Abstract
In recent years, fever control in critically ill patients by medications and/or external cooling has gained widespread use, notably in patients suffering from neurological injuries. Nevertheless, such a strategy in septic patients is not supported by relevant data. Indeed, in response to sepsis, experimental and clinical studies argue that fever plays a key role in increasing the clearance of microorganisms, the immune response and the heat shock response. Moreover, fever is a cornerstone diagnostic sign in clinical practice, which aids in early and appropriate therapy, and allows physicians to follow the infection course. After discussing the physiological aspects of fever production, the present review aims to delineate the advantages and drawbacks of fever in septic patients. Finally, the treatment of fever by pharmacological and/or physical means is discussed with regards to their drawbacks, which argues for their careful use in septic patients in the absence of clinical relevance.
Introduction
Fever is a nonspecific response to various types of infectious or non-infectious stimuli. The incidence in ICUs ranges from 23 to 70% and is related to an infectious process in only one-half of the cases [1-3]. In the past two decades, numerous studies have underlined the deleterious effects of fever on outcome, especially in neurological diseases, leading to active treatment of fever with medications and/or various physical means [4]. However, the rationale for extending such a strategy to septic patients is not supported by clinical data, and several lines of evidence suggest that fever in these patients may be helpful to fight the infectious process. Some practices, such as fever-induced discomfort and/or favoured febrile seizures, have been reconsidered [5-8]. Moreover, fever is a cornerstone diagnostic sign in clinical practice that helps to start early appropriate therapy and to follow the infection course. Besides, sepsis biomarkers (that is, pro-calcitonin, C-reactive protein) have to prove their relevance [9]. Finally, antipyretic therapies have side effects that must be taken into account when physicians decide to control fever.
The objective of the present review is to delineate the advantages and drawbacks of fever in septic patients. The main side effects of antipyretic therapies are also emphasised.
Definition and pathophysiology of fever
The core body temperature is tightly regulated around a set point by homeostatic mechanisms under normal physiological conditions. Nevertheless, there is a female hormonal-induced variation and a diurnal variation. So, Mackowiak and colleagues found that the mean temperature was 36.8°C, with a range of 35.6 to 38.2°C, the temperature being lower in the morning than in the evening [1].
Fever is an upregulation of the hypothalamic temperature and is often difficult to differentiate from hyperthermic syndromes. In the latter, the setpoint remains unchanged but involves a dysregulation of peripheral mechanisms of heat production or loss. The threshold value of fever differs between epidemiological ICU studies, ranging from 38.3 to 38.5°C [2,10,11], but a threshold value of ≥38.3°C has been recommended by several societies for the diagnosis of fever [12,13]. This definition has to be considered with regards to the methods used to determine the temperature. Indeed, the core temperature is important as a core to peripheral temperature gradient is common in critically ill patients, especially in those who are hypovolaemic, have a low cardiac output or are peripherally vasoconstricted. In the ICU, the temperature reference is provided by the thermistance of the pulmonary artery catheter, but most of the patients have no such device in place. In addition, comparison with other methods of temperature measurement is far from being well correlated. Accordingly, it has been shown among different methods of temperature measurement that better accuracy was obtained for the urinary or oesophageal temperature [14,15].
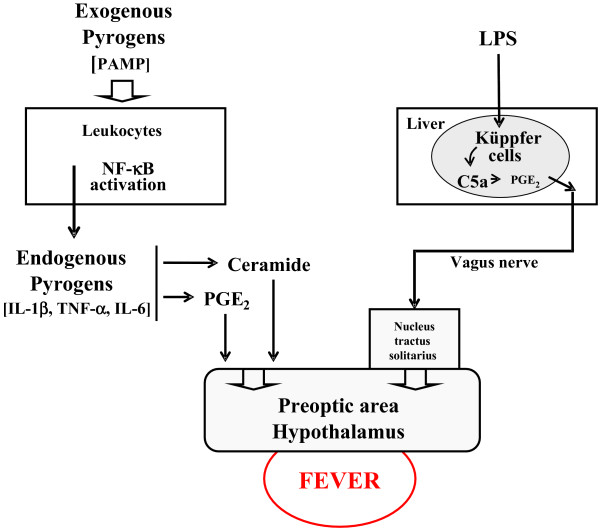
Fever is a preserved phylogenetic response to a wide variety of infectious and non-infectious triggers, which induce, by different methods, upregulation of the thermostatic setpoint in the preoptic area of the hypothalamus, finally resulting in fever. Several methods of activation of the hypothalamus have been described (Figure 1). Classically, the pathogen-associated molecular patterns (called exogenous pyrogens) stimulate leukocytes, which in turn produce cytokines (called endogenous pyrogens), mainly IL-1β, TNFα and IL-6 [16]. These endogenous pyrogens activate the febrile response indirectly, by inducing secretion of prostaglandin E2 in the organum vasculosum of the lamina propria located just below the preoptic area of the hypothalamus. Two other methods are recognised. The first, recently described, is also mediated by IL-1β but involves ceramide production by an enzymatic pathway (neutral sphingomyelinase) [17]. Ceramide therefore acts as a second messenger in place of prostaglandin E2, which explains the early rise in core temperature [17]. The remaining method is neuronal and independent of cytokine production. The Küppfer cells stimulated by lipopolysaccharide produce prostaglandin E2, which in turn elicits a hypothalamic response through a neural pathway mediated by the vagus nerve and the nucleus tractus solitarius [18,19]. These pathophysiological considerations explain why fever may be induced by inflammation or infection.
Figure 1.
Proposed methods of activation of the hypothalamus. LPS, lipopolysaccharide; PAMP, pathogen-associated molecular pattern; PGE2, prostaglandin E2.
Beneficial effects of fever
While many years of clinical observations and several published observational studies suggest fever is beneficial to the host, it is important to emphasise the lack of robust clinical evidence concerning the assessment of fever's benefits. How fever could influence outcome in septic patients is a key issue that remains debated because of the limited value of studies that included a heterogeneous population of patients with different levels of severity of sepsis. Nevertheless, a set of arguments can help enlighten this issue. Indeed, there are direct arguments that refer to the beneficial effects of fever per se and indirect arguments that reflect the noxious effects of fever suppression.
Direct arguments
Fever effects on infectious agents
Fever has an impact on microorganism growth. Human pathogen infectious agents usually grow under optimal temperatures of around 35 to 37°C [20]. In experimental meningitis, the elevated body temperature increases the pneumococci growth time in cerebral spinal fluid when compared with a blunted febrile response induced by urethane [21]. Similarly, an in vitro study on Plasmodium falciparum concluded that febrile temperatures play a role in inhibiting parasite growth [22]. Experimentally, increasing the temperature from 35 to 41.5°C on 432 strains of bacteria revealed a progressive rise in the activity of antimicrobial agents (17 antimicrobial agents tested) and a reduction in the minimum inhibitory concentrations [23].
Effects of fever on immunity and heat shock response
Fever is also known to modulate the cellular immune response and to induce the heat shock response. Hyperthermic preconditioning of a rat model of peritonitis reduced the severity of infection, prevented a decrease in the number of CD4 lymphocytes and B cells, and decreased the serum level of the proinflammatory cytokine TNFα [24]. Furthermore, other studies reported an increase in the mobility of polymorphonuclear cells, in the phagocytosis speed, in lymphocyte recruitment, in adherence of T-helper lymphocytes to L-selectin, in immunoglobulin levels and in TNFα cytotoxicity in response to elevated temperature [25].
Heat shock proteins are critical for cellular protection in reducing endothelial and organ damage during several stresses including fever. Recent data demonstrated that a heat shock response can downregulate the activity of NF-κB, modulating the immune response [26]. Reduced mortality and organ injury were reported after heat pretreatment in a rat model of intra-abdominal sepsis and sepsis-induced lung injury, with increased levels of HSP-72 in the lungs and heart of the heat-treated animals [27]. More recently, in a sheep model of peritonitis, febrile animals had a longer survival time with concomitant higher HSP-70 levels when compared with the other animals [28].
Clinical data
Direct clinical evidence is supported by old studies and more recent studies. A retrospective analysis of 218 patients with Gram-negative bacilli bacteraemia reported significantly higher survival in patients who developed fever on the day of bacteraemia [29]. The mortality of patients with spontaneous bacterial peritonitis was reduced when the body temperature was >38°C [30]. In the same disease, a positive correlation between body temperature increase and survival has been shown [31]. In elderly patients with community-acquired pneumonia, a higher mortality rate was observed in patients who lacked fever when compared with patients who developed a febrile response (29% vs. 4%) [32]. More recently, the multicenter French AmarCand study pointed out that fever >38.2°C was a protective factor in invasive Candida spp. infections in the ICU [33]. In a selected population of ICU-infected patients, both hypothermia and fever increased morbidity and mortality rates, but patients with hypothermia had a higher mortality when compared with those who had fever (80% vs. 47%) [11]. In a similar selected population, Arons and colleagues reported an increased mortality in hypothermic patients. Interestingly, the inflammatory response was increased in these patients when compared with febrile patients, suggesting a protective effect of fever per se [34].
Indirect arguments
Experimental data
Beneficial effects of fever are reported in several experimental studies. Ectothermic desert lizards (Dipsosaurus dorsalis) infected by Aeromonas hydrophilia had a greater survival rate when they were placed in a warm environment [35]. Subsequently, in the same model, the suppression of fever by an injection of sodium salicylate was demonstrated to dramatically increase mortality [36]. Similarly, in a murine bacterial peritonitis model, increasing the core temperature by housing mice in a 35.5°C ambient temperature led to an improved survival rate when compared with animals placed in a cooler environment. Moreover, TNFα expression was suppressed in the early 48 hours and IFNγ expression was delayed. Interestingly, after animal sacrifice, significantly lower concentrations of bacteria per organ were observed in animals with fever when compared with cooled animals [37].
Su and colleagues explored the effects of controlling fever with paracetamol or external cooling in a sheep septic shock model. The febrile animals had better respiratory function and a prolonged survival time [28]. Finally, a recent meta-analysis that included eight studies on influenza-infected animals reported an increased risk of mortality when the animals received various anti-pyretic treatments (odds ratio = 1.34, 95% confidence interval = 1.04 to 1.73) [38].
Clinical data
Several clinical studies indirectly advocate a beneficial effect of fever. For instance, in a placebo-controlled trial, Graham and colleagues compared the effects of aspirin and paracetamol on virus shedding, immune response and clinical status in rhinovirus-infected volunteers. In the aspirin and paracetamol group, a longer duration of virus shedding and suppression of serum-neutralising antibody response were observed [5]. In addition, another randomised trial showed that treatment of fever with paracetamol in P. falciparum malaria-infected children prolonged the parasite clearance time when compared with untreated children [39]. A more recent study demonstrated that prophylactic administration of antipyretic drugs at the time of vaccination induced a delayed and lower antibody response to several vaccine antigens, although paracetamol similarly affected antibody response in children with or without fever [40]. Finally, a randomised study in febrile surgical and trauma critically ill patients to assess the impact of antipyretic therapy on infection development was interrupted after the first interim analysis, because of higher mortality in the antipyretic group (seven deaths vs. one death, P = 0.06). Moreover, the infection rate tended to be higher in the treated group (4 ± 6 per patient vs. 3 ± 2 per patient, P = 0.26) [41].
These indirect data reinforce the concept that fever may play a role in the survival of septic patients, although the impact of antipyretics on morbidity cannot be excluded.
Detrimental effects of fever
Even though the febrile response seems useful in the adaptive reaction to a stressful situation, it could cause several detrimental effects on clinical outcomes. Indeed, fever increases metabolic demand and consequently oxygen consumption of different organs, notably the brain and the heart, and worsens pre-existing disease. For instance, in neurological injuries, fever is now a wellrecognised factor of secondary cerebral insult and contributes to deterioration of the clinical outcome [4]. In acute ischaemic stroke, studies suggest that fever is strongly associated with significant morbidity and a mortality increase up to 20% [42,43]. A similar issue is raised in traumatic brain injuries in which fever is responsible for overwhelming secondary brain injuries [44]. In neurological injuries, therefore, the control of fever is a major therapeutic axis to prevent worsening of the primary lesions, despite the lack of prospective studies that assess the impact of a normothermia strategy on the outcome [45].
Myocardial injuries are another disease category in which fever can be deleterious. Because of increased oxygen consumption, patients with underlying heart diseases, especially coronary disease and ischaemic cardiomyopathy, are more exposed to the systemic effects of fever. In a swine model of acute myocardial infarction, an elevation of body temperature up to 39°C provoked an increased infarct size [46]. Similarly, in febrile critically ill patients, the reduction of fever from 39 to 37°C induced a decrease of oxygen consumption and unloaded the cardiorespiratory system, which favoured resuscitation of patients who had limited oxygen delivery [47]. In these situations, the benefits of fever control when an infectious process is ongoing must be counterbalanced by the inherent benefits of fever. However, no clinical data are available to support such an approach.
The discomfort from fever is usually claimed to justify fever treatment, although it is not clear whether the discomfort is due to fever per se or rather to the neuroendocrine and/or metabolic response to an infectious process [8,48]. Similarly, the preventive treatment of fever to avoid febrile seizures in children remains a largely debated and controversial issue [6].
Finally, it has been hypothesised that fever could induce collateral tissue damage as a consequence of enhanced microbial killing mechanisms. In a mouse model of Gram-negative bacterial pneumonia, fever tended to worsen survival despite enhanced innate host defence and successful elimination of pathogens. The authors found that the reduced survival was accompanied by increased vascular pulmonary injury, enhanced accumulation of neutrophils and increased levels of cytokines in the bronchoalveolar lavage [49]. Indeed, the same process could also initiate injury to host tissues, suggesting the fact that the ultimate effect of fever is determined by the balance between accelerated pathogen clearance and collateral tissue injury. At a high fever level (>40 to 41°C), however, the beneficial immunomodulatory effect could be outweighed by the deleterious metabolic/inflammatory effect of fever.
Side effects of antipyretic treatments
Despite a lack of experimental and clinical data, febrile ICU patients are frequently treated to lower their fever response [50]. Methods of treatment include direct cooling and/or antipyretic medications such as non-steroidal anti-inflammatory drugs (NSAIDs) and paracetamol. These treatments may delay early diagnosis and appropriate therapy of major infections, and they carry their own undesirable side effects (bleeding, hypotension, hepatic and renal toxicity). These consequences must be taken into account when fever-reducing therapy is initiated in critically ill patients.
Paracetamol
The most serious adverse effect of paracetamol is a life-threatening hepatic necrosis related to overdosage. This necrosis leads to hepatocellular injury in relation to the toxic N-acetyl-p-benzo-quinone imine metabolite when the capacity of glutathione is exceeded. In normal use, paracetamol is safe - but it is noteworthy that acute hepatitis may occur in ICU patients who have reduced glutathione reserves, such as in alcoholics and/or malnourished patients [51]. In addition, clinical evidence suggests that the same metabolic pathway could be involved in the kidney and plays a role in analgesic-associated nephropathy [52].
Interestingly, in a randomised single-blind study, healthy volunteers who received paracetamol (4 g daily for 14 days) experienced a significant increase of serum alanine aminotransferases when compared with placebo [53]. The incidence of maximum alanine aminotransferase increased more than three times the upper normal value in approximately one-third of treated patients. The clinical significance of the alanine aminotransferase elevation is unclear but the implication in ICU patients warrants further investigation.
In contrast to NSAIDs, paracetamol usually is not considered to influence platelet function. However, intra venous paracetamol has been shown to inhibit platelet cyclooxygenase-1 in a dose-dependent anti-aggregatory manner in healthy volunteers [54].
Finally, the potential for paracetamol to produce cardiovascular toxicity is low. Blood pressure was significantly reduced, however, after administration of 1 g paracetamol by mouth or feeding tube [55]. More recently, in 14 febrile critically ill patients, Hersch and colleagues administered an intravenous bolus of propacetamol, 2 g over 15 to 20 minutes, and showed that blood pressure was significantly decreased 15 minutes after infusion. Noteworthy, the systolic blood pressure dropped to <90 mmHg in approximately one-third of patients, requiring both fluid administration and norepinephrine escalade or infusion [56].
Nonsteroidal anti-inflammatory drugs
The main side effect of NSAIDs, gastrointestinal bleeding, derives from their capacity to inhibit cyclooxygenase. NSAIDs with a high affinity for cyclooxygenase-1 are 10 times more likely to induce a gastrointestinal event such as mucosal lesions, a perforated ulcer or gastrointestinal bleeding [57]. NSAIDs are also known to have adverse effects on kidney function through inhibition of prostaglandin synthesis, notably when used in situations in which the renin-angiotensin system is stimulated, such as volume depletion, pre-existing renal failure or concomitant nephrotoxic agents [58,59]. Of note, some NSAIDs may cause vasospasm in patients who have previous coronary artery disease [60].
Risk factors for severe NSAID-induced adverse effects include high dosage, advanced age, concomitant use of steroids or anticoagulants and short duration of therapy, situations that are frequently observed in ICU patients [61].
Physical methods
Physical cooling is usually indicated for the treatment of hyperthermia and fever, but its use remains controversial because of the propensity to induce sympathetic activation, cutaneous vasoconstriction and shivering [62]. As a first consequence, in febrile patients the capacity of external cooling to lower the core temperature may be limited by thermoregulatory mechanisms aiming to maintain the elevated body temperature [63]. Second, if shivering is present, physical cooling causes a rise in oxygen consumption and may be deleterious. In volunteers, induction of fever and active external cooling increased oxygen consumption up to 40% and was associated with a significant increase in catecholamine levels [62]. Therefore, when external cooling is used in the ICU, it is frequently necessary to inhibit shivering by administering therapeutic myorelaxant medication [47]. Moreover, the use of a hypothermia blanket in febrile ICU patients has been shown to induce a large temperature fluctuation and frequent rebound hypothermia [64].
Extracorporeal mechanisms
Although techniques such as extracorporeal membrane oxygenation, haemodialysis or plasmapheresis are not specifically used to decrease fever, they generally lead to normothermia in febrile patients. However, the impact of such consequences remains elusive.
Conclusion
In light of these concerns, healthcare providers have to consider carefully whether to use an antipyretic technique and/or agent in ICU patients by weighing up the risks and the possible benefits.
Conclusion
Fever is a basic response triggered by an infectious or a non-infectious process. The balance of benefit to harm of fever in septic ICU patients is complex. This balance is likely to be dependent on the stage and severity of the infection, on the intensity of the immune response, on the extent of systemic inflammatory response-induced collateral tissue damage as well as on the underlying physiological reserve of the patient (Table 1). On the other hand, the widespread use of antipyretic methods in ICU patients is not supported by clinical data and fever control may be harmful, particularly when an infectious disease is progressing. We await appropriately designed, prospective randomised trials to define patient groups likely to benefit from or be harmed by antipyretic treatment. The decision to introduce an antipyretic therapy should be well balanced according to the presence of neurological injuries and/or underlying cardiac disease and the absence of sepsis.
Table 1.
Summary of the beneficial and detrimental effects of fever
| Beneficial effects | Detrimental effects |
|---|---|
| On invading microorganism | Increased metabolic demand and oxygen consumption (myocardial and neurological injuries) |
| Reduced growth/prolonged growth time | Source of patients' discomfort? |
| Increased antibiotic sensitivity/reduced minimal inhibitory concentration | Children's seizures? (Controversial) |
| Accelerated immune response | Collateral tissue damage? |
| Increased mobility of polymorphonuclear cells | |
| Increased phagocytosis | |
| Increased T-helper cell adherence | |
| Prevention of lymphocytes cell reduction (CD4 T cells and B cells) | |
| Attenuated immune response/protection against the collateral damage | |
| Increased heat shock protein causing a decrease of NF-κB | |
| Reduced TNFα | |
| Reduced IFNγ |
Abbreviations
HSP: heat shock protein; ICU: intensive care unit; IFN: interferon; IL: interleukin; NF: nuclear factor; NSAID: nonsteroidal anti-inflammatory drug; TNF: tumour necrosis factor.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Yoann Launey, Email: yoann.launey@chu-rennes.fr.
Nicolas Nesseler, Email: nicolas.nesseler@chu-rennes.fr.
Yannick Mallédant, Email: yannick.malledant@chu-rennes.fr.
Philippe Seguin, Email: philippe.seguin@chu-rennes.fr.
References
- Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6°F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992;268:1578–1580. doi: 10.1001/jama.268.12.1578. [DOI] [PubMed] [Google Scholar]
- Circiumaru B, Baldock G, Cohen J. A prospective study of fever in the intensive care unit. Intensive Care Med. 1999;25:668–673. doi: 10.1007/s001340050928. [DOI] [PubMed] [Google Scholar]
- Laupland KB. Fever in the critically ill medical patient. Crit Care Med. 2009;37:S273–S278. doi: 10.1097/CCM.0b013e3181aa6117. [DOI] [PubMed] [Google Scholar]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162:1277–1282. doi: 10.1093/infdis/162.6.1277. [DOI] [PubMed] [Google Scholar]
- Fetveit A. Assessment of febrile seizures in children. Eur J Pediatr. 2008;167:17–27. doi: 10.1007/s00431-007-0577-x. [DOI] [PubMed] [Google Scholar]
- Lenhardt R, Negishi C, Sessler DI, Ozaki M, Ettinger K, Bastanmehr H, Lobo E. The effect of pyrogen administration on sweating and vasoconstriction thresholds during desflurane anesthesia. Anesthesiology. 1999;90:1587–1595. doi: 10.1097/00000542-199906000-00014. [DOI] [PubMed] [Google Scholar]
- Gozzoli V, Schöttker P, Suter PM, Ricou B. Is it worth treating fever in intensive care unit patients? Preliminary results from a randomized trial of the effect of external cooling. Arch Intern Med. 2001;161:121–123. doi: 10.1001/archinte.161.1.121. [DOI] [PubMed] [Google Scholar]
- Pierrakos C, Vincent J. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupland KB, Shahpori R, Kirkpatrick AW, Ross T, Gregson DB, Stelfox HT. Occurrence and outcome of fever in critically ill adults. Crit Care Med. 2008;36:1531–1535. doi: 10.1097/CCM.0b013e318170efd3. [DOI] [PubMed] [Google Scholar]
- Peres Bota D, Lopes Ferreira F, Melot C, Vincent JL. Body temperature alterations in the critically ill. Intensive Care Med. 2004;30:811–816. doi: 10.1007/s00134-004-2166-z. [DOI] [PubMed] [Google Scholar]
- O'Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, Linden P, Maki DG, Nierman D, Pasculle W, Masur H. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36:1330–1349. doi: 10.1097/CCM.0b013e318169eda9. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- Moran JL, Peter JV, Solomon PJ, Grealy B, Smith T, Ashforth W, Wake M, Peake SL, Peisach AR. Tympanic temperature measurements: are they reliable in the critically ill? A clinical study of measures of agreement. Crit Care Med. 2007;35:155–164. doi: 10.1097/01.CCM.0000250318.31453.CB. [DOI] [PubMed] [Google Scholar]
- Lefrant J, Muller L, de La Coussaye JE, Benbabaali M, Lebris C, Zeitoun N, Mari C, Saïssi G, Ripart J, Eledjam J. Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med. 2003;29:414–418. doi: 10.1007/s00134-002-1619-5. [DOI] [PubMed] [Google Scholar]
- Netea MG, Kullberg BJ, Van der Meer JW. Circulating cytokines as mediators of fever. Clin Infect Dis. 2000;31(Suppl 5):S178–S184. doi: 10.1086/317513. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1β. Proc Natl Acad Sci USA. 2006;103:2904–2908. doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlik V, Li Z, Goorha S, Ballou LR, Blatteis CM. LPS-activated complement, not LPS per se, triggers the early release of PGE2 by Kupffer cells. Am J Physiol Regul Integr Comp Physiol. 2005;289:R332–R339. doi: 10.1152/ajpregu.00567.2004. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Székely M. Neural route of pyrogen signaling to the brain. Clin Infect Dis. 2000;31(Suppl 5):S162–S167. doi: 10.1086/317515. [DOI] [PubMed] [Google Scholar]
- Prescott LM. Microbiology. Boston, MA: WCB/McGraw-Hill; 1999. [Google Scholar]
- Small PM, Täuber MG, Hackbarth CJ, Sande MA. Influence of body temperature on bacterial growth rates in experimental pneumococcal meningitis in rabbits. Infect Immun. 1986;52:484–487. doi: 10.1128/iai.52.2.484-487.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D. Febrile temperatures can synchronize the growth of Plasmodium falciparum in vitro. J Exp Med. 1989;169:357–361. doi: 10.1084/jem.169.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak PA, Marling-Cason M, Cohen RL. Effects of temperature on antimicrobial susceptibility of bacteria. J Infect Dis. 1982;145:550–553. doi: 10.1093/infdis/145.4.550. [DOI] [PubMed] [Google Scholar]
- Ozveri ES, Bekraki A, Cingi A, Yuksel M, Demiralp EE, Yegen BC, Aktan AO. The effect of hyperthermic preconditioning on the immune system in rat peritonitis. Intensive Care Med. 1999;25:1155–1159. doi: 10.1007/s001340051028. [DOI] [PubMed] [Google Scholar]
- Hasday JD, Singh IS. Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperones. 2000;5:471–480. doi: 10.1379/1466-1268(2000)005<0471:FATHSR>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Andersson R. NF-κB activation and inhibition: a review. Shock. 2002;18:99–106. doi: 10.1097/00024382-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Villar J, Ribeiro SP, Mullen JB, Kuliszewski M, Post M, Slutsky AS. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med. 1994;22:914–921. [PubMed] [Google Scholar]
- Su F, Nguyen ND, Wang Z, Cai Y, Rogiers P, Vincent J. Fever control in septic shock: beneficial or harmful? Shock. 2005;23:516–520. [PubMed] [Google Scholar]
- Bryant RE, Hood AF, Hood CE, Koenig MG. Factors affecting mortality of Gram-negative rod bacteremia. Arch Intern Med. 1971;127:120–128. doi: 10.1001/archinte.127.1.120. [DOI] [PubMed] [Google Scholar]
- Weinstein MP, Iannini PB, Stratton CW, Eickhoff TC. Spontaneous bacterial peritonitis. A review of 28 cases with emphasis on improved survival and factors influencing prognosis. Am J Med. 1978;64:592–598. doi: 10.1016/0002-9343(78)90578-8. [DOI] [PubMed] [Google Scholar]
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology. 1982;2:399–407. doi: 10.1002/hep.1840020402. [DOI] [PubMed] [Google Scholar]
- Ahkee S, Srinath L, Ramirez J. Community-acquired pneumonia in the elderly: association of mortality with lack of fever and leukocytosis. South Med J. 1997;90:296–298. doi: 10.1097/00007611-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Leroy O, Gangneux J, Montravers P, Mira J, Gouin F, Sollet J, Carlet J, Reynes J, Rosenheim M, Regnier B, Lortholary O. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005-2006) Crit Care Med. 2009;37:1612–1618. doi: 10.1097/CCM.0b013e31819efac0. [DOI] [PubMed] [Google Scholar]
- Arons MM, Wheeler AP, Bernard GR, Christman BW, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson W, Wright P, Dupont WD, Swindell BB. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Ibuprofen in Sepsis Study Group. Crit Care Med. 1999;27:699–707. doi: 10.1097/00003246-199904000-00020. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975;188:166–168. doi: 10.1126/science.1114347. [DOI] [PubMed] [Google Scholar]
- Bernheim HA, Kluger MJ. Fever: effect of drug-induced antipyresis on survival. Science. 1976;193:237–239. doi: 10.1126/science.935867. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun. 2000;68:1265–1270. doi: 10.1128/IAI.68.3.1265-1270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyers S, Weatherall M, Shirtcliffe P, Perrin K, Beasley R. The effect on mortality of antipyretics in the treatment of influenza infection: systematic review and meta-analysis. J R Soc Med. 2010;103:403–411. doi: 10.1258/jrsm.2010.090441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandts CH, Ndjavé M, Graninger W, Kremsner PG. Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet. 1997;350:704–709. doi: 10.1016/S0140-6736(97)02255-1. [DOI] [PubMed] [Google Scholar]
- Prymula R, Siegrist C, Chlibek R, Zemlickova H, Vackova M, Smetana J, Lommel P, Kaliskova E, Borys D, Schuerman L. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet. 2009;374:1339–1350. doi: 10.1016/S0140-6736(09)61208-3. [DOI] [PubMed] [Google Scholar]
- Schulman CI, Namias N, Doherty J, Manning RJ, Li P, Elhaddad A, Lasko D, Amortegui J, Dy CJ, Dlugasch L, Baracco G, Cohn SM. The effect of antipyretic therapy upon outcomes in critically ill patients: a randomized, prospective study. Surg Infect (Larchmt) 2005;6:369–375. doi: 10.1089/sur.2005.6.369. [DOI] [PubMed] [Google Scholar]
- Saini M, Saqqur M, Kamruzzaman A, Lees KR, Shuaib A. Effect of hyperthermia on prognosis after acute ischemic stroke. Stroke. 2009;40:3051–3059. doi: 10.1161/STROKEAHA.109.556134. [DOI] [PubMed] [Google Scholar]
- Hajat C, Hajat S, Sharma P. Effects of poststroke pyrexia on stroke outcome: a meta-analysis of studies in patients. Stroke. 2000;31:410–414. doi: 10.1161/01.str.31.2.410. [DOI] [PubMed] [Google Scholar]
- Jiang J, Gao G, Li W, Yu M, Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma. 2002;19:869–874. doi: 10.1089/08977150260190456. [DOI] [PubMed] [Google Scholar]
- Badjatia N. Hyperthermia and fever control in brain injury. Crit Care Med. 2009;37:S250–S257. doi: 10.1097/CCM.0b013e3181aa5e8d. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Klassen CL, Ishibashi Y, Herrlinger SH, Pavek TJ, Bache RJ. Effect of temperature on myocardial infarction in swine. Am J Physiol. 1996;270:H1189–H1199. doi: 10.1152/ajpheart.1996.270.4.H1189. [DOI] [PubMed] [Google Scholar]
- Manthous CA, Hall JB, Olson D, Singh M, Chatila W, Pohlman A, Kushner R, Schmidt GA, Wood LD. Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med. 1995;151:10–14. doi: 10.1164/ajrccm.151.1.7812538. [DOI] [PubMed] [Google Scholar]
- Betts RF Chapman SW Penn RL Reese and Betts' A Practical Approach to Infectious Diseases 2003Philadelphia, PA: Lippincott Williams & Wilkins; 984766 [Google Scholar]
- Rice P, Martin E, He J, Frank M, DeTolla L, Hester L, O'Neill T, Manka C, Benjamin I, Nagarsekar A, Singh I, Hasday JD. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental Gramnegative bacterial pneumonia. J Immunol. 2005;174:3676–3685. doi: 10.4049/jimmunol.174.6.3676. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Wheeler AP, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson WJ, Wright PE, Christman BW, Dupont WD, Higgins SB, Swindell BB. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med. 1997;336:912–918. doi: 10.1056/NEJM199703273361303. [DOI] [PubMed] [Google Scholar]
- Moling O, Cairon E, Rimenti G, Rizza F, Pristerá R, Mian P. Severe hepatotoxicity after therapeutic doses of acetaminophen. Clin Ther. 2006;28:755–760. doi: 10.1016/j.clinthera.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Blantz RC. Acetaminophen: acute and chronic effects on renal function. Am J Kidney Dis. 1996;28:S3–S6. doi: 10.1016/S0272-6386(96)90561-2. [DOI] [PubMed] [Google Scholar]
- Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- Munsterhjelm E, Munsterhjelm NM, Niemi TT, Ylikorkala O, Neuvonen PJ, Rosenberg PH. Dose-dependent inhibition of platelet function by acetaminophen in healthy volunteers. Anesthesiology. 2005;103:712–717. doi: 10.1097/00000542-200510000-00009. [DOI] [PubMed] [Google Scholar]
- Boyle M, Hundy S, Torda TA. Paracetamol administration is associated with hypotension in the critically ill. Aust Crit Care. 1997;10:120–122. doi: 10.1016/S1036-7314(97)70414-4. [DOI] [PubMed] [Google Scholar]
- Hersch M, Raveh D, Izbicki G. Effect of intravenous propacetamol on blood pressure in febrile critically ill patients. Pharmacotherapy. 2008;28:1205–1210. doi: 10.1592/phco.28.10.1205. [DOI] [PubMed] [Google Scholar]
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991;115:787–796. doi: 10.7326/0003-4819-115-10-787. [DOI] [PubMed] [Google Scholar]
- Pirson Y, van Ypersele de Strihou C. Renal side effects of nonsteroidal antiinflammatory drugs: clinical relevance. Am J Kidney Dis. 1986;8:338–344. doi: 10.1016/s0272-6386(86)80108-1. [DOI] [PubMed] [Google Scholar]
- Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106:13S–24S. doi: 10.1016/S0002-9343(99)00113-8. [DOI] [PubMed] [Google Scholar]
- Greisman LA, Mackowiak PA. Fever: beneficial and detrimental effects of antipyretics. Curr Opin Infect Dis. 2002;15:241–245. doi: 10.1097/00001432-200206000-00005. [DOI] [PubMed] [Google Scholar]
- Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- Lenhardt R, Negishi C, Sessler DI, Vuong K, Bastanmehr H, Kim JS, Bjorksten AR. The effects of physical treatment on induced fever in humans. Am J Med. 1999;106:550–555. doi: 10.1016/S0002-9343(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Kurz A, Sessler DI, Christensen R, Dechert M. Heat balance and distribution during the core-temperature plateau in anesthetized humans. Anesthesiology. 1995;83:491–499. doi: 10.1097/00000542-199509000-00007. [DOI] [PubMed] [Google Scholar]
- O'Donnell J, Axelrod P, Fisher C, Lorber B. Use and effectiveness of hypothermia blankets for febrile patients in the intensive care unit. Clin Infect Dis. 1997;24:1208–1213. doi: 10.1086/513660. [DOI] [PubMed] [Google Scholar]