Abstract
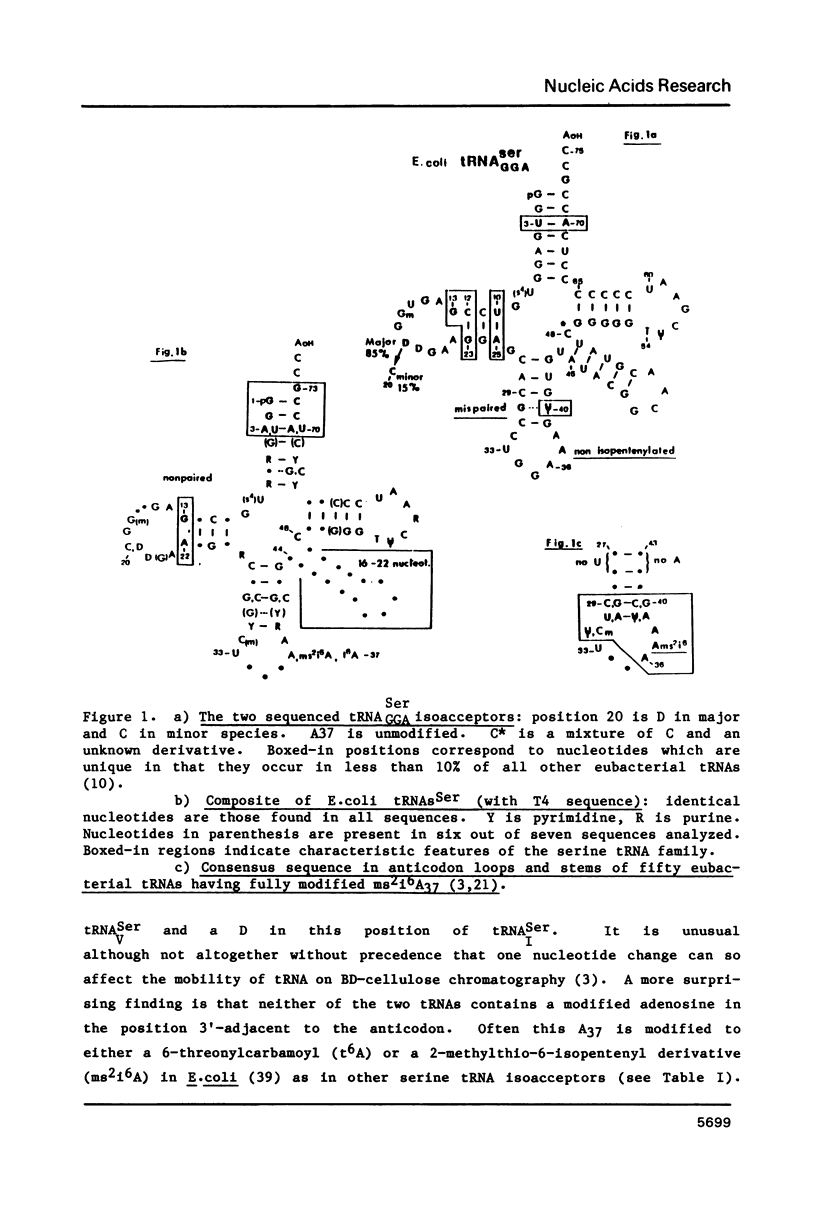
We have determined the nucleotide sequence of the major species of E. coli tRNASer and of a minor species having the same GGA anticodon. These two tRNAs should recognize the UCC and UCU codons, the most widely used codons for serine in the highly expressed genes of E. coli. The two sequences differ in only one position of the D-loop. Neither tRNA has a modified adenosine in the position 3'-adjacent to the anticodon. This can be rationalized on the basis of a structural constraint in the anticodon stem and may be related to optimization of the codon-anticodon interaction. Comparison of all E.coli serine tRNAs (and that encoded by bacteriophage T4) reveals characteristic (possibly functional) features. Evolutionary analysis suggests an eubacterial origin of the T4 tRNASer gene and the existence of a recent common ancestor for the tRNASerGGA and tRNASerGUC genes.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. J., Burrows W. J., Skoog F., Roy K. L., Söll D. Cytokinins: distribution in transfer RNA species of Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):834–841. doi: 10.1073/pnas.63.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J., Söll D., Burrows W. J., Skoog F. Identification of the cytokinin-active ribonucleosides in pure Escherichia coli tRNA species. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1448–1453. doi: 10.1073/pnas.67.3.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Ames B. N. A modified nucleotide in tRNA as a possible regulator of aerobiosis: synthesis of cis-2-methyl-thioribosylzeatin in the tRNA of Salmonella. Cell. 1984 Feb;36(2):523–531. doi: 10.1016/0092-8674(84)90245-9. [DOI] [PubMed] [Google Scholar]
- Cedergren R. J., LaRue B., Sankoff D., Lapalme G., Grosjean H. Convergence and minimal mutation criteria for evaluating early events in tRNA evolution. Proc Natl Acad Sci U S A. 1980 May;77(5):2791–2795. doi: 10.1073/pnas.77.5.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedergren R. J., Sankoff D., LaRue B., Grosjean H. The evolving tRNA molecule. CRC Crit Rev Biochem. 1981;11(1):35–104. doi: 10.3109/10409238109108699. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Piper P. W. Compilation of mutant suppressor tRNA sequences. Nucleic Acids Res. 1982 Jan 22;10(2):r83–r91. doi: 10.1093/nar/10.2.762-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers D. M., Seno T., Söll G. Is there a discriminator site in transfer RNA? Proc Natl Acad Sci U S A. 1972 Oct;69(10):3063–3067. doi: 10.1073/pnas.69.10.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S. P., Yarus M., Soll L. The effect of an Escherichia coli regulatory mutation on transfer RNA structure. J Mol Biol. 1979 Nov 25;135(1):111–126. doi: 10.1016/0022-2836(79)90343-7. [DOI] [PubMed] [Google Scholar]
- Goddard J. P. The structures and functions of transfer RNA. Prog Biophys Mol Biol. 1977;32(3):233–308. [PubMed] [Google Scholar]
- Grosjean H., Cedergren R. J., McKay W. Structure in tRNA data. Biochimie. 1982 Jun;64(6):387–397. doi: 10.1016/s0300-9084(82)80576-2. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Sankoff D., Jou W. M., Fiers W., Cedergren R. J. Bacteriophage MS2 RNA: a correlation between the stability of the codon: anticodon interaction and the choice of code words. J Mol Evol. 1978 Dec 29;12(2):113–119. doi: 10.1007/BF01733262. [DOI] [PubMed] [Google Scholar]
- Gu X. R., Nicoghosian K., Cedergren R. J., Wong J. T. Sequences of halobacterial tRNAs and the paucity of U in the first position of their anticodons. Nucleic Acids Res. 1983 Aug 25;11(16):5433–5442. doi: 10.1093/nar/11.16.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Rapid print-readout technique for sequencing of RNA's containing modified nucleotides. Nucleic Acids Res. 1979 Aug 10;6(11):3443–3458. doi: 10.1093/nar/6.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Wilhelm R. C. Genetic mapping and dominance of the amber suppressor, Su1 (supD), in Escherichia coli K-12. J Bacteriol. 1970 Jul;103(1):32–36. doi: 10.1128/jb.103.1.32-36.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T., Ozeki H. Gross map location of Escherichia coli transfer RNA genes. J Mol Biol. 1977 Dec 5;117(2):419–446. doi: 10.1016/0022-2836(77)90136-x. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Clark B. F. The nucleotide sequence of a serine transfer ribonucleic acid from Escherichia coli. J Biol Chem. 1973 Oct 10;248(19):6663–6673. [PubMed] [Google Scholar]
- Ishikura H., Yamada Y., Nishimura S. Structure of serine tRNA from Escherichia coli. I. Purification of serine tRNA's with different codon responses. Biochim Biophys Acta. 1971 Jan 28;228(2):471–481. doi: 10.1016/0005-2787(71)90052-9. [DOI] [PubMed] [Google Scholar]
- McLennan B. D., Buck M., Humphreys J., Griffiths E. Iron-related modification of bacterial transfer RNA. Nucleic Acids Res. 1981 Jun 11;9(11):2629–2640. doi: 10.1093/nar/9.11.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz P., Amstutz H., Kohli J., Leupold U. Recombination between dispersed serine tRNA genes in Schizosaccharomyces pombe. Nature. 1982 Nov 18;300(5889):225–231. doi: 10.1038/300225a0. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather N. E., Murgola E. J., Mims B. H. Nucleotide substitution in the amino acid acceptor stem of lysine transfer RNA causes missense suppression. J Mol Biol. 1984 Jan 15;172(2):177–184. doi: 10.1016/s0022-2836(84)80036-4. [DOI] [PubMed] [Google Scholar]
- Roy K. L., Söll D. Purification of five serine transfer ribonucleic acid species from Escherichia coli and their acylation by homologous and heterologous seryl transfer ribonucleic acid synthetases. J Biol Chem. 1970 Mar 25;245(6):1394–1400. [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Smith D. W., McNamara A. L., Rice M., Hatfield D. L. The effects of a post-transcriptional modification on the function of tRNALys isoaccepting species in translation. J Biol Chem. 1981 Oct 10;256(19):10033–10036. [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1984;12 (Suppl):r1–57. [PMC free article] [PubMed] [Google Scholar]
- Steege D. A. A nucleotide change in the anticodon of an Escherichia coli serine transfer RNA results in supD-amber suppression. Nucleic Acids Res. 1983 Jun 11;11(11):3823–3832. doi: 10.1093/nar/11.11.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundharadas G., Katze J. R., Söll D., Konigsberg W., Lengyel P. On the recognition of serine transfer RNA's specific for unrelated codons by the same seryl-transfer RNA synthetase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):693–700. doi: 10.1073/pnas.61.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorbjarnardóttir S., Dingermann T., Rafnar T., Andrésson O. S., Söll D., Eggertsson G. Leucine tRNA family of Escherichia coli: nucleotide sequence of the supP(Am) suppressor gene. J Bacteriol. 1985 Jan;161(1):219–222. doi: 10.1128/jb.161.1.219-222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorbjarnardóttir S., Uemura H., Dingermann T., Rafnar T., Thorsteinsdóttir S., Söll D., Eggertsson G. Escherichia coli supH suppressor: temperature-sensitive missense suppression caused by an anticodon change in tRNASer2. J Bacteriol. 1985 Jan;161(1):207–211. doi: 10.1128/jb.161.1.207-211.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuriaux P. Distribution of family-specific sequence homologies in six families of isoaccepting tRNAs. Biochimie. 1983 Oct;65(10):585–588. doi: 10.1016/s0300-9084(83)80109-6. [DOI] [PubMed] [Google Scholar]
- Tsang T. H., Buck M., Ames B. N. Sequence specificity of tRNA-modifying enzymes. An analysis of 258 tRNA sequences. Biochim Biophys Acta. 1983 Nov 17;741(2):180–196. doi: 10.1016/0167-4781(83)90058-1. [DOI] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Neill R. J., Landsberg R., Ames B. N. Pseudouridylation of tRNAs and its role in regulation in Salmonella typhimurium. J Biol Chem. 1979 Jun 25;254(12):5111–5119. [PubMed] [Google Scholar]
- Uemura H., Imai M., Ohtsuka E., Ikehara M., Söll D. E. coli initiator tRNA analogs with different nucleotides in the discriminator base position. Nucleic Acids Res. 1982 Oct 25;10(20):6531–6539. doi: 10.1093/nar/10.20.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Grosjean H. Effect of threonylcarbamoyl modification (t6A) in yeast tRNA Arg III on codon-anticodon and anticodon-anticodon interactions. A thermodynamic and kinetic evaluation. Eur J Biochem. 1981 May;116(1):207–213. doi: 10.1111/j.1432-1033.1981.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Ishikura H. Identification of a modified nucleoside in Escherichia coli tRNA1Ser as 2'-O-methylcytidine. Biochim Biophys Acta. 1975 Sep 1;402(3):285–287. doi: 10.1016/0005-2787(75)90265-8. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Yarus M. Translational efficiency of transfer RNA's: uses of an extended anticodon. Science. 1982 Nov 12;218(4573):646–652. doi: 10.1126/science.6753149. [DOI] [PubMed] [Google Scholar]



