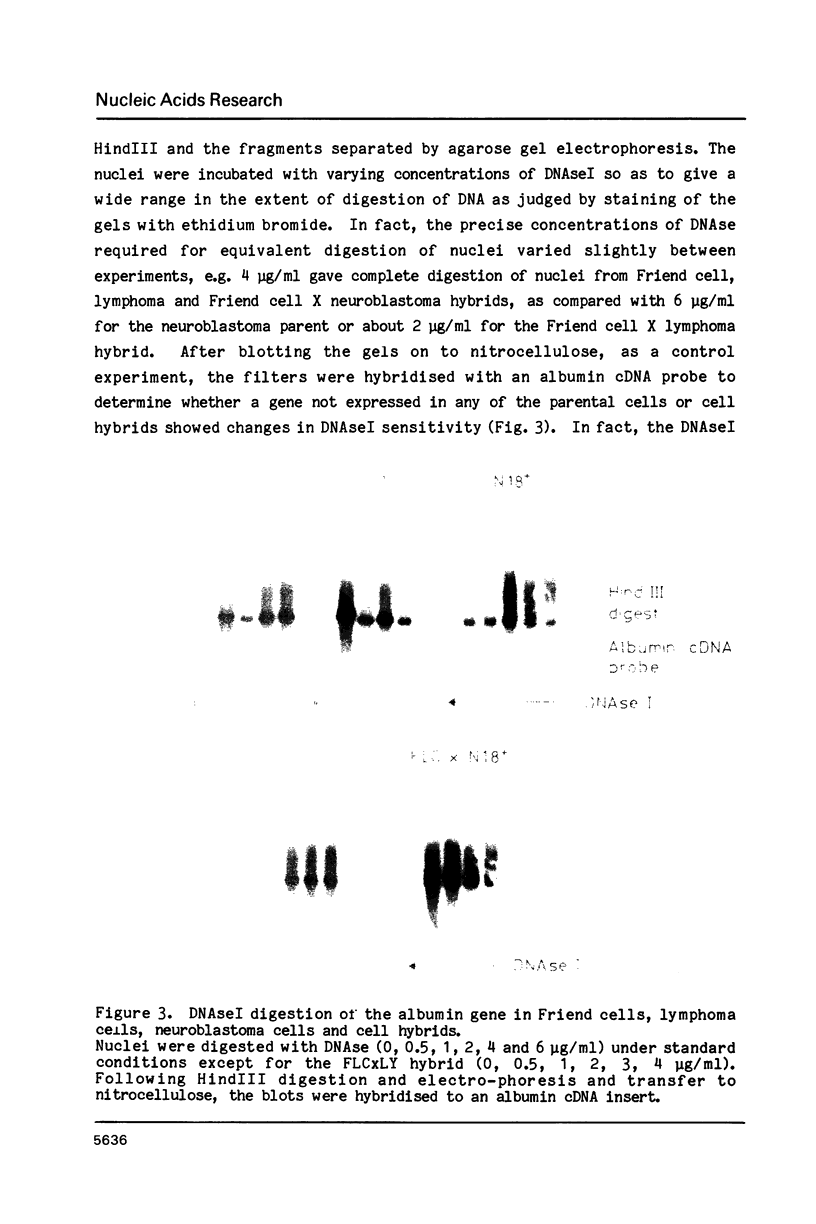
Red blood cell differentiation involves the coordinate expression of a set of polypeptides some of which are erythroid-specific (the abundant globins as well as minor species such as glycophorin, carbonic anhydrase I and the RBC lipoxygenase) whereas others are found also in a subset of other cells, e.g. beta spectrin and a 19 kd polypeptide (ep 19) found in adult liver and kidney as well as erythroid cells. To investigate the genetic mechanisms involved in the regulation of these classes of genes, the expression of lipoxygenase, ep 19 and beta globin mRNAs was investigated in cell hybrids between mouse erythroid (Friend) cells and mouse T-lymphoma or neuroblastoma cells. All three mRNAs are expressed or repressed together in cell hybrids between the Friend cell and lymphoma or neuroblastoma cells respectively. Moreover, studies of the chromatin structure surrounding the genes reveal that erythroid cell-specific DNaseI hypersensitive sites within the ep 19 and beta major globin genes are lost in the Friend cell X neuroblastoma hybrids whereas they are retained in the Friend cell X lymphoma cell hybrids. This implies that the trans-acting mechanism responsible for regulating the RBC phenotype in these cell hybrids acts at the level of the early chromatin changes thought to reflect a pre-activation stage in gene expression.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affara N., Goldfarb P. S., Yang Q. S., Harrison P. R. Patterns of expression of erythroblast non-globin mRNAs. Nucleic Acids Res. 1983 Feb 25;11(4):931–945. doi: 10.1093/nar/11.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan M., Harrison P. Co-expression of differentiation markers in hybrids between Friend cells and lymphoid cells and the influence of the cell shape. Cell. 1980 Feb;19(2):437–447. doi: 10.1016/0092-8674(80)90518-8. [DOI] [PubMed] [Google Scholar]
- Allan M., Lanyon W. G., Paul J. Multiple origins of transcription in the 4.5 Kb upstream of the epsilon-globin gene. Cell. 1983 Nov;35(1):187–197. doi: 10.1016/0092-8674(83)90221-0. [DOI] [PubMed] [Google Scholar]
- Allan M., Zhu J. D., Montague P., Paul J. Differential response of multiple epsilon-globin cap sites to cis- and trans-acting controls. Cell. 1984 Sep;38(2):399–407. doi: 10.1016/0092-8674(84)90495-1. [DOI] [PubMed] [Google Scholar]
- Axelrod D. E., Gopalakrishnan T. V., Willing M., Anderson W. F. Maintenance of hemoglobin inducibility in somatic cell hybrids of tetraploid (2S) mouse erythroleukemia cells with mouse or human fibroblasts. Somatic Cell Genet. 1978 Mar;4(2):157–168. doi: 10.1007/BF01538981. [DOI] [PubMed] [Google Scholar]
- Coggins L. W., Vass J. K., Stinson M. A., Lanyon W. G., Paul J. A B1 repetitive sequence near the mouse beta-major globin gene. Gene. 1982 Jan;17(1):113–116. doi: 10.1016/0378-1119(82)90107-x. [DOI] [PubMed] [Google Scholar]
- Davidson R. L. Gene expression in somatic cell hybrids. Annu Rev Genet. 1974;8:195–218. doi: 10.1146/annurev.ge.08.120174.001211. [DOI] [PubMed] [Google Scholar]
- Davis F. M., Adelberg E. A. Use of somatic cell hybrids for analysis of the differentiated state. Bacteriol Rev. 1973 Jun;37(2):197–214. doi: 10.1128/br.37.2.197-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth A., Hendrick D. Activation of phenotypic expression of human globin genes from nonerythroid cells by chromosome-dependent transfer to tetraploid mouse erythroleukemia cells. Proc Natl Acad Sci U S A. 1979 May;76(5):2185–2189. doi: 10.1073/pnas.76.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson B. M., Felsenfeld G. Specific factor conferring nuclease hypersensitivity at the 5' end of the chicken adult beta-globin gene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):95–99. doi: 10.1073/pnas.81.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb P. S., O'Prey J., Affara N., Yang Q. S., Harrison P. R. Isolation of non-globin genes expressed preferentially in mouse erythroid cells. Nucleic Acids Res. 1983 Jun 11;11(11):3517–3530. doi: 10.1093/nar/11.11.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Propagation of globin DNAase I-hypersensitive sites in absence of factors required for induction: a possible mechanism for determination. Cell. 1982 Aug;30(1):131–139. doi: 10.1016/0092-8674(82)90019-8. [DOI] [PubMed] [Google Scholar]
- Harrison P. R., Affara N., McNab A., Paul J. Erythroid differentiation in a Friend erythroleukemic cell X lymphoma hybrid cell line is limited, possibly due to reduced hem levels. Exp Cell Res. 1977 Oct 15;109(2):237–246. doi: 10.1016/0014-4827(77)90002-7. [DOI] [PubMed] [Google Scholar]
- Harrison P. R. Molecular analysis of erythropoiesis. A current appraisal. Exp Cell Res. 1984 Dec;155(2):321–344. doi: 10.1016/0014-4827(84)90194-0. [DOI] [PubMed] [Google Scholar]
- Jongstra J., Reudelhuber T. L., Oudet P., Benoist C., Chae C. B., Jeltsch J. M., Mathis D. J., Chambon P. Induction of altered chromatin structures by simian virus 40 enhancer and promoter elements. Nature. 1984 Feb 23;307(5953):708–714. doi: 10.1038/307708a0. [DOI] [PubMed] [Google Scholar]
- Keene M. A., Corces V., Lowenhaupt K., Elgin S. C. DNase I hypersensitive sites in Drosophila chromatin occur at the 5' ends of regions of transcription. Proc Natl Acad Sci U S A. 1981 Jan;78(1):143–146. doi: 10.1073/pnas.78.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Ley T. J., Chiang Y. L., Haidaris D., Anagnou N. P., Wilson V. L., Anderson W. F. DNA methylation and regulation of the human beta-globin-like genes in mouse erythroleukemia cells containing human chromosome 11. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6618–6622. doi: 10.1073/pnas.81.21.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M. W., Featherstone M. S., Kaplan H. Activation of teratocarcinoma-derived hemoglobin genes in teratocarcinoma-Friend cell hybrids. Cell. 1978 Dec;15(4):1323–1330. doi: 10.1016/0092-8674(78)90057-0. [DOI] [PubMed] [Google Scholar]
- McBurney M., Craig J., Stedman D., Featherstone M. Expression of globin genes in teratocarcinoma--Friend cell hybrids. Exp Cell Res. 1981 Feb;131(2):277–282. doi: 10.1016/0014-4827(81)90232-9. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Shermoen A. W., Heemskerk J., Beckendorf S. K. DNA sequence changes in an upstream DNase I-hypersensitive region are correlated with reduced gene expression. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1063–1067. doi: 10.1073/pnas.80.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffery M., Rifkind R. A., Marks P. A. Murine erythroleukemia cell differentiation: DNase I hypersensitivity and DNA methylation near the globin genes. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1180–1184. doi: 10.1073/pnas.79.4.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wu C., Gilbert W. Tissue-specific exposure of chromatin structure at the 5' terminus of the rat preproinsulin II gene. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1577–1580. doi: 10.1073/pnas.78.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]